Abstract
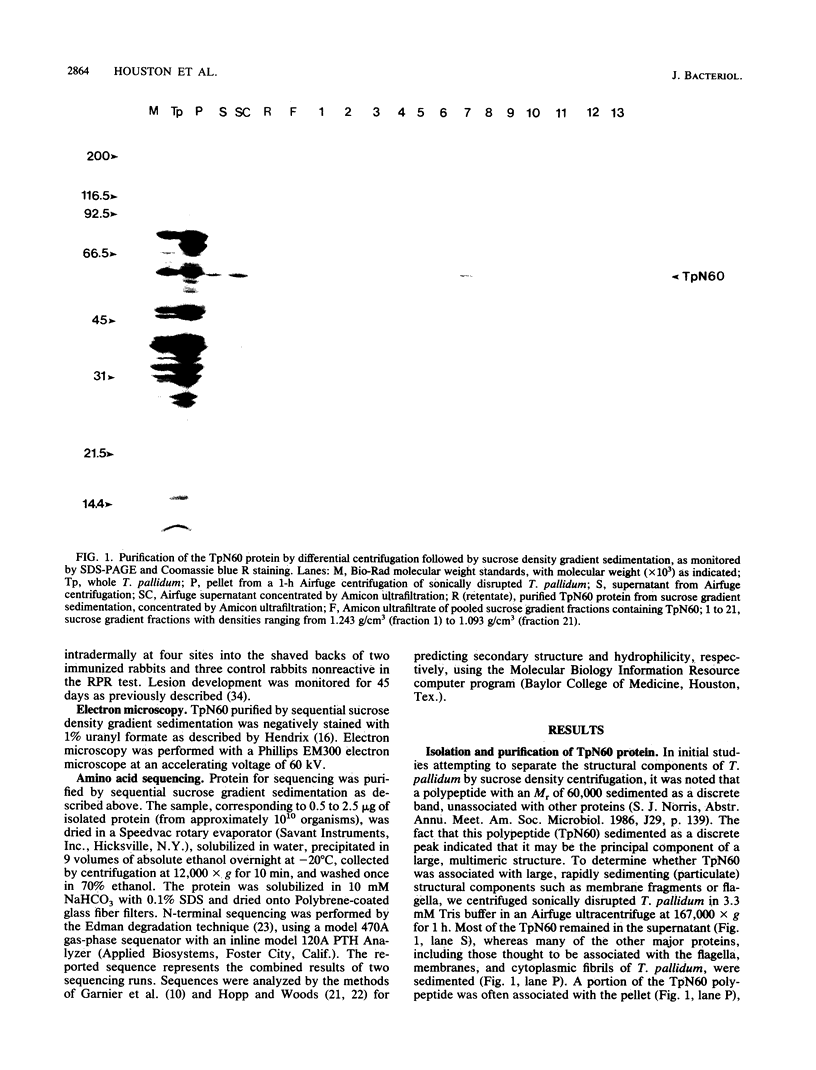
A native structure containing the major 60-kilodalton common antigen polypeptide (designated TpN60) was isolated from Treponema pallidum subsp. pallidum (Nichols strain) through a combination of differential centrifugation and sucrose density gradient sedimentation. Gel filtration chromatography indicated that this structure is a high-molecular-weight homo-oligomer of TpN60. Antisera to TpN60 reacted with the groEL polypeptide of Escherichia coli, as determined by immunoperoxidase staining of two-dimensional electroblots. Electron microscopy of the isolated complex revealed a ringlike structure with a diameter of approximately 16 nm which was very similar in appearance to the groEL protein. Comparison of the N-terminal amino acid sequence of TpN60 with the deduced sequences of the E. coli groEL protein, related chaperonin proteins from mycobacteria and Coxiella burnetti, the hsp60 protein of Saccharomyces cerevisiae, the wheat ribulose bisphosphate carboxylase-oxygenase-subunit-binding protein (alpha subunit), and the human P1 mitochondrial protein indicated sequence identity at 8 of 22 to 10 of 22 residues (36 to 45% identity). We conclude that the oligomer of TpN60 is homologous to the groEL protein and related chaperonins found in a wide variety of procaryotes and eucaryotes and thus may represent a heat shock protein involved in protein folding and assembly.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bangsborg J. M., Collins M. T., Høiby N., Hindersson P. Cloning and expression of the Legionella micdadei "common antigen" in Escherichia coli. APMIS. 1989 Jan;97(1):14–22. [PubMed] [Google Scholar]
- Carrascosa J. L., García J. A., Salas M. A protein similar to Escherichia coli gro EL is present in Bacillus subtilis. J Mol Biol. 1982 Jul 15;158(4):731–737. doi: 10.1016/0022-2836(82)90257-1. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar G. N., Tilly K., Woolford C., Hendrix R., Georgopoulos C. Purification and properties of the groES morphogenetic protein of Escherichia coli. J Biol Chem. 1986 Sep 15;261(26):12414–12419. [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Dudani A. K., Gupta R. S. Immunological characterization of a human homolog of the 65-kilodalton mycobacterial antigen. Infect Immun. 1989 Sep;57(9):2786–2793. doi: 10.1128/iai.57.9.2786-2793.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayet O., Ziegelhoffer T., Georgopoulos C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989 Mar;171(3):1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Hanff P. A., Norris S. J., Lovett M. A., Miller J. N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 1984 Oct-Dec;11(4):275–286. doi: 10.1097/00007435-198410000-00003. [DOI] [PubMed] [Google Scholar]
- Hansen K., Bangsborg J. M., Fjordvang H., Pedersen N. S., Hindersson P. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen common to a wide range of bacteria. Infect Immun. 1988 Aug;56(8):2047–2053. doi: 10.1128/iai.56.8.2047-2053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W. Purification and properties of groE, a host protein involved in bacteriophage assembly. J Mol Biol. 1979 Apr 15;129(3):375–392. doi: 10.1016/0022-2836(79)90502-3. [DOI] [PubMed] [Google Scholar]
- Hindersson P., Knudsen J. D., Axelsen N. H. Cloning and expression of treponema pallidum common antigen (Tp-4) in Escherichia coli K12. J Gen Microbiol. 1987 Mar;133(3):587–596. doi: 10.1099/00221287-133-3-587. [DOI] [PubMed] [Google Scholar]
- Hindersson P., Petersen C. S., Pedersen N. S., Høiby N., Axelsen N. H. Immunological cross-reaction between antigen Tp-4 of Treponema pallidum and an antigen common to a wide range of bacteria. Acta Pathol Microbiol Immunol Scand B. 1984 Aug;92(4):183–188. doi: 10.1111/j.1699-0463.1984.tb02818.x. [DOI] [PubMed] [Google Scholar]
- Hoffman P. S., Butler C. A., Quinn F. D. Cloning and temperature-dependent expression in Escherichia coli of a Legionella pneumophila gene coding for a genus-common 60-kilodalton antigen. Infect Immun. 1989 Jun;57(6):1731–1739. doi: 10.1128/iai.57.6.1731-1739.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P. Protein surface analysis. Methods for identifying antigenic determinants and other interaction sites. J Immunol Methods. 1986 Apr 3;88(1):1–18. doi: 10.1016/0022-1759(86)90045-1. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. A computer program for predicting protein antigenic determinants. Mol Immunol. 1983 Apr;20(4):483–489. doi: 10.1016/0161-5890(83)90029-9. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaijser B. Immunological studies of an antigen common to many gram-negative bacteria with special reference to E. coli. Characterization and biological significance. Int Arch Allergy Appl Immunol. 1975;48(1):72–81. doi: 10.1159/000231293. [DOI] [PubMed] [Google Scholar]
- Kusukawa N., Yura T. Heat shock protein GroE of Escherichia coli: key protective roles against thermal stress. Genes Dev. 1988 Jul;2(7):874–882. doi: 10.1101/gad.2.7.874. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger M., Smogór W. Artificial immunization of rabbits against syphilis. I. Effect of increasing doses of treponemes given by the intramuscular route. Br J Vener Dis. 1969 Dec;45(4):308–312. doi: 10.1136/sti.45.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. N. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by -irradiation. J Immunol. 1973 May;110(5):1206–1215. [PubMed] [Google Scholar]
- NELSON R. A., Jr, DIESENDRUCK J. A., ZHEUTLIN H. E. C., STACK P. S., BARNETT M. Studies on treponemal immobilizing antibodies in syphilis. I. Techniques of measurement and factors influencing immobilization. J Immunol. 1951 Jun;66(6):667–685. [PubMed] [Google Scholar]
- Norris S. J., Miller J. N., Sykes J. A., Fitzgerald T. J. Influence of oxygen tension, sulfhydryl compounds, and serum on the motility and virulence of Treponema pallidum (Nichols strain) in a cell-free system. Infect Immun. 1978 Dec;22(3):689–697. doi: 10.1128/iai.22.3.689-697.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Pedersen N. S., Axelsen N. H., Petersen C. S. Antigenic analysis of Treponema pallidum: cross-reactions between individual antigens of T. pallidum and T. Reiter. Scand J Immunol. 1981;13(2):143–150. doi: 10.1111/j.1365-3083.1981.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Penn C. W., Rhodes J. G. Surface-associated antigens of Treponema pallidum concealed by an inert outer layer. Immunology. 1982 May;46(1):9–16. [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Norgard M. V., Schulz W. W. Outer membrane ultrastructure explains the limited antigenicity of virulent Treponema pallidum. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2051–2055. doi: 10.1073/pnas.86.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading D. S., Hallberg R. L., Myers A. M. Characterization of the yeast HSP60 gene coding for a mitochondrial assembly factor. Nature. 1989 Feb 16;337(6208):655–659. doi: 10.1038/337655a0. [DOI] [PubMed] [Google Scholar]
- Sand Petersen C., Strandberg Pedersen N., Axelsen N. H. Isolation and characterization of TR-c, an antigen of the Reiter treponeme precipitating with antibodies in syphilis. Scand J Immunol. 1981 May;15(5):459–465. doi: 10.1111/j.1365-3083.1982.tb00671.x. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky D., Hertz J. B., Høiby N., Jensen K., Mansa B., Pedersen V. B., Samra Z. An antigen common to a wide range of bacteria. 2. A biochemical study of a "common antigen" from Pseudomonas aeruginosa. Acta Pathol Microbiol Scand B. 1980 Oct;88(5):253–260. doi: 10.1111/j.1699-0463.1980.tb02637.x. [DOI] [PubMed] [Google Scholar]
- Stamm L. V., Hodinka R. L., Wyrick P. B., Bassford P. J., Jr Changes in the cell surface properties of Treponema pallidum that occur during in vitro incubation of freshly extracted organisms. Infect Immun. 1987 Sep;55(9):2255–2261. doi: 10.1128/iai.55.9.2255-2261.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M. H., Williams J. C. A heat shock operon in Coxiella burnetti produces a major antigen homologous to a protein in both mycobacteria and Escherichia coli. J Bacteriol. 1988 Mar;170(3):1227–1234. doi: 10.1128/jb.170.3.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E. M., Zampighi G. A., Blanco D. R., Miller J. N., Lovett M. A. Demonstration of rare protein in the outer membrane of Treponema pallidum subsp. pallidum by freeze-fracture analysis. J Bacteriol. 1989 Sep;171(9):5005–5011. doi: 10.1128/jb.171.9.5005-5011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]