Abstract
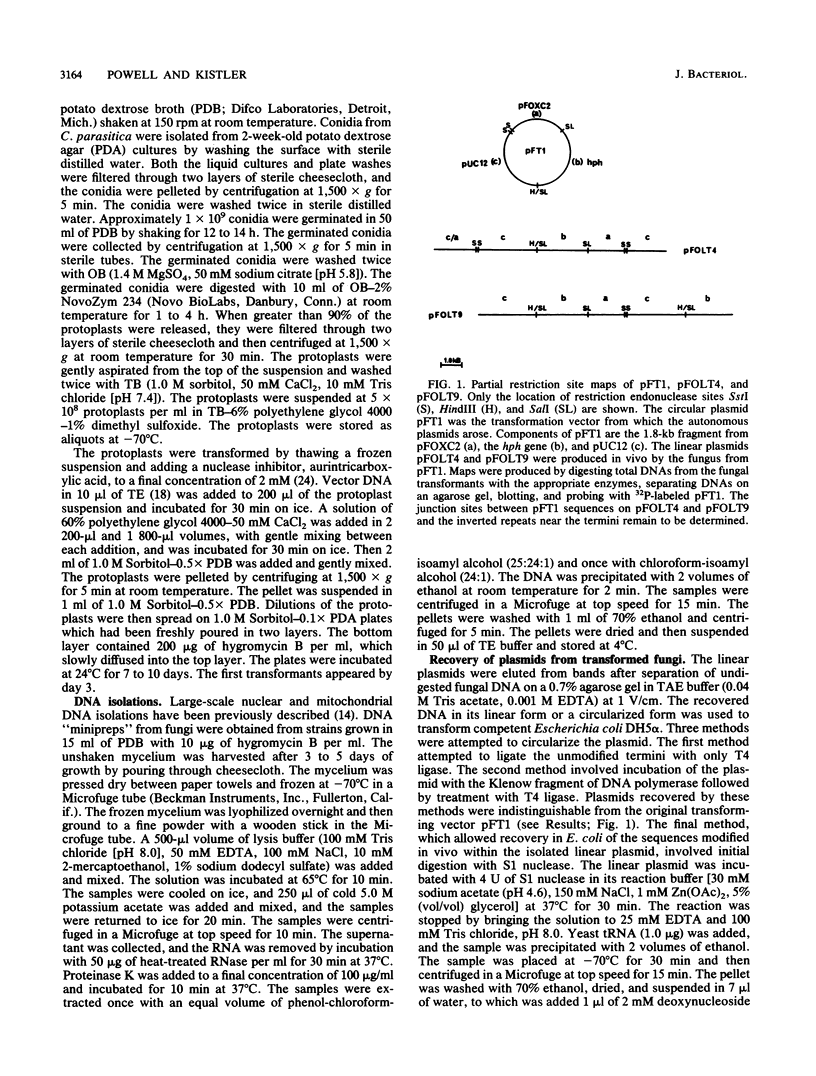
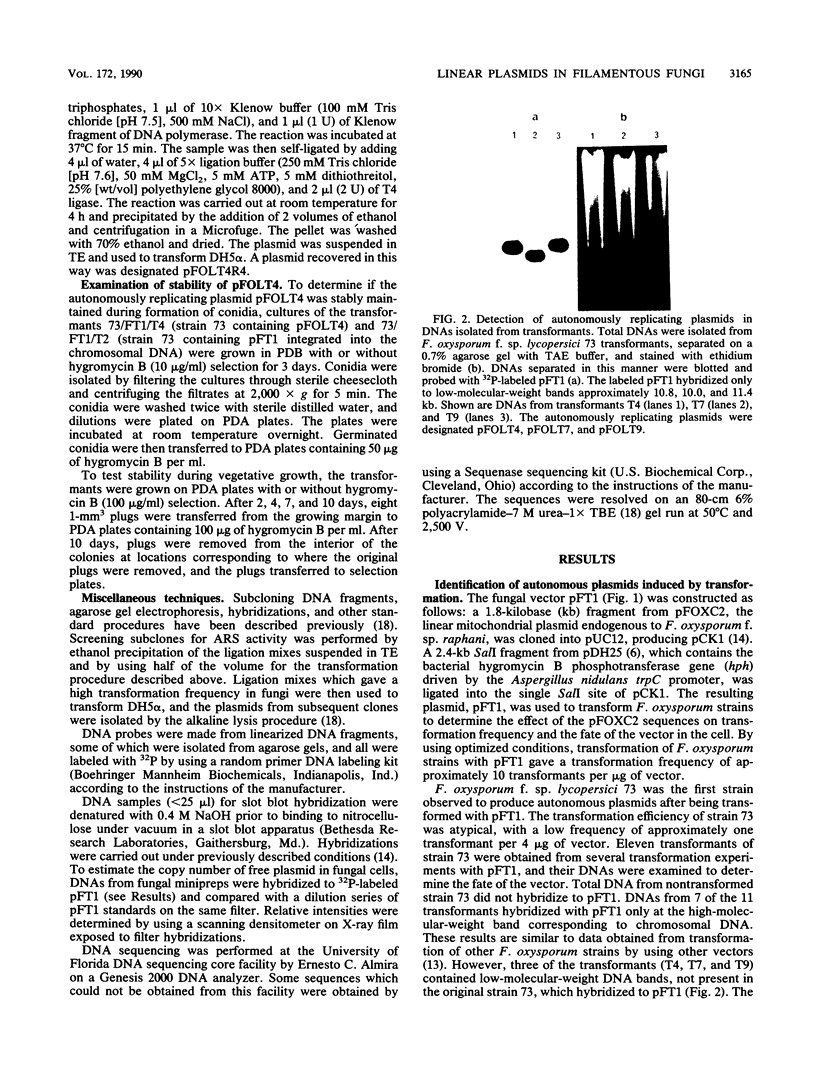
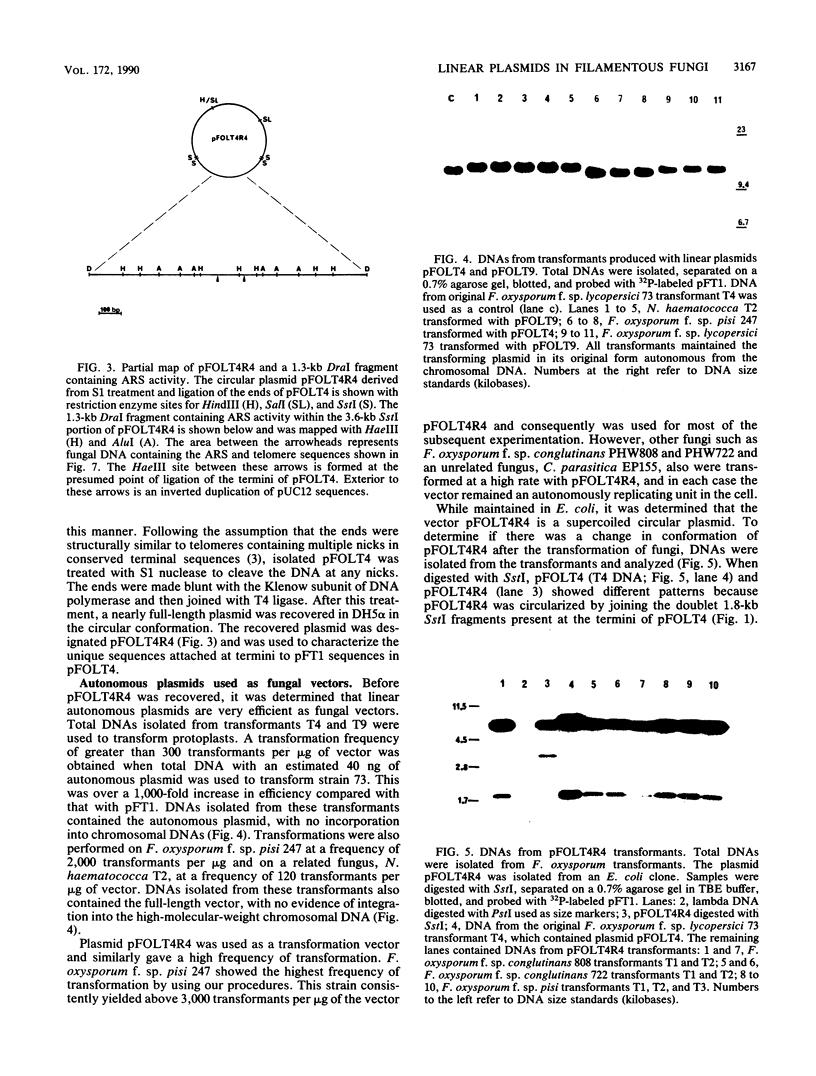
Particular combinations of fungal strains and transformation vectors allow for fungal rearrangement of normally integrative plasmids, resulting in the creation of linear self-replicating plasmids in Fusarium oxysporum. The rearrangement results in the addition of fungal DNA, including telomere consensus sequences, to plasmid termini. The mechanism by which this rearrangement occurs is unclear, but it has similarities to extrachromosomal gene amplification. A DNA fragment which allows for linear autonomous replication upon reintroduction to the fungus was subcloned and sequenced. This DNA sequence contains the repeated telomeric sequence TTAGGG flanked by a region of twofold symmetry consisting primarily of pUC12 DNA. Isolation and identification of this sequence is the first step toward development of vectors that function as artificial chromosomes in filamentous fungi. This sequence was shown to promote autonomous replication and enhance transformation in several strains of F. oxysporum, Nectria haematococca, and Cryphonectria parasitica.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackburn E. H., Challoner P. B. Identification of a telomeric DNA sequence in Trypanosoma brucei. Cell. 1984 Feb;36(2):447–457. doi: 10.1016/0092-8674(84)90238-1. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H., Gall J. G. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978 Mar 25;120(1):33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomeres: do the ends justify the means? Cell. 1984 May;37(1):7–8. doi: 10.1016/0092-8674(84)90295-2. [DOI] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell. 1987 Nov 6;51(3):463–471. doi: 10.1016/0092-8674(87)90642-8. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Li Y. Y., Feldman J., Jayaram M., Abraham J., Nasmyth K. A., Hicks J. B. Localization and sequence analysis of yeast origins of DNA replication. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1165–1173. doi: 10.1101/sqb.1983.047.01.132. [DOI] [PubMed] [Google Scholar]
- Cullen D., Leong S. A., Wilson L. J., Henner D. J. Transformation of Aspergillus nidulans with the hygromycin-resistance gene, hph. Gene. 1987;57(1):21–26. doi: 10.1016/0378-1119(87)90172-7. [DOI] [PubMed] [Google Scholar]
- Fincham J. R. Transformation in fungi. Microbiol Rev. 1989 Mar;53(1):148–170. doi: 10.1128/mr.53.1.148-170.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney J., Henderson E. R., Blackburn E. H. Identification of the telomeric sequence of the acellular slime molds Didymium iridis and Physarum polycephalum. Nucleic Acids Res. 1987 Nov 25;15(22):9143–9152. doi: 10.1093/nar/15.22.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. M., Lambowitz A. M., Rambosek J. A., Kinsey J. A. Transformation of Neurospora crassa with recombinant plasmids containing the cloned glutamate dehydrogenase (am) gene: evidence for autonomous replication of the transforming plasmid. Mol Cell Biol. 1984 Oct;4(10):2041–2051. doi: 10.1128/mcb.4.10.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989 Jan 26;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Spotila L. D., Nawotka K. A., el-Assouli S. M., Davis L. R. The in vivo replication origin of the yeast 2 microns plasmid. Cell. 1987 Nov 6;51(3):473–481. doi: 10.1016/0092-8674(87)90643-x. [DOI] [PubMed] [Google Scholar]
- Kearsey S. Structural requirements for the function of a yeast chromosomal replicator. Cell. 1984 May;37(1):299–307. doi: 10.1016/0092-8674(84)90326-x. [DOI] [PubMed] [Google Scholar]
- Kistler H. C., Leong S. A. Linear plasmidlike DNA in the plant pathogenic fungus Fusarium oxysporum f. sp. conglutinans. J Bacteriol. 1986 Aug;167(2):587–593. doi: 10.1128/jb.167.2.587-593.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari L., Decallonne J. R., Meyer J. A. Deoxyribonucleic acid metabolism and nuclear division during spore germination in Fusarium oxysporum. J Gen Microbiol. 1975 Jun;88(2):245–252. doi: 10.1099/00221287-88-2-245. [DOI] [PubMed] [Google Scholar]
- Lundblad V., Szostak J. W. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989 May 19;57(4):633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paietta J., Marzluf G. A. Plasmid recovery from transformants and the isolation of chromosomal DNA segments improving plasmid replication in Neurospora crassa. Curr Genet. 1985;9(5):383–388. doi: 10.1007/BF00421609. [DOI] [PubMed] [Google Scholar]
- Perrot M., Barreau C., Bégueret J. Nonintegrative transformation in the filamentous fungus Podospora anserina: stabilization of a linear vector by the chromosomal ends of Tetrahymena thermophila. Mol Cell Biol. 1987 May;7(5):1725–1730. doi: 10.1128/mcb.7.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Hill C. W. Recombination between repeated genes in microorganisms. Annu Rev Genet. 1988;22:147–168. doi: 10.1146/annurev.ge.22.120188.001051. [DOI] [PubMed] [Google Scholar]
- Rambosek J. A., Kinsey J. A. An unstable mutant gene of the am locus of Neurospora results from a small duplication. Gene. 1984 Jan;27(1):101–107. doi: 10.1016/0378-1119(84)90242-7. [DOI] [PubMed] [Google Scholar]
- Russell P. J., Rodland K. D. Magnification of rRNA gene number in a Neurospora crassa strain with a partial deletion of the nucleolus organizer. Chromosoma. 1986;93(4):337–340. doi: 10.1007/BF00327592. [DOI] [PubMed] [Google Scholar]
- Schechtman M. G. Isolation of telomere DNA from Neurospora crassa. Mol Cell Biol. 1987 Sep;7(9):3168–3177. doi: 10.1128/mcb.7.9.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shampay J., Szostak J. W., Blackburn E. H. DNA sequences of telomeres maintained in yeast. Nature. 1984 Jul 12;310(5973):154–157. doi: 10.1038/310154a0. [DOI] [PubMed] [Google Scholar]
- Stinchcomb D. T., Struhl K., Davis R. W. Isolation and characterisation of a yeast chromosomal replicator. Nature. 1979 Nov 1;282(5734):39–43. doi: 10.1038/282039a0. [DOI] [PubMed] [Google Scholar]
- Stohl L. L., Lambowitz A. M. Construction of a shuttle vector for the filamentous fungus Neurospora crassa. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1058–1062. doi: 10.1073/pnas.80.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez T., Eslava A. P. Transformation of Phycomyces with a bacterial gene for kanamycin resistance. Mol Gen Genet. 1988 Apr;212(1):120–123. doi: 10.1007/BF00322453. [DOI] [PubMed] [Google Scholar]
- Tsukuda T., Carleton S., Fotheringham S., Holloman W. K. Isolation and characterization of an autonomously replicating sequence from Ustilago maydis. Mol Cell Biol. 1988 Sep;8(9):3703–3709. doi: 10.1128/mcb.8.9.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton J. D., Paquin C. E., Kaneko K., Williamson V. M. Resistance to antimycin A in yeast by amplification of ADH4 on a linear, 42 kb palindromic plasmid. Cell. 1986 Sep 12;46(6):857–863. doi: 10.1016/0092-8674(86)90067-x. [DOI] [PubMed] [Google Scholar]
- Williamson D. H. The yeast ARS element, six years on: a progress report. Yeast. 1985 Sep;1(1):1–14. doi: 10.1002/yea.320010102. [DOI] [PubMed] [Google Scholar]