Abstract
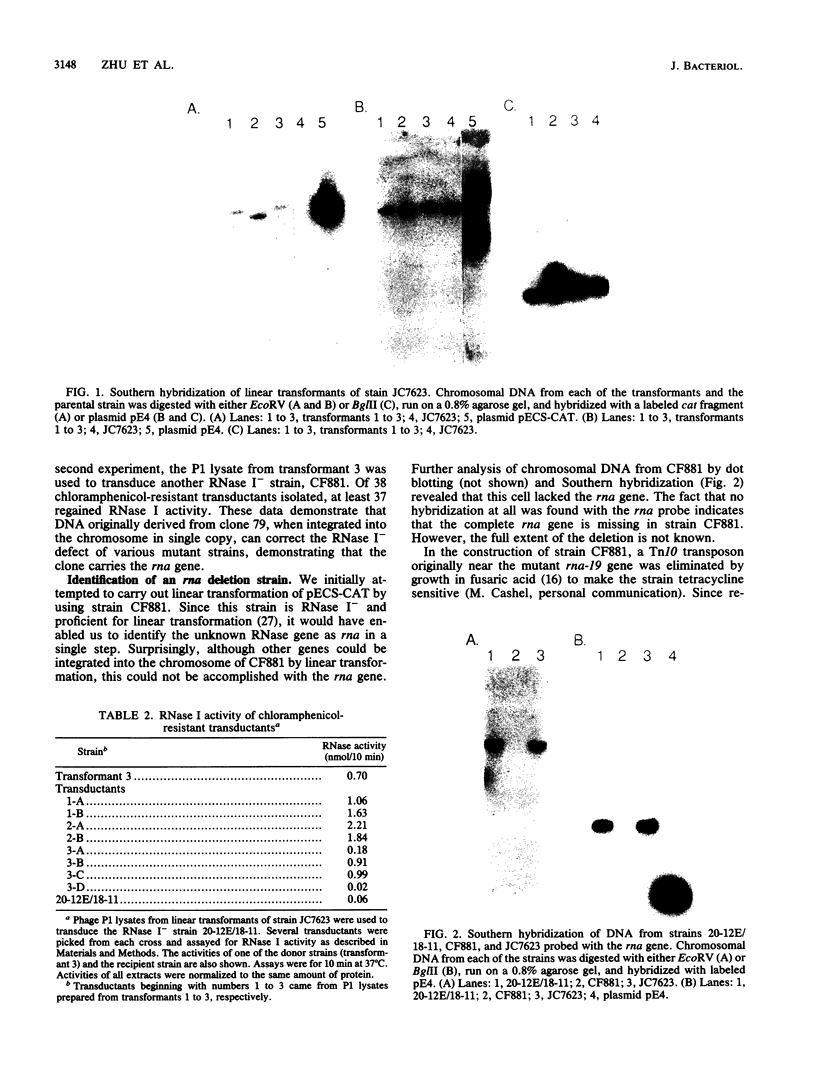
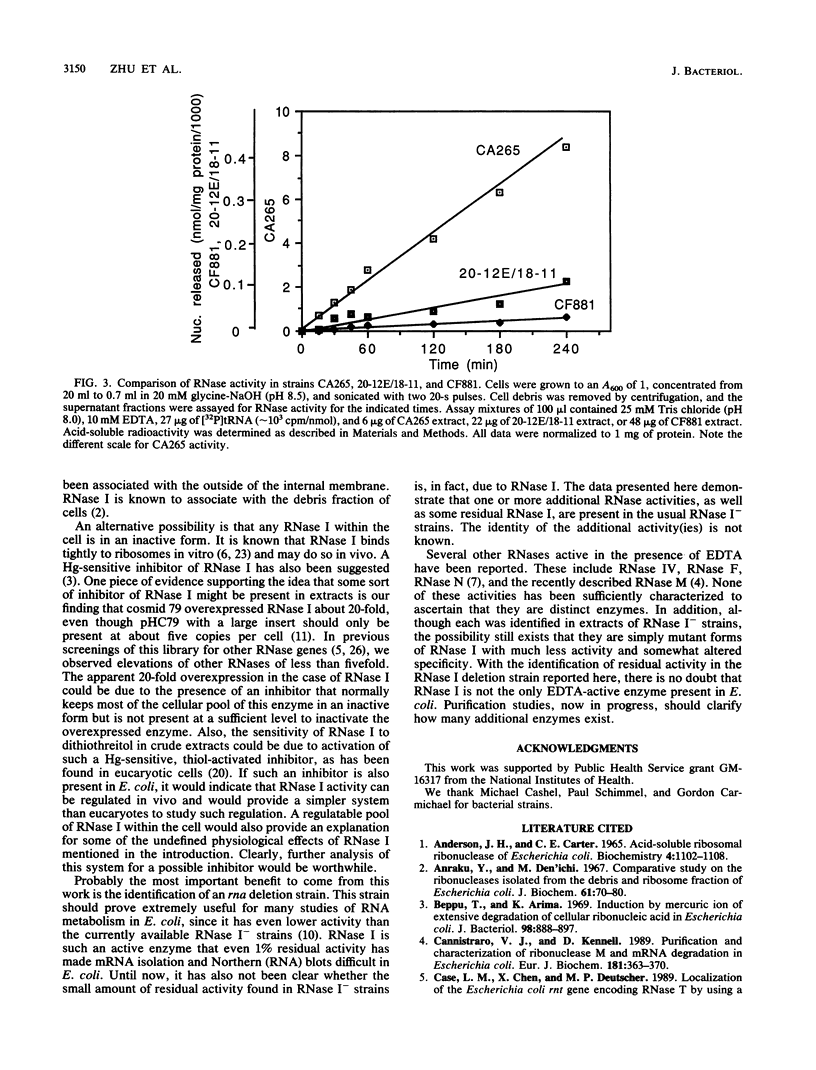
The cloning and overexpression of the Escherichia coli rna gene encoding RNase I are described. Only a single copy of the rna gene is present on the E. coli chromosome. Although cells with as much as a 100-fold increase in RNase I activity were constructed, little effect on cell growth was observed. Overexpressed RNase I was found in the periplasmic space to the same degree (approximately 85%) as wild-type enzyme, suggesting no limitation in RNase I transport. The rna clone was used to identify a deletion strain totally lacking the rna gene. The normal growth of this strain showed that RNase I is not essential for cell viability. Extracts from the RNase I deletion strain still retained a low level of RNase activity in the presence of EDTA, conclusively demonstrating the existence of additional EDTA-active RNases in E. coli. The possibility of a RNase I inhibitor is also discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. H., Carter C. E. Acid-soluble ribosomal ribonuclease of Escherichia coli. Biochemistry. 1965 Jun;4(6):1102–1108. doi: 10.1021/bi00882a019. [DOI] [PubMed] [Google Scholar]
- Anraku Y., Mizuno D. Comparative study on the ribonucleases isolated from the debris and ribosome fraction of Escherichia coli. J Biochem. 1967 Jan;61(1):70–80. doi: 10.1093/oxfordjournals.jbchem.a128522. [DOI] [PubMed] [Google Scholar]
- Beppu T., Arima K. Induction by mercuric ion of extensive degradation of cellular ribonucleic acid in Escherichia coli. J Bacteriol. 1969 Jun;98(3):888–897. doi: 10.1128/jb.98.3.888-897.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannistraro V. J., Kennell D. Purification and characterization of ribonuclease M and mRNA degradation in Escherichia coli. Eur J Biochem. 1989 May 1;181(2):363–370. doi: 10.1111/j.1432-1033.1989.tb14733.x. [DOI] [PubMed] [Google Scholar]
- Case L. M., Chen X. N., Deutscher M. P. Localization of the Escherichia coli rnt gene encoding RNase T by using a combination of physical and genetic mapping. J Bacteriol. 1989 Oct;171(10):5736–5737. doi: 10.1128/jb.171.10.5736-5737.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Burma D. P. Association of ribonuclease I with ribosomes and their subunits. J Biol Chem. 1972 Nov 10;247(21):6795–6801. [PubMed] [Google Scholar]
- Deutscher M. P. E. coli RNases: making sense of alphabet soup. Cell. 1985 Apr;40(4):731–732. doi: 10.1016/0092-8674(85)90330-7. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P., Marlor C. W., Zaniewski R. RNase T is responsible for the end-turnover of tRNA in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6427–6430. doi: 10.1073/pnas.82.19.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELSON D. Latent enzymic activity of a ribonucleoprotein isolated from Escherichia coli. Biochim Biophys Acta. 1959 Dec;36:372–386. doi: 10.1016/0006-3002(59)90179-9. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Ito R., Ohnishi Y. The roles of RNA polymerase and RNAase I in stable RNA degradation in Escherichia coli carrying the srnB+ gene. Biochim Biophys Acta. 1983 Jan 20;739(1):27–34. doi: 10.1016/0167-4781(83)90040-4. [DOI] [PubMed] [Google Scholar]
- Kaplan R., Apirion D. The involvement of ribonuclease I, ribonuclease II, and polynucleotide phosphorylase in the degradation of stable ribonucleic acid during carbon starvation in Escherichia coli. J Biol Chem. 1974 Jan 10;249(1):149–151. [PubMed] [Google Scholar]
- Kaplan S. Screening procedure for Escherichia coli mutants deficient in ribonuclease I. J Bacteriol. 1973 Feb;113(2):1081–1083. doi: 10.1128/jb.113.2.1081-1083.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Reichardt K., Botstein D. Inversions and deletions of the Salmonella chromosome generated by the translocatable tetracycline resistance element Tn10. J Mol Biol. 1979 Jan 5;127(1):89–115. doi: 10.1016/0022-2836(79)90461-3. [DOI] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEU H. C., HEPPEL L. A. THE RELEASE OF RIBONUCLEASE INTO THE MEDIUM WHEN ESCHERICHIA COLI CELLS ARE CONVERTED TO SPEROPLASTS. J Biol Chem. 1964 Nov;239:3893–3900. [PubMed] [Google Scholar]
- ROTH J. S. Ribonuclease. VII. Partial purification and characterization of a ribonuclease inhibitor in rat liver supernatant fraction. J Biol Chem. 1958 Apr;231(2):1085–1095. [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Zaniewski R., Petkaitis E., Deutscher M. P. A multiple mutant of Escherichia coli lacking the exoribonucleases RNase II, RNase D, and RNase BN. J Biol Chem. 1984 Oct 10;259(19):11651–11653. [PubMed] [Google Scholar]
- Zhang J. R., Deutscher M. P. Cloning, characterization, and effects of overexpression of the Escherichia coli rnd gene encoding RNase D. J Bacteriol. 1988 Feb;170(2):522–527. doi: 10.1128/jb.170.2.522-527.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Deutscher M. P. tRNA nucleotidyltransferase is not essential for Escherichia coli viability. EMBO J. 1987 Aug;6(8):2473–2477. doi: 10.1002/j.1460-2075.1987.tb02528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]