Abstract
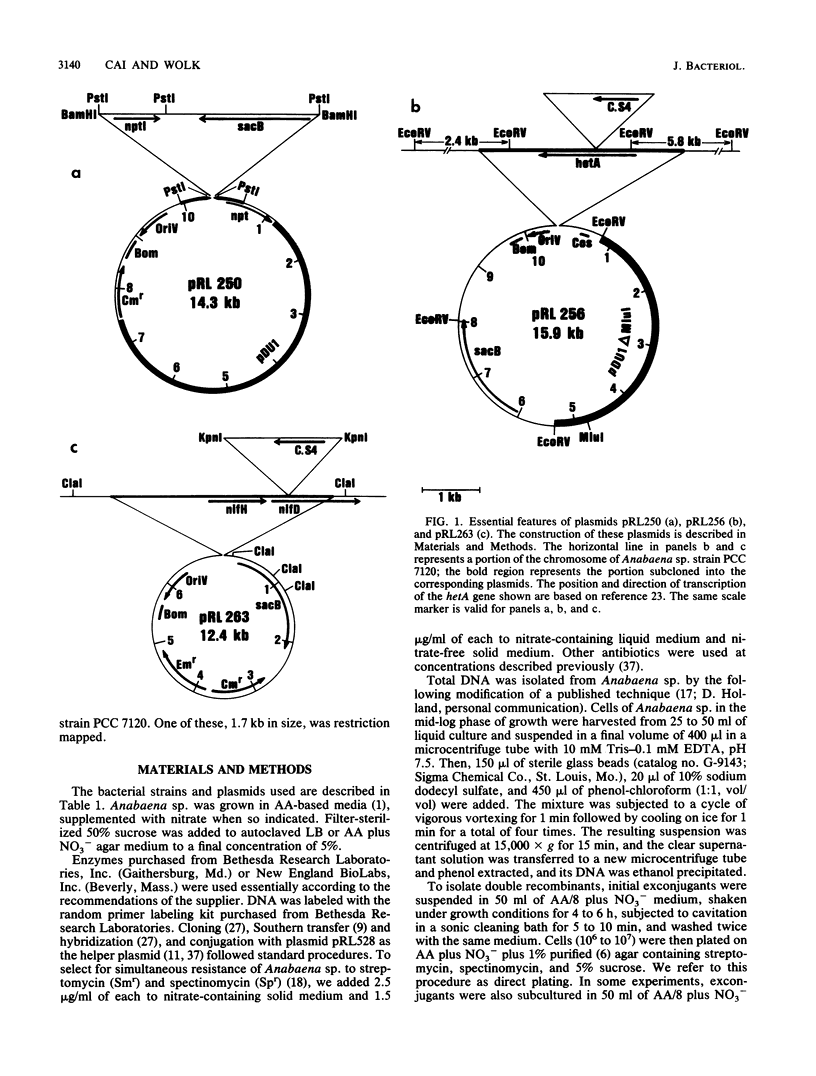
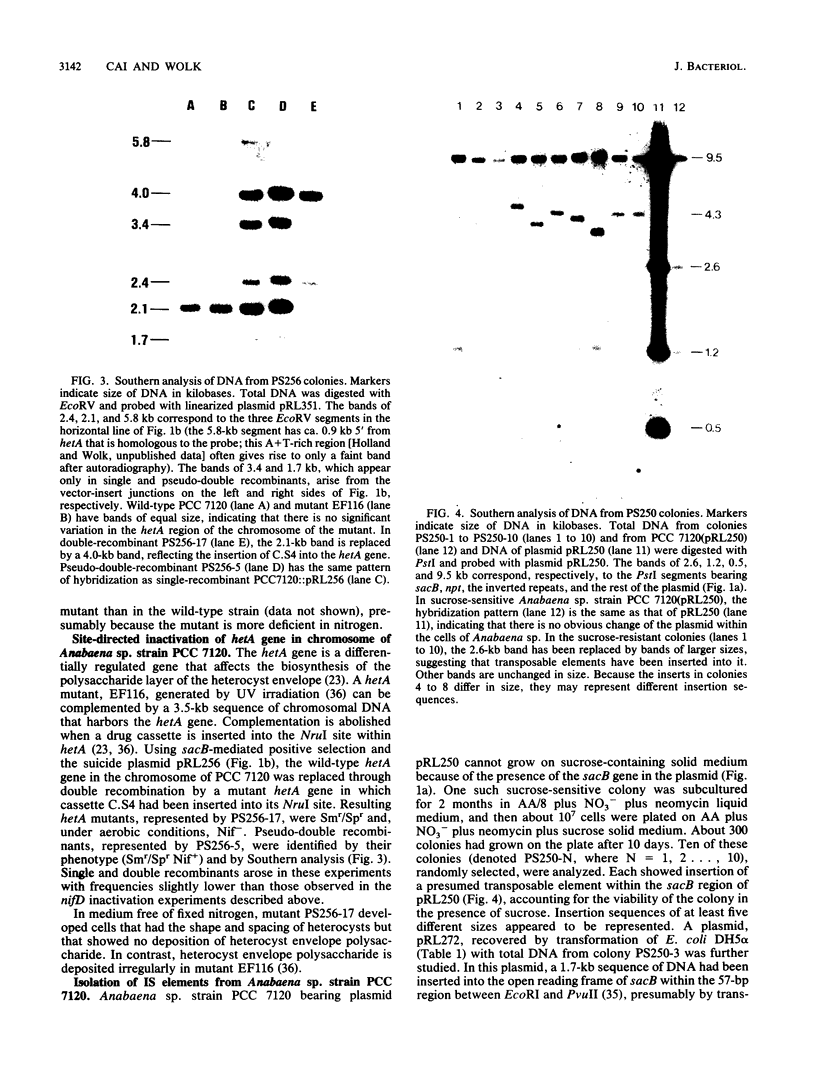
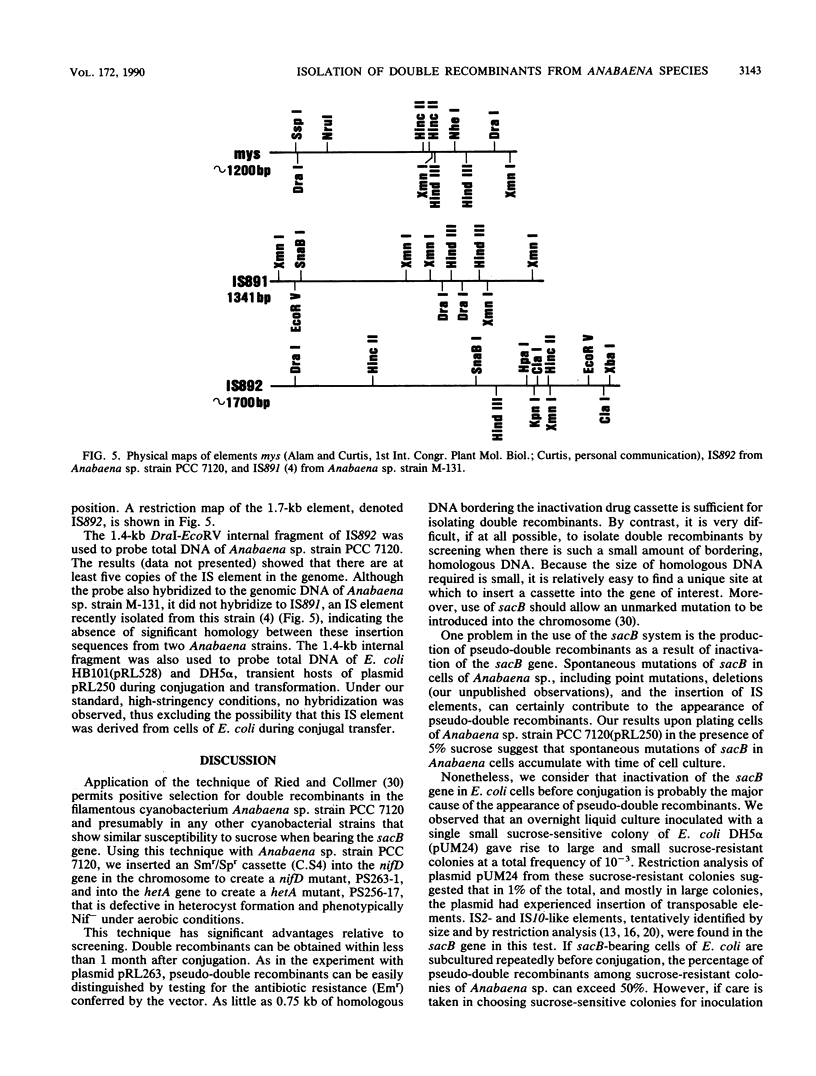
Use of the sacB gene (J. L. Ried and A. Collmer, Gene 57:239-246, 1987) provides a simple, effective, positive selection for double recombinants in Anabaena sp. strain PCC 7120, a filamentous cyanobacterium. This gene, which encodes the secretory levansucrase of Bacillus subtilis, was inserted into the vector portion of a suicide plasmid bearing a mutant version of a chromosomal gene. Cells of colonies in which such a plasmid had integrated into the Anabaena chromosome through single recombination were plated on solid medium containing 5% sucrose. Under this condition, the presence of the sacB gene is lethal. A small fraction of the cells from initially sucrose-sensitive colonies became sucrose resistant; the majority of these sucrose-resistant derivatives had undergone a second recombinational event in which the sacB-containing vector had been lost and the wild-type form of the chromosomal gene had been replaced by the mutant form. By the use of this technique, we mutated two selected genes in the chromosome of Anabaena sp. strain PCC 7120. The conditionally lethal nature of the sacB gene was also used to detect insertion sequences from this Anabaena strain. Sucrose-resistant colonies derived from cells bearing a sacB-containing autonomously replicating plasmid were analyzed. Five different, presumed insertion sequences were found to have inserted into the sacB gene of the plasmids in these colonies. One of them, denoted IS892, was characterized by physical mapping. It is 1.7 kilobases in size and is present in at least five copies in the genome of Anabaena sp. strain PCC 7120.
Full text
PDF



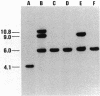
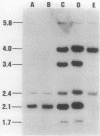
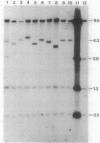
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. B., Arnon D. I. Studies on Nitrogen-Fixing Blue-Green Algae. I. Growth and Nitrogen Fixation by Anabaena Cylindrica Lemm. Plant Physiol. 1955 Jul;30(4):366–372. doi: 10.1104/pp.30.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymerich S., Gonzy-Tréboul G., Steinmetz M. 5'-noncoding region sacR is the target of all identified regulation affecting the levansucrase gene in Bacillus subtilis. J Bacteriol. 1986 Jun;166(3):993–998. doi: 10.1128/jb.166.3.993-998.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUN A. C., WOOD H. N. On the activation of certain essential biosynthetic systems in cells of Vinca rosea L. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1776–1782. doi: 10.1073/pnas.48.10.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft I., Wolk C. P. Characterization of an insertion sequence (IS891) of novel structure from the cyanobacterium Anabaena sp. strain M-131. J Bacteriol. 1989 Nov;171(11):5949–5954. doi: 10.1128/jb.171.11.5949-5954.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft I., Wolk C. P., Oren E. V. Physical and genetic maps of the genome of the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1989 Nov;171(11):5940–5948. doi: 10.1128/jb.171.11.5940-5948.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith J., Silhavy T. J. Genetic analysis of protein export in Escherichia coli. Methods Enzymol. 1983;97:3–11. doi: 10.1016/0076-6879(83)97114-8. [DOI] [PubMed] [Google Scholar]
- Craig I. W., Leach C. K., Carr N. G. Studies with deoxyribonucleic acid from blue-green algae. Arch Mikrobiol. 1969;65(3):218–227. doi: 10.1007/BF00407105. [DOI] [PubMed] [Google Scholar]
- Currier T. C., Haury J. F., Wolk C. P. Isolation and preliminary characterization of auxotrophs of a filamentous Cyanobacterium. J Bacteriol. 1977 Mar;129(3):1556–1562. doi: 10.1128/jb.129.3.1556-1562.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. Carolyne Davis: achieving in a new frontier. Interview by Michele Ward-Schaefer. Health Matrix. 1986 Spring;4(1):62–65. [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988 Aug 15;68(1):119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 1988;167:747–754. doi: 10.1016/0076-6879(88)67086-8. [DOI] [PubMed] [Google Scholar]
- Fiandt M., Szybalski W., Malamy M. H. Polar mutations in lac, gal and phage lambda consist of a few IS-DNA sequences inserted with either orientation. Mol Gen Genet. 1972;119(3):223–231. doi: 10.1007/BF00333860. [DOI] [PubMed] [Google Scholar]
- Gay P., Le Coq D., Steinmetz M., Berkelman T., Kado C. I. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J Bacteriol. 1985 Nov;164(2):918–921. doi: 10.1128/jb.164.2.918-921.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay P., Le Coq D., Steinmetz M., Ferrari E., Hoch J. A. Cloning structural gene sacB, which codes for exoenzyme levansucrase of Bacillus subtilis: expression of the gene in Escherichia coli. J Bacteriol. 1983 Mar;153(3):1424–1431. doi: 10.1128/jb.153.3.1424-1431.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., Sommer H., Saedler H. Nucleotide sequence of the transposable DNA-element IS2. Nucleic Acids Res. 1979 Mar;6(3):1111–1122. doi: 10.1093/nar/6.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Robinson S. J., Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985 Apr 4;314(6010):419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Wiest D. R. Genome rearrangement and nitrogen fixation in Anabaena blocked by inactivation of xisA gene. Science. 1988 Dec 9;242(4884):1421–1423. doi: 10.1126/science.3144039. [DOI] [PubMed] [Google Scholar]
- Golden S. S. Mutagenesis of cyanobacteria by classical and gene-transfer-based methods. Methods Enzymol. 1988;167:714–727. doi: 10.1016/0076-6879(88)67083-2. [DOI] [PubMed] [Google Scholar]
- Halling S. M., Simons R. W., Way J. C., Walsh R. B., Kleckner N. DNA sequence organization of IS10-right of Tn10 and comparison with IS10-left. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2608–2612. doi: 10.1073/pnas.79.8.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D., Wolk C. P. Identification and characterization of hetA, a gene that acts early in the process of morphological differentiation of heterocysts. J Bacteriol. 1990 Jun;172(6):3131–3137. doi: 10.1128/jb.172.6.3131-3137.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klier A., Fouet A., Débarbouillé M., Kunst F., Rapoport G. Distinct control sites located upstream from the levansucrase gene of Bacillus subtilis. Mol Microbiol. 1987 Sep;1(2):233–241. doi: 10.1111/j.1365-2958.1987.tb00517.x. [DOI] [PubMed] [Google Scholar]
- Porter R. D. Transformation in cyanobacteria. Crit Rev Microbiol. 1986;13(2):111–132. doi: 10.3109/10408418609108736. [DOI] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Ried J. L., Collmer A. An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in gram-negative bacteria by marker exchange-eviction mutagenesis. Gene. 1987;57(2-3):239–246. doi: 10.1016/0378-1119(87)90127-2. [DOI] [PubMed] [Google Scholar]
- Schmetterer G., Wolk C. P. Identification of the region of cyanobacterial plasmid pDU1 necessary for replication in Anabaena sp. strain M-131. Gene. 1988;62(1):101–109. doi: 10.1016/0378-1119(88)90583-5. [DOI] [PubMed] [Google Scholar]
- Seed B. Purification of genomic sequences from bacteriophage libraries by recombination and selection in vivo. Nucleic Acids Res. 1983 Apr 25;11(8):2427–2445. doi: 10.1093/nar/11.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P., Huang H. V. Homologous recombination in Escherichia coli: dependence on substrate length and homology. Genetics. 1986 Mar;112(3):441–457. doi: 10.1093/genetics/112.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Cohen-Bazire G. Phototrophic prokaryotes: the cyanobacteria. Annu Rev Microbiol. 1977;31:225–274. doi: 10.1146/annurev.mi.31.100177.001301. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Le Coq D., Aymerich S., Gonzy-Tréboul G., Gay P. The DNA sequence of the gene for the secreted Bacillus subtilis enzyme levansucrase and its genetic control sites. Mol Gen Genet. 1985;200(2):220–228. doi: 10.1007/BF00425427. [DOI] [PubMed] [Google Scholar]
- Wolk C. P., Cai Y., Cardemil L., Flores E., Hohn B., Murry M., Schmetterer G., Schrautemeier B., Wilson R. Isolation and complementation of mutants of Anabaena sp. strain PCC 7120 unable to grow aerobically on dinitrogen. J Bacteriol. 1988 Mar;170(3):1239–1244. doi: 10.1128/jb.170.3.1239-1244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk C. P., Vonshak A., Kehoe P., Elhai J. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1561–1565. doi: 10.1073/pnas.81.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]