Abstract
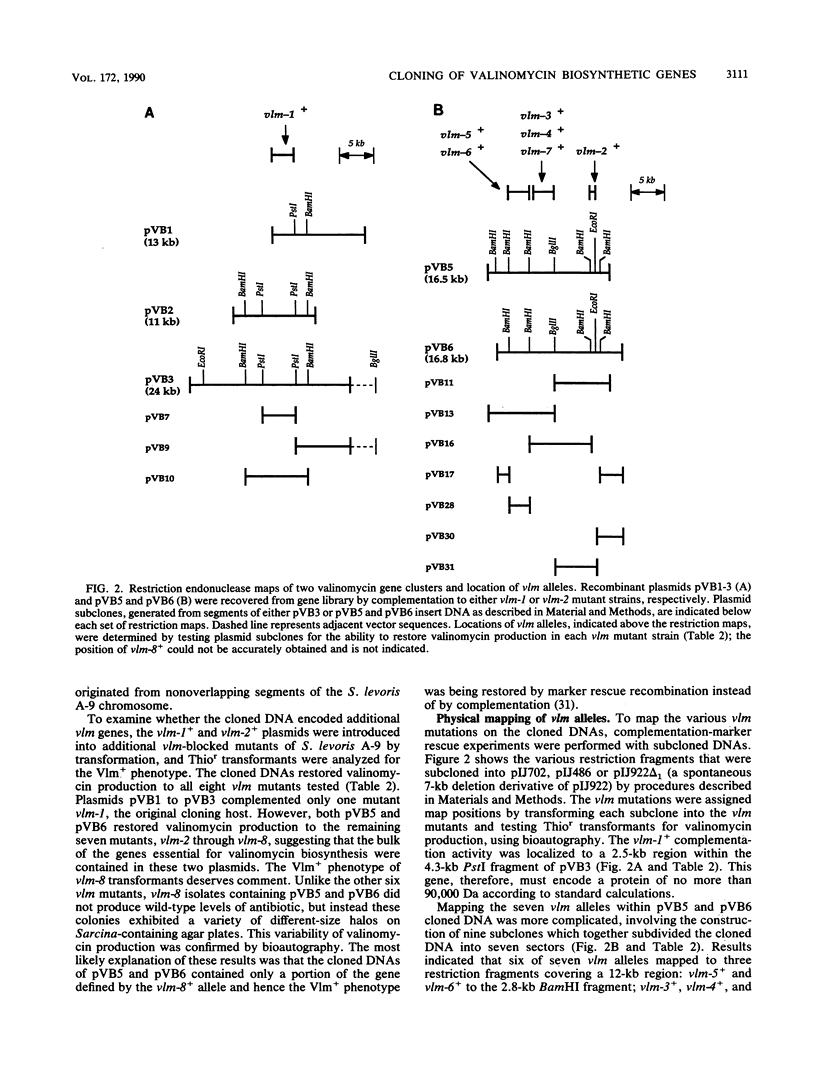
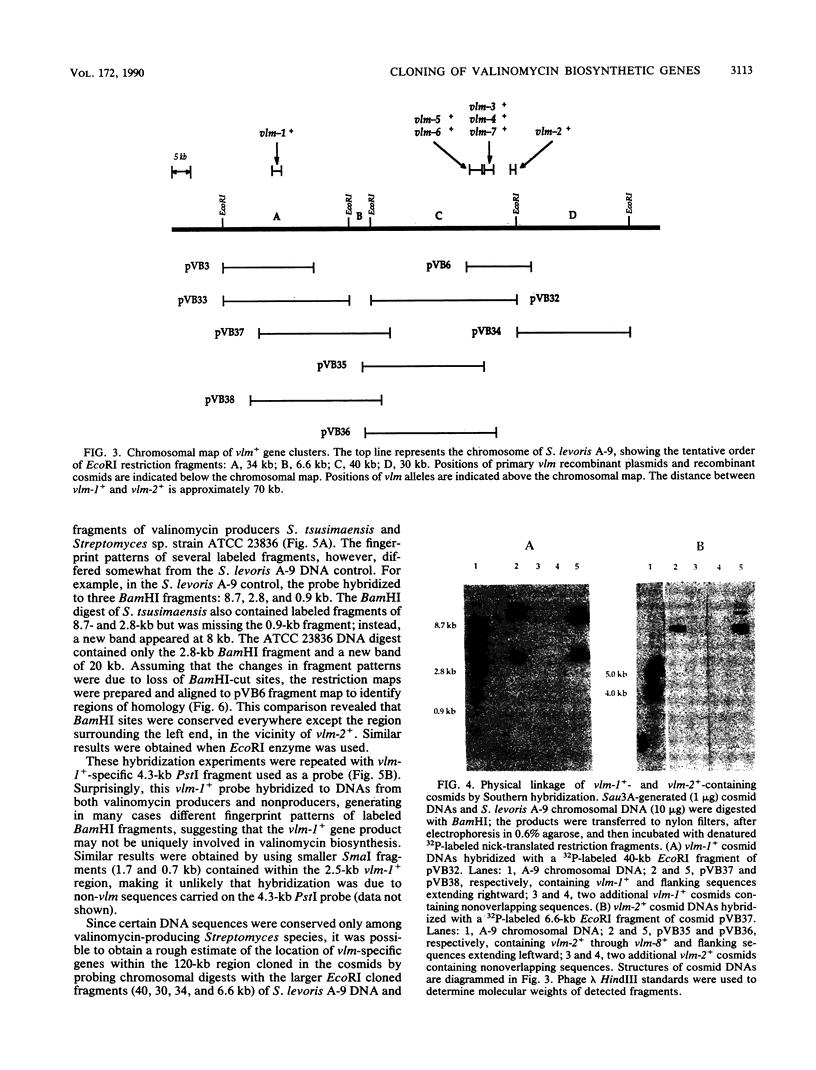
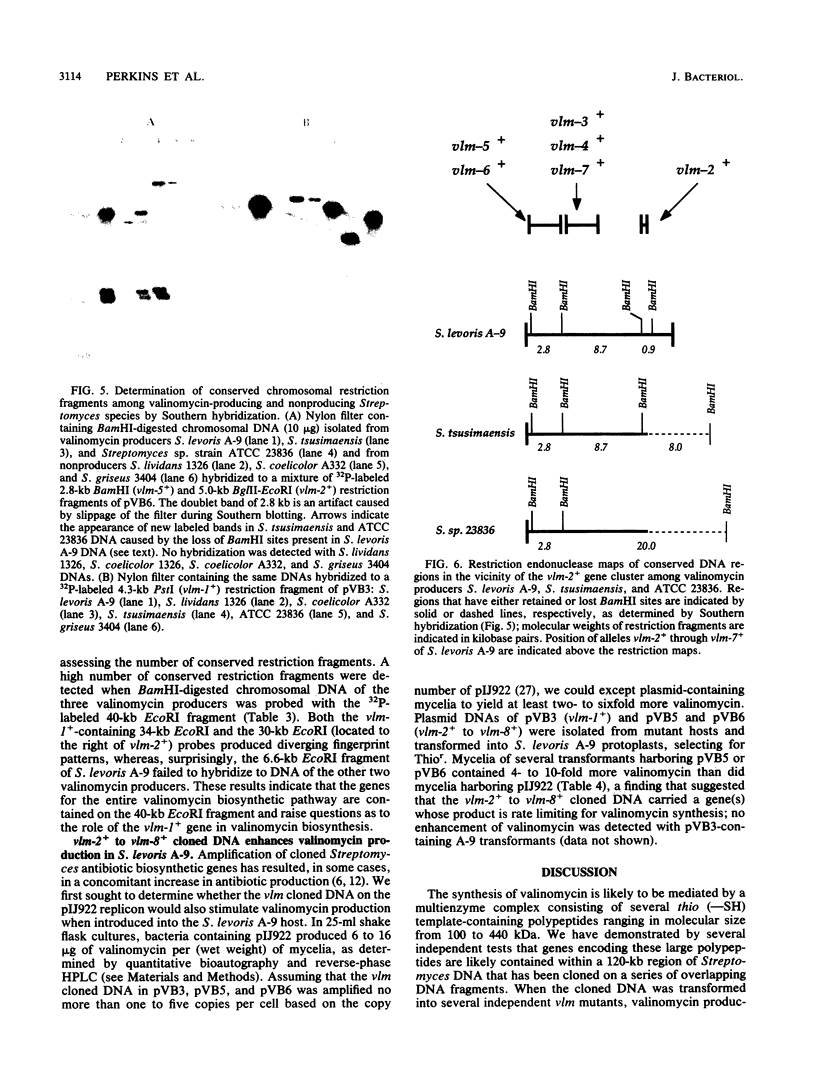
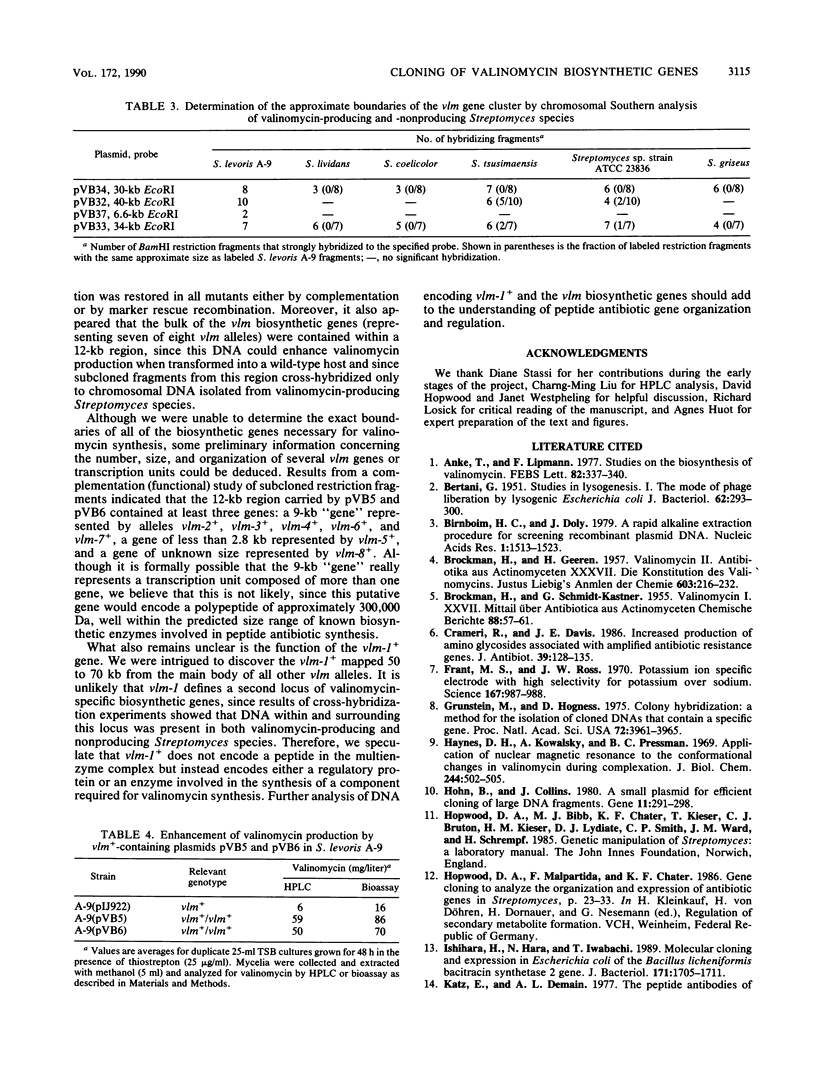
We have identified genes from Streptomyces levoris A-9 involved in the biosynthesis of the peptide antibiotic valinomycin. Two segments of chromosomal DNA were recovered from genomic libraries, constructed by using the low-copy-number plasmid pIJ922, by complementation of valinomycin-deficient (vlm) mutants of S. levoris A-9. One set of plasmids restored valinomycin production to only one mutant, that carrying vlm-1, whereas a second set of plasmids restored productivity to seven vlm mutants, those carrying vlm-2 through vlm-8. Additional complementation studies using subcloned restriction enzyme fragments showed that the vlm-1+ gene was contained within a 2.5-kilobase (kb) DNA region, whereas alleles vlm-2+ through vlm-8+ were contained in a 12-kb region, representing at least three genes. Physical mapping experiments based on the isolation of cosmid clones showed that the two vlm loci were 50 to 70 kb apart. Southern hybridization experiments demonstrated that the vlm-2+ gene cluster was highly conserved among other valinomycin-producing Streptomyces strains, whereas the vlm-1+ gene was ubiquitous among Streptomyces species tested. Increasing the copy number of the vlm-2+ gene cluster in S. levoris A-9 by the introduction of low-copy-number recombinant plasmids resulted in a concomitant increase in the level of valinomycin production.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anke T., Lipmann F. Studies on the biosynthesis of valinomycin. FEBS Lett. 1977 Oct 15;82(2):337–340. doi: 10.1016/0014-5793(77)80615-7. [DOI] [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crameri R., Davies J. E. Increased production of aminoglycosides associated with amplified antibiotic resistance genes. J Antibiot (Tokyo) 1986 Jan;39(1):128–135. doi: 10.7164/antibiotics.39.128. [DOI] [PubMed] [Google Scholar]
- Frant M. S., Ross J. W., Jr Potassium ion specific electrode with high selectivity for potassium over sodium. Science. 1970 Feb 13;167(3920):987–988. doi: 10.1126/science.167.3920.987. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes D. H., Kowalsky A., Pressman B. C. Application of nuclear magnetic resonance to the conformational changes in valinomycin during complexation. J Biol Chem. 1969 Jan 25;244(2):502–505. [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Hara N., Iwabuchi T. Molecular cloning and expression in Escherichia coli of the Bacillus licheniformis bacitracin synthetase 2 gene. J Bacteriol. 1989 Mar;171(3):1705–1711. doi: 10.1128/jb.171.3.1705-1711.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Kleinkauf H., von Döhren H. Nucleic acid independent synthesis of peptides. Curr Top Microbiol Immunol. 1981;91:129–177. doi: 10.1007/978-3-642-68058-8_6. [DOI] [PubMed] [Google Scholar]
- Krause M., Marahiel M. A. Organization of the biosynthesis genes for the peptide antibiotic gramicidin S. J Bacteriol. 1988 Oct;170(10):4669–4674. doi: 10.1128/jb.170.10.4669-4674.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Marahiel M. A., von Döhren H., Kleinkauf H. Molecular cloning of an ornithine-activating fragment of the gramicidin S synthetase 2 gene from Bacillus brevis and its expression in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1120–1125. doi: 10.1128/jb.162.3.1120-1125.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krätzschmar J., Krause M., Marahiel M. A. Gramicidin S biosynthesis operon containing the structural genes grsA and grsB has an open reading frame encoding a protein homologous to fatty acid thioesterases. J Bacteriol. 1989 Oct;171(10):5422–5429. doi: 10.1128/jb.171.10.5422-5429.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laland S. G., Zimmer T. L. The protein thiotemplate mechanism of synthesis for the peptide antibiotics produced by Bacillus brevis. Essays Biochem. 1973;9:31–57. [PubMed] [Google Scholar]
- Lee S. G., Lipmann F. Tyrocidine synthetase system. Methods Enzymol. 1975;43:585–602. doi: 10.1016/0076-6879(75)43121-4. [DOI] [PubMed] [Google Scholar]
- Lipmann F., Gevers W., Kleinkauf H., Roskoski R., Jr Polypeptide synthesis on protein templates: the enzymatic synthesis of gramicidin S and tyrocidine. Adv Enzymol Relat Areas Mol Biol. 1971;35:1–34. doi: 10.1002/9780470122808.ch1. [DOI] [PubMed] [Google Scholar]
- Lydiate D. J., Malpartida F., Hopwood D. A. The Streptomyces plasmid SCP2*: its functional analysis and development into useful cloning vectors. Gene. 1985;35(3):223–235. doi: 10.1016/0378-1119(85)90001-0. [DOI] [PubMed] [Google Scholar]
- MACDONALD J. C. Biosynthesis of valinomycin. Can J Microbiol. 1960 Feb;6:27–34. doi: 10.1139/m60-005. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Molecular cloning of the whole biosynthetic pathway of a Streptomyces antibiotic and its expression in a heterologous host. 1984 May 31-Jun 6Nature. 309(5967):462–464. doi: 10.1038/309462a0. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Physical and genetic characterisation of the gene cluster for the antibiotic actinorhodin in Streptomyces coelicolor A3(2). Mol Gen Genet. 1986 Oct;205(1):66–73. doi: 10.1007/BF02428033. [DOI] [PubMed] [Google Scholar]
- Marahiel M. A., Krause M., Skarpeid H. J. Cloning of the tyrocidine synthetase 1 gene from Bacillus brevis and its expression in Escherichia coli. Mol Gen Genet. 1985;201(2):231–236. doi: 10.1007/BF00425664. [DOI] [PubMed] [Google Scholar]
- Marahiel M. A., Zuber P., Czekay G., Losick R. Identification of the promoter for a peptide antibiotic biosynthesis gene from Bacillus brevis and its regulation in Bacillus subtilis. J Bacteriol. 1987 May;169(5):2215–2222. doi: 10.1128/jb.169.5.2215-2222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittenhuber G., Weckermann R., Marahiel M. A. Gene cluster containing the genes for tyrocidine synthetases 1 and 2 from Bacillus brevis: evidence for an operon. J Bacteriol. 1989 Sep;171(9):4881–4887. doi: 10.1128/jb.171.9.4881-4887.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ristow H., Salnikow J., Kleinkauf H. Biosynthesis of valinomycin. FEBS Lett. 1974 Jun 1;42(2):127–130. doi: 10.1016/0014-5793(74)80768-4. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. DNA cloning in Streptomyces: resistance genes from antibiotic-producing species. Nature. 1980 Jul 31;286(5772):525–527. doi: 10.1038/286525a0. [DOI] [PubMed] [Google Scholar]
- Ward J. M., Janssen G. R., Kieser T., Bibb M. J., Buttner M. J., Bibb M. J. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol Gen Genet. 1986 Jun;203(3):468–478. doi: 10.1007/BF00422072. [DOI] [PubMed] [Google Scholar]
- Youngman P. J., Perkins J. B., Losick R. Genetic transposition and insertional mutagenesis in Bacillus subtilis with Streptococcus faecalis transposon Tn917. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2305–2309. doi: 10.1073/pnas.80.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]