Abstract
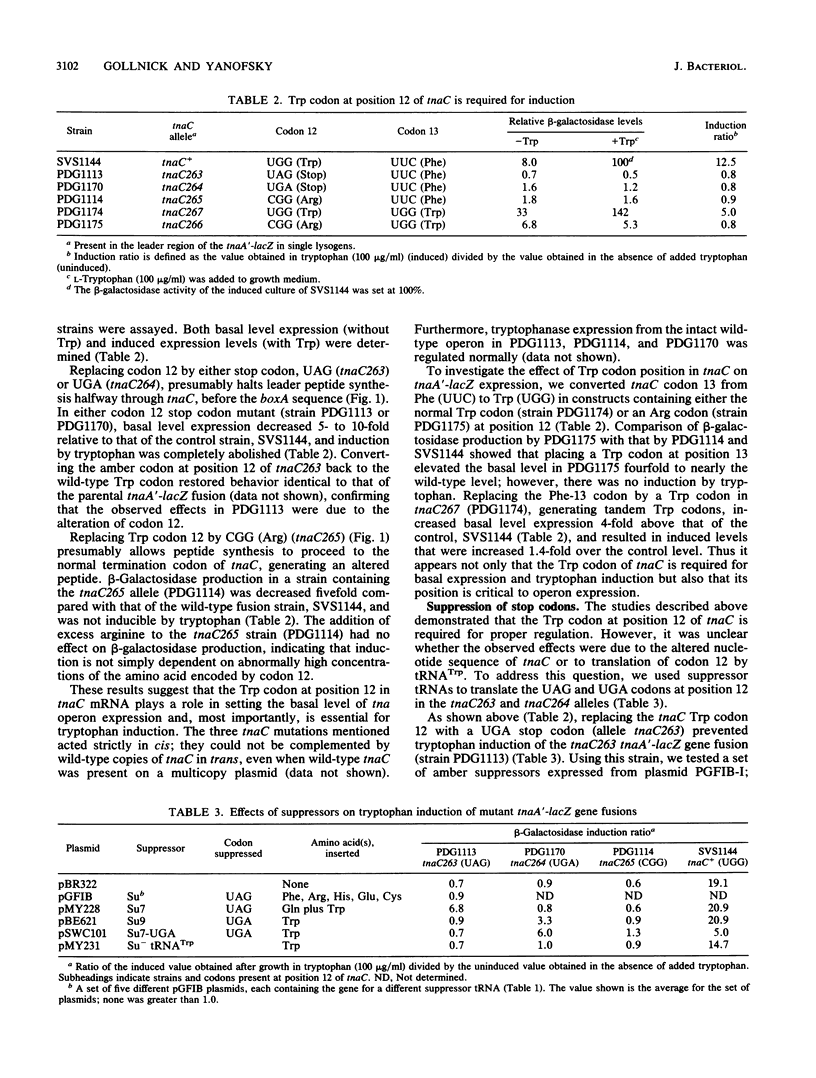
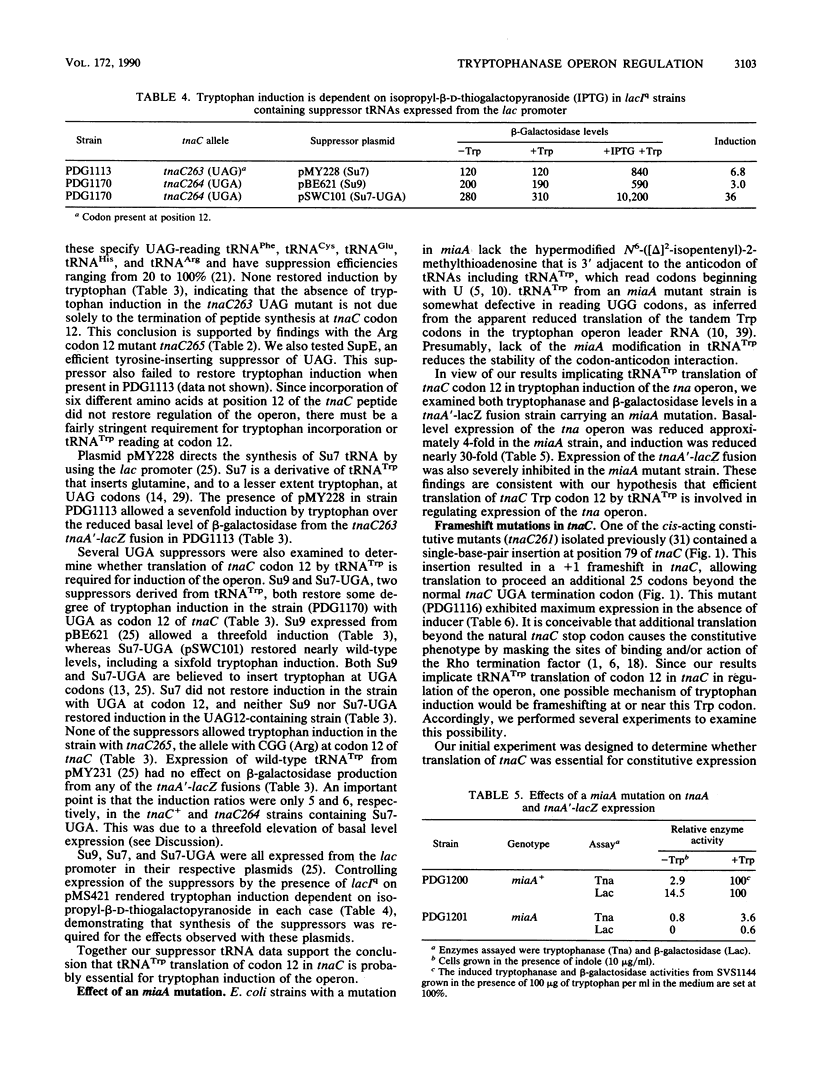
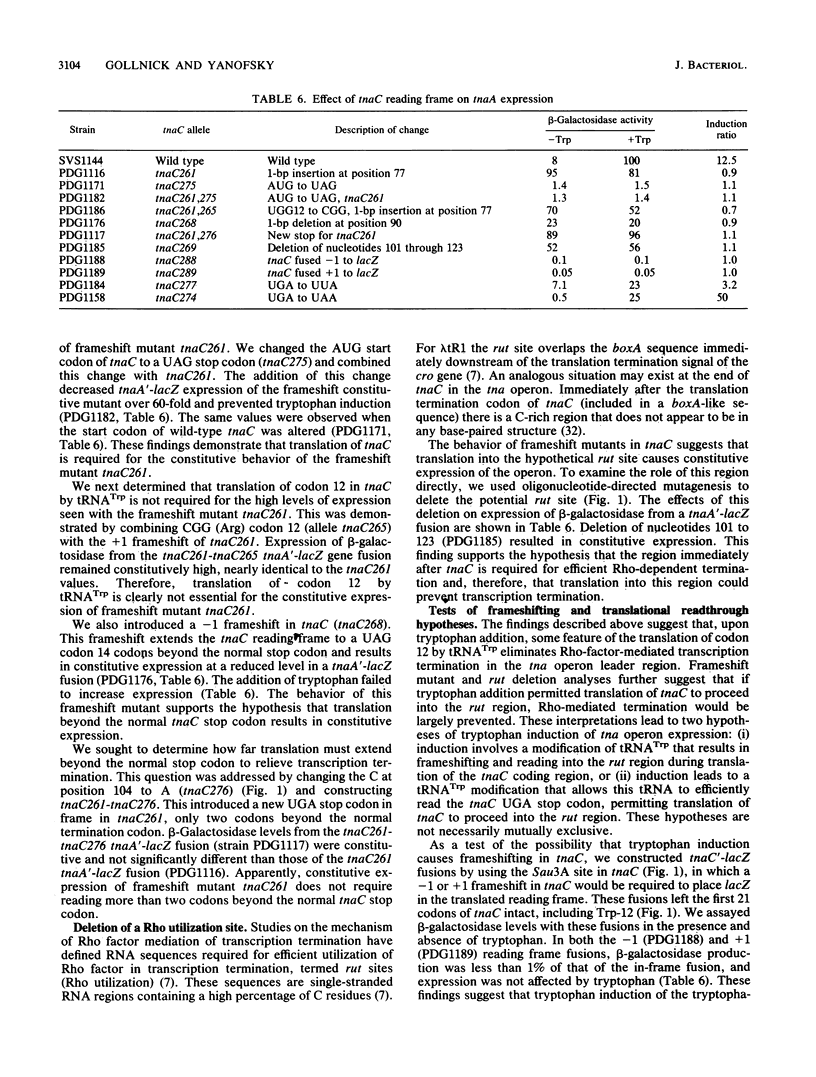
Tryptophanase (tna) operon expression in Escherichia coli is induced by tryptophan. This response is mediated by features of a 319-base-pair leader region preceding the major structural genes of the operon. Translation of the coding region (tnaC) for a 24-amino-acid leader peptide is essential for induction. We have used site-directed mutagenesis to investigate the role of the single Trp codon, at position 12 in tnaC, in regulation of the operon. Codon 12 was changed to either a UAG or UGA stop codon or to a CGG arginine codon. Induction by tryptophan was eliminated by any of these changes. Studies with suppressor tRNAs indicated that tRNA(Trp) translation of codon 12 in tnaC is essential for induction of the operon. Reduction of tna expression by a miaA mutation supports a role for translation by tRNA(Trp) in regulation of the operon. Frameshift mutations and suppression that allows translation of tnaC to proceed beyond the normal stop codon result in constitutive tna operon expression. Deletion of a potential site for Rho factor utilization just beyond tnaC also results in partial constitutive expression. These studies suggest possible models for tryptophan induction of tna operon expression involving tRNA(Trp)-mediated frame shifting or readthrough at the tnaC stop codon.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews C., Richardson J. P. Transcription termination factor rho mediates simultaneous release of RNA transcripts and DNA template from complexes with Escherichia coli RNA polymerase. J Biol Chem. 1985 May 10;260(9):5826–5831. [PubMed] [Google Scholar]
- Bilezikian J. P., Kaempfer R. O., Magasanik B. Mechanism of tryptophanase induction in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):495–506. doi: 10.1016/0022-2836(67)90054-x. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Ames B. N. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982 Aug 25;257(16):9759–9769. [PubMed] [Google Scholar]
- Botsford J. L., DeMoss R. D. Catabolite repression of tryptophanase in Escherichia coli. J Bacteriol. 1971 Jan;105(1):303–312. doi: 10.1128/jb.105.1.303-312.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillet J., Droogmans L. Molecular cloning of the Escherichia coli miaA gene involved in the formation of delta 2-isopentenyl adenosine in tRNA. J Bacteriol. 1988 Sep;170(9):4147–4152. doi: 10.1128/jb.170.9.4147-4152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceruzzi M. A., Bektesh S. L., Richardson J. P. Interaction of rho factor with bacteriophage lambda cro gene transcripts. J Biol Chem. 1985 Aug 5;260(16):9412–9418. [PubMed] [Google Scholar]
- Chen C. Y., Richardson J. P. Sequence elements essential for rho-dependent transcription termination at lambda tR1. J Biol Chem. 1987 Aug 15;262(23):11292–11299. [PubMed] [Google Scholar]
- Deeley M. C., Yanofsky C. Nucleotide sequence of the structural gene for tryptophanase of Escherichia coli K-12. J Bacteriol. 1981 Sep;147(3):787–796. doi: 10.1128/jb.147.3.787-796.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. M., Yudkin M. D. Location of the gene for the low-affinity tryptophan-specific permease of Escherichia coli. Biochem J. 1982 May 15;204(2):617–619. doi: 10.1042/bj2040617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S. P., Yarus M., Soll L. The effect of an Escherichia coli regulatory mutation on transfer RNA structure. J Mol Biol. 1979 Nov 25;135(1):111–126. doi: 10.1016/0022-2836(79)90343-7. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G. Novel mechanisms of translational control in Saccharomyces cerevisiae. Trends Genet. 1988 Jun;4(6):169–174. doi: 10.1016/0168-9525(88)90023-6. [DOI] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J Mol Biol. 1971 Jun 14;58(2):439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- Knowlton R. G., Soll L., Yarus M. Dual specificity of su+ 7 tRNA. Evidence for translational discrimination. J Mol Biol. 1980 Jun 5;139(4):705–720. doi: 10.1016/0022-2836(80)90056-x. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Morgan W. D., Bear D. G., Litchman B. L., von Hippel P. H. RNA sequence and secondary structure requirements for rho-dependent transcription termination. Nucleic Acids Res. 1985 May 24;13(10):3739–3754. doi: 10.1093/nar/13.10.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON W. A., SNELL E. E. CATALYTIC PROPERTIES OF TRYPTOPHANASE, A MULTIFUNCTIONAL PYRIDOXAL PHOSPHATE ENZYME. Proc Natl Acad Sci U S A. 1964 Mar;51:382–389. doi: 10.1073/pnas.51.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON W. A., SNELL E. E. FORMATION AND INTERRELATIONSHIPS OF TRYPTOPHANASE AND TRYPTOPHAN SYNTHETASES IN ESCHERICHIA COLI. J Bacteriol. 1965 Feb;89:355–364. doi: 10.1128/jb.89.2.355-364.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J., Masson J. M., Kleina L. G., Abelson J., Miller J. H. Construction of two Escherichia coli amber suppressor genes: tRNAPheCUA and tRNACysCUA. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6548–6552. doi: 10.1073/pnas.83.17.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyunoya H., Lusty C. J. Sequence of the small subunit of yeast carbamyl phosphate synthetase and identification of its catalytic domain. J Biol Chem. 1984 Aug 10;259(15):9790–9798. [PubMed] [Google Scholar]
- Phillips R. S., Miles E. W., Cohen L. A. Differential inhibition of tryptophan synthase and of tryptophanase by the two diastereoisomers of 2,3-dihydro-L-tryptophan. Implications for the stereochemistry of the reaction intermediates. J Biol Chem. 1985 Nov 25;260(27):14665–14670. [PubMed] [Google Scholar]
- Raftery L. A., Egan J. B., Cline S. W., Yarus M. Defined set of cloned termination suppressors: in vivo activity of isogenetic UAG, UAA, and UGA suppressor tRNAs. J Bacteriol. 1984 Jun;158(3):849–859. doi: 10.1128/jb.158.3.849-859.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Houman F., Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Davis B. D. Interaction of secreted nascent chains with surrounding membrane in Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5922–5925. doi: 10.1073/pnas.75.12.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll L. Mutational alterations of tryptophan-specific transfer RNA that generate translation suppressors of the UAA, UAG and UGA nonsense codons. J Mol Biol. 1974 Jun 25;86(2):233–243. doi: 10.1016/0022-2836(74)90015-1. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Olmsted R. A., Venkatesan S., Coligan J. E., Collins P. L. Fusion glycoprotein of human parainfluenza virus type 3: nucleotide sequence of the gene, direct identification of the cleavage-activation site, and comparison with other paramyxoviruses. Virology. 1986 Jul 15;152(1):241–251. doi: 10.1016/0042-6822(86)90388-0. [DOI] [PubMed] [Google Scholar]
- Stewart V., Landick R., Yanofsky C. Rho-dependent transcription termination in the tryptophanase operon leader region of Escherichia coli K-12. J Bacteriol. 1986 Apr;166(1):217–223. doi: 10.1128/jb.166.1.217-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V., Yanofsky C. Evidence for transcription antitermination control of tryptophanase operon expression in Escherichia coli K-12. J Bacteriol. 1985 Nov;164(2):731–740. doi: 10.1128/jb.164.2.731-740.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V., Yanofsky C. Role of leader peptide synthesis in tryptophanase operon expression in Escherichia coli K-12. J Bacteriol. 1986 Jul;167(1):383–386. doi: 10.1128/jb.167.1.383-386.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Snell E. E. Reversibility of the tryptophanase reaction: synthesis of tryptophan from indole, pyruvate, and ammonia. Proc Natl Acad Sci U S A. 1972 May;69(5):1086–1090. doi: 10.1073/pnas.69.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M., Feller A., Messenguy F., Piérard A. The leader peptide of yeast gene CPA1 is essential for the translational repression of its expression. Cell. 1987 Jun 19;49(6):805–813. doi: 10.1016/0092-8674(87)90618-0. [DOI] [PubMed] [Google Scholar]
- Werner M., Feller A., Piérard A. Nucleotide sequence of yeast gene CP A1 encoding the small subunit of arginine-pathway carbamoyl-phosphate synthetase. Homology of the deduced amino acid sequence to other glutamine amidotransferases. Eur J Biochem. 1985 Jan 15;146(2):371–381. doi: 10.1111/j.1432-1033.1985.tb08663.x. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Kelley R. L., Horn V. Repression is relieved before attenuation in the trp operon of Escherichia coli as tryptophan starvation becomes increasingly severe. J Bacteriol. 1984 Jun;158(3):1018–1024. doi: 10.1128/jb.158.3.1018-1024.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Mutations affecting tRNATrp and its charging and their effect on regulation of transcription termination at the attenuator of the tryptophan operon. J Mol Biol. 1977 Jul 15;113(4):663–677. doi: 10.1016/0022-2836(77)90229-7. [DOI] [PubMed] [Google Scholar]
- Yonath A., Leonard K. R., Wittmann H. G. A tunnel in the large ribosomal subunit revealed by three-dimensional image reconstruction. Science. 1987 May 15;236(4803):813–816. doi: 10.1126/science.3576200. [DOI] [PubMed] [Google Scholar]



