Abstract
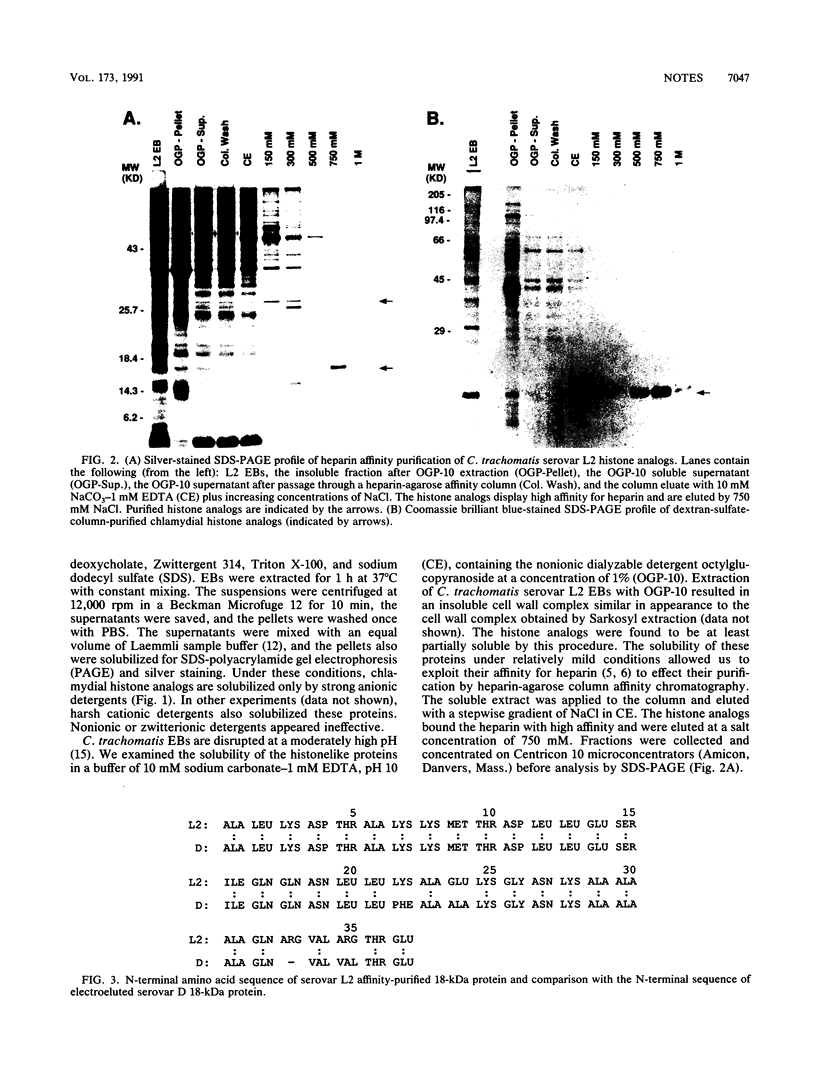
DNA-binding proteins specific to Chlamydia trachomatis elementary bodies have been described and recently characterized as procaryotic histone analogs. I have developed an affinity purification procedure for the 18-kDa histone analog, Hc1, based on its affinity for polyanions. The availability of highly purified Hc1 has allowed for determination of its N-terminal amino acid sequence and should prove useful in studies of its biological function. The variable C. trachomatis histone analog not obtained by this procedure was electrophoresed onto Immobilon paper for sequencing. The N terminus of the variable histone was conserved among C. trachomatis serotypes L2, D, and B and was distinct from that of Hc1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bavoil P., Ohlin A., Schachter J. Role of disulfide bonding in outer membrane structure and permeability in Chlamydia trachomatis. Infect Immun. 1984 May;44(2):479–485. doi: 10.1128/iai.44.2.479-485.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Poffenroth L., Wilt J. C., Kordová N. Ultrastructural studies of the nucleoids of the pleomorphic forms of Chlamydia psittaci 6BC: a comparison with bacteria. Can J Microbiol. 1976 Jan;22(1):16–28. doi: 10.1139/m76-003. [DOI] [PubMed] [Google Scholar]
- Gray G. J., Kaul R., Roy K. L., Wenman W. M. Isolation and molecular characterization of the ribosomal protein L6 homolog from Chlamydia trachomatis. J Bacteriol. 1991 Mar;173(5):1663–1669. doi: 10.1128/jb.173.5.1663-1669.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Baehr W., Ying Y. Chlamydia trachomatis developmentally regulated protein is homologous to eukaryotic histone H1. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3937–3941. doi: 10.1073/pnas.88.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T. Identification and properties of chlamydial polypeptides that bind eucaryotic cell surface components. J Bacteriol. 1986 Jan;165(1):13–20. doi: 10.1128/jb.165.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Todd W. J., Caldwell H. D. Disulfide-mediated interactions of the chlamydial major outer membrane protein: role in the differentiation of chlamydiae? J Bacteriol. 1985 Jan;161(1):25–31. doi: 10.1128/jb.161.1.25-31.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Allan I., Pearce J. H. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol. 1984 Jan;157(1):13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul R., Roy K. L., Wenman W. M. Cloning, expression, and primary structure of a Chlamydia trachomatis binding protein. J Bacteriol. 1987 Nov;169(11):5152–5156. doi: 10.1128/jb.169.11.5152-5156.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle G. T., Painter S. D., Blankenship J. E., Kurosky A. Proteolytic processing of egg-laying hormone-related precursors in Aplysia. Identification of peptide regions critical for biological activity. J Biol Chem. 1988 Jul 5;263(19):9223–9237. [PubMed] [Google Scholar]
- Narita T., Wyrick P. B., Manire G. P. Effect of alkali on the structure of cell envelopes of Chlamydia psittaci elementary bodies. J Bacteriol. 1976 Jan;125(1):300–307. doi: 10.1128/jb.125.1.300-307.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Jones R. B. Disulfide-linked oligomers of the major outer membrane protein of chlamydiae. J Bacteriol. 1983 May;154(2):998–1001. doi: 10.1128/jb.154.2.998-1001.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S., Kaul R., Wenman W. M. Identification and nucleotide sequence of a developmentally regulated gene encoding a eukaryotic histone H1-like protein from Chlamydia trachomatis. J Bacteriol. 1991 May;173(9):2818–2822. doi: 10.1128/jb.173.9.2818-2822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagar E. A., Stephens R. S. Developmental-form-specific DNA-binding proteins in Chlamydia spp. Infect Immun. 1988 Jul;56(7):1678–1684. doi: 10.1128/iai.56.7.1678-1684.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenman W. M., Meuser R. U. Chlamydia trachomatis elementary bodies possess proteins which bind to eucaryotic cell membranes. J Bacteriol. 1986 Feb;165(2):602–607. doi: 10.1128/jb.165.2.602-607.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]





