Abstract
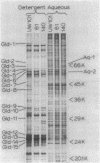
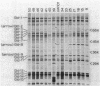
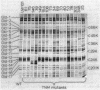
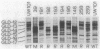
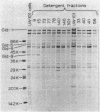
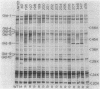
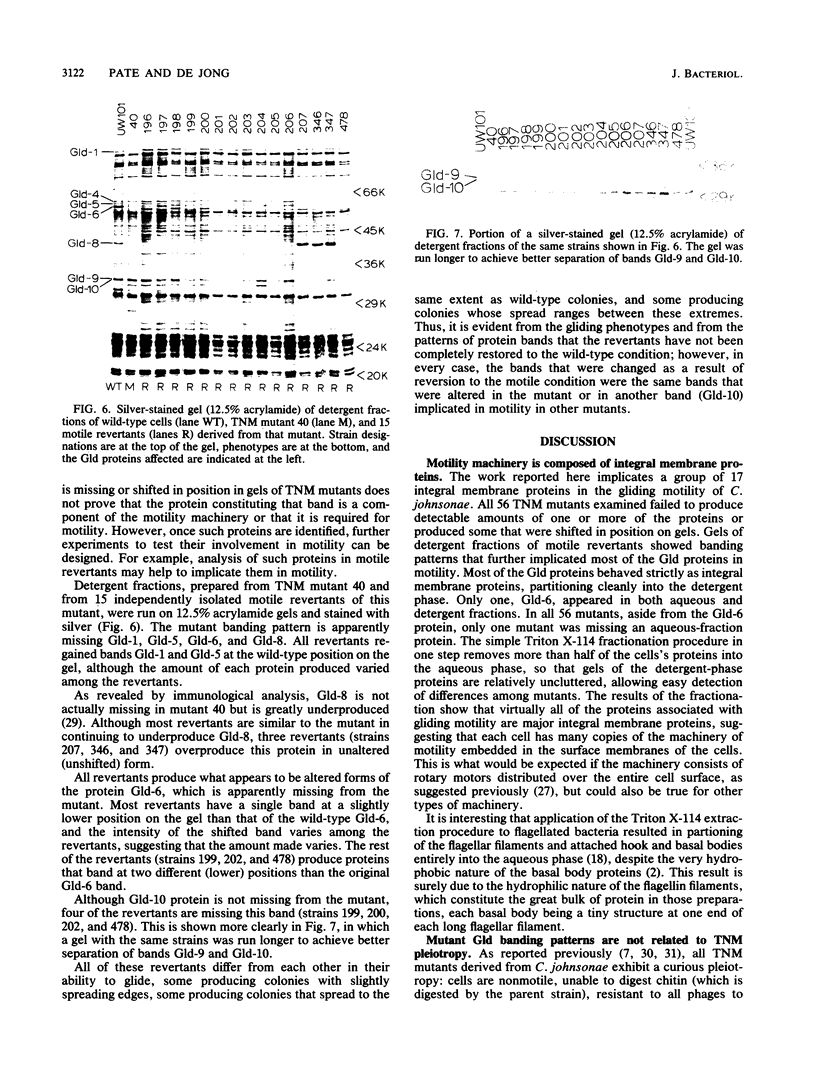
Nonmotile mutants of the gliding bacterium Cytophaga johnsonae were examined to identify proteins that might be involved in gliding motility. Wild-type and mutant cell proteins were solubilized and fractionated by using Triton X-114, and the proteins that partitioned into the aqueous phase or the detergent phase were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis for proteins that differed between wild-type and mutant cells. Seventeen proteins, ranging in size from 16 to 150 kilodaltons, were implicated by this technique as having some relationship to gliding and were designated Gld-1 through Gld-17. All Gld proteins behaved as integral membrane proteins, partitioning into the detergent phase. All 56 mutants examined exhibited changes in 1 or more of the Gld proteins, with the number of proteins altered in any mutant varying from 1 to 11. Several lines of evidence suggested that proteins Gld-12 through Gld-15 are glycoproteins. Analysis of banding patterns of detergent-fraction proteins of motile revertants supported the idea that the Gld proteins have a role in gliding motility.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa S. I., Dean G. E., Jones C. J., Macnab R. M., Yamaguchi S. Purification and characterization of the flagellar hook-basal body complex of Salmonella typhimurium. J Bacteriol. 1985 Mar;161(3):836–849. doi: 10.1128/jb.161.3.836-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood R. A. Gliding motility and the dynamics of flagellar membrane glycoproteins in Chlamydomonas reinhardtii. J Protozool. 1988 Nov;35(4):552–558. doi: 10.1111/j.1550-7408.1988.tb04151.x. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Chang L. E., Pate J. L., Betzig R. J. Isolation and characterization of nonspreading mutants of the gliding bacterium Cytophaga johnsonae. J Bacteriol. 1984 Jul;159(1):26–35. doi: 10.1128/jb.159.1.26-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M., Keller K. H., Weisberg D. Experimental observations consistent with a surface tension model of gliding motility of Myxococcus xanthus. J Bacteriol. 1983 Sep;155(3):1367–1371. doi: 10.1128/jb.155.3.1367-1371.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzandu J. K., Deh M. E., Barratt D. L., Wise G. E. Detection of erythrocyte membrane proteins, sialoglycoproteins, and lipids in the same polyacrylamide gel using a double-staining technique. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1733–1737. doi: 10.1073/pnas.81.6.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Leadbetter E. R. Sulfonolipids of gliding bacteria. Structure of the N-acylaminosulfonates. J Biol Chem. 1984 Mar 10;259(5):2982–2990. [PubMed] [Google Scholar]
- Hamaguchi H., Cleve H. Solubilization of human erythrocyte membrane glycoproteins and separation of the MN glycoprotein from a glycoprotein with I, S, and A activity. Biochim Biophys Acta. 1972 Sep 29;278(2):271–280. doi: 10.1016/0005-2795(72)90232-2. [DOI] [PubMed] [Google Scholar]
- Kalmokoff M. L., Jarrell K. F., Koval S. F. Isolation of flagella from the archaebacterium Methanococcus voltae by phase separation with Triton X-114. J Bacteriol. 1988 Apr;170(4):1752–1758. doi: 10.1128/jb.170.4.1752-1758.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller K. H., Grady M., Dworkin M. Surface tension gradients: feasible model for gliding motility of Myxococcus xanthus. J Bacteriol. 1983 Sep;155(3):1358–1366. doi: 10.1128/jb.155.3.1358-1366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda Y. Transcriptional control of flagellar genes in Escherichia coli K-12. J Bacteriol. 1986 Dec;168(3):1315–1318. doi: 10.1128/jb.168.3.1315-1318.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapidus I. R., Berg H. C. Gliding motility of Cytophaga sp. strain U67. J Bacteriol. 1982 Jul;151(1):384–398. doi: 10.1128/jb.151.1.384-398.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeba P. Y. Isolation of a surface glycoprotein from Myxococcus xanthus. J Bacteriol. 1986 May;166(2):644–650. doi: 10.1128/jb.166.2.644-650.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Stephens K., Hartzell P., Kaiser D. Gliding motility in Myxococcus xanthus: mgl locus, RNA, and predicted protein products. J Bacteriol. 1989 Feb;171(2):819–830. doi: 10.1128/jb.171.2.819-830.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]