Abstract
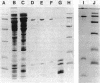
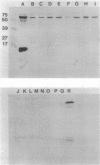
Morganella morganii, a very common cause of catheter-associated bacteriuria, was previously classified with the genus Proteus on the basis of urease production. M. morganii constitutively synthesizes a urease distinct from that of other uropathogens. The enzyme, purified 175-fold by passage through DEAE-Sepharose, phenyl-Sepharose, Mono-Q, and Superose 6 chromatography resins, was found to have a native molecular size of 590 kilodaltons and was composed of three distinct subunits with apparent molecular sizes of 63, 15, and 6 kilodaltons, respectively. Amino-terminal analysis of the subunit polypeptides revealed a high degree of conservation of amino acid sequence between jack bean and Proteus mirabilis ureases. Km for urea equalled 0.8 mM. Antiserum prepared against purified enzyme inhibited activity by 43% at a 1:2 dilution after 1 h of incubation. All urease activity was immunoprecipitated from cytosol by a 1:16 dilution. Antiserum did not precipitate ureases of other species except for one Providencia rettgeri strain but did recognize the large subunits of ureases of Providencia and Proteus species on Western blots (immunoblots). Thirteen urease-positive cosmid clones of Morganella chromosomal DNA shared a 3.5-kilobase (kb) BamHI fragment. Urease gene sequences were localized to a 7.1-kb EcoRI-SalI fragment. Tn5 mutagenesis revealed that between 3.3 and 6.6 kb of DNA were necessary for enzyme activity. A Morganella urease DNA probe did not hybridize with gene sequences of other species tested. Morganella urease antiserum recognized identical subunit polypeptides on Western blots of cytosol from the wild-type strain and Escherichia coli bearing the recombinant clone which corresponded to those seen in denatured urease. Although the wild-type strain and recombinant clone produced equal amounts of urease protein, the clone produced less than 1% of the enzyme activity of the wild-type strain.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barriere S. L., Flaherty J. F. Third-generation cephalosporins: a critical evaluation. Clin Pharm. 1984 Jul-Aug;3(4):351–373. [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignani G., Belgrano E., Puppo P., Cichero A., Giuliani L. Hydroxyurea in the management of chronic urea-splitting urinary infections. Br J Urol. 1980 Aug;52(4):316–320. doi: 10.1111/j.1464-410x.1980.tb08924.x. [DOI] [PubMed] [Google Scholar]
- Cox C. E. Aztreonam therapy for complicated urinary tract infections caused by multidrug-resistant bacteria. Rev Infect Dis. 1985 Nov-Dec;7 (Suppl 4):S767–S771. doi: 10.1093/clinids/7.supplement_4.s767. [DOI] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Hickman F. W., Brenner D. J., Schreiber M., Rickenbach D. G. Unusual Enterobacteriaceae. "Proteus rettgeri" that "change" into Providencia stuartii. J Clin Microbiol. 1977 Oct;6(4):373–378. doi: 10.1128/jcm.6.4.373-378.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUO M. M., LIU P. V. SEROLOGICAL SPECIFICITIES OF UREASES OF PROTEUS SPECIES. J Gen Microbiol. 1965 Mar;38:417–422. doi: 10.1099/00221287-38-3-417. [DOI] [PubMed] [Google Scholar]
- Griffin H. G., Foster T. J., Falkiner F. R., Carr M. E., Coleman D. C. Molecular analysis of multiple-resistance plasmids transferred from gram-negative bacteria isolated in a urological unit. Antimicrob Agents Chemother. 1985 Sep;28(3):413–418. doi: 10.1128/aac.28.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Mobley H. L. Genetic and biochemical diversity of ureases of Proteus, Providencia, and Morganella species isolated from urinary tract infection. Infect Immun. 1987 Sep;55(9):2198–2203. doi: 10.1128/iai.55.9.2198-2203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Mobley H. L. Proteus mirabilis urease: genetic organization, regulation, and expression of structural genes. J Bacteriol. 1988 Aug;170(8):3342–3349. doi: 10.1128/jb.170.8.3342-3349.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Mobley H. L. Proteus mirabilis urease: nucleotide sequence determination and comparison with jack bean urease. J Bacteriol. 1989 Dec;171(12):6414–6422. doi: 10.1128/jb.171.12.6414-6422.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Madsen P. O. Treatment of urinary tract infections with cefotaxime: noncomparative and prospective comparative trials. Rev Infect Dis. 1982 Sep-Oct;4 (Suppl):S416–S420. doi: 10.1093/clinids/4.supplement_2.s416. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Cortesia M. J., Rosenthal L. E., Jones B. D. Characterization of urease from Campylobacter pylori. J Clin Microbiol. 1988 May;26(5):831–836. doi: 10.1128/jcm.26.5.831-836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Hausinger R. P. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989 Mar;53(1):85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Warren J. W. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987 Nov;25(11):2216–2217. doi: 10.1128/jcm.25.11.2216-2217.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos M., Jr, Nguyen N. Y., Liu T. Y. Reproducible high yield sequencing of proteins electrophoretically separated and transferred to an inert support. J Biol Chem. 1988 May 5;263(13):6005–6008. [PubMed] [Google Scholar]
- Mulrooney S. B., Lynch M. J., Mobley H. L., Hausinger R. P. Purification, characterization, and genetic organization of recombinant Providencia stuartii urease expressed by Escherichia coli. J Bacteriol. 1988 May;170(5):2202–2207. doi: 10.1128/jb.170.5.2202-2207.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Utsunomiya M., Ichikawa Y., Koide T., Takaha M., Mimaki T., Sonoda T. Xanthine calculi in the patient with the Lesch-Nyhan syndrome associated with urinary tract infection. Urol Int. 1985;40(3):138–140. doi: 10.1159/000281067. [DOI] [PubMed] [Google Scholar]
- Rosenstein I. J., Hamilton-Miller J. M., Brumfitt W. Role of urease in the formation of infection stones: comparison of ureases from different sources. Infect Immun. 1981 Apr;32(1):32–37. doi: 10.1128/iai.32.1.32-37.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior B. W., Bradford N. C., Simpson D. S. The ureases of Proteus strains in relation to virulence for the urinary tract. J Med Microbiol. 1980 Nov;13(4):507–512. doi: 10.1099/00222615-13-4-507. [DOI] [PubMed] [Google Scholar]
- Senior B. W. Proteus morgani is less frequently associated with urinary tract infections than Proteus mirabilis--an explanation. J Med Microbiol. 1983 Aug;16(3):317–322. doi: 10.1099/00222615-16-3-317. [DOI] [PubMed] [Google Scholar]
- Silverman D. E., Stamey T. A. Management of infection stones: the Stanford experience. Medicine (Baltimore) 1983 Jan;62(1):44–51. doi: 10.1097/00005792-198301000-00004. [DOI] [PubMed] [Google Scholar]
- Todd M. J., Hausinger R. P. Purification and characterization of the nickel-containing multicomponent urease from Klebsiella aerogenes. J Biol Chem. 1987 May 5;262(13):5963–5967. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. W., Tenney J. H., Hoopes J. M., Muncie H. L., Anthony W. C. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982 Dec;146(6):719–723. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]