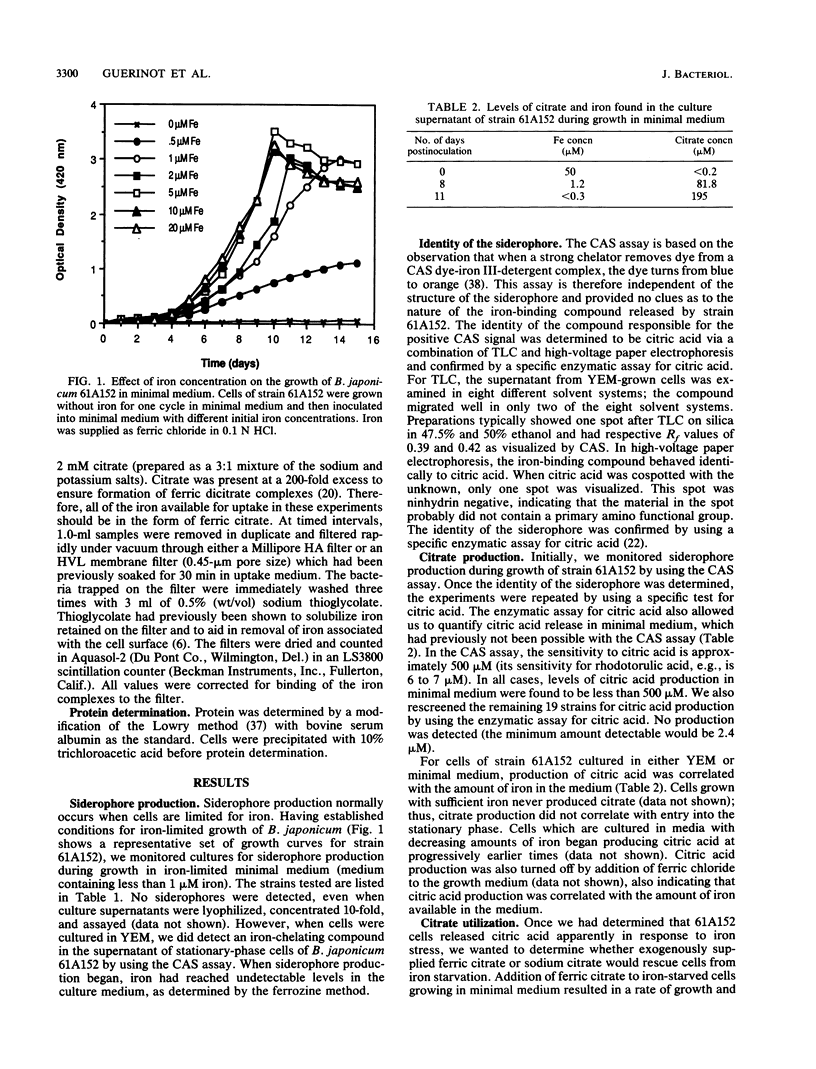
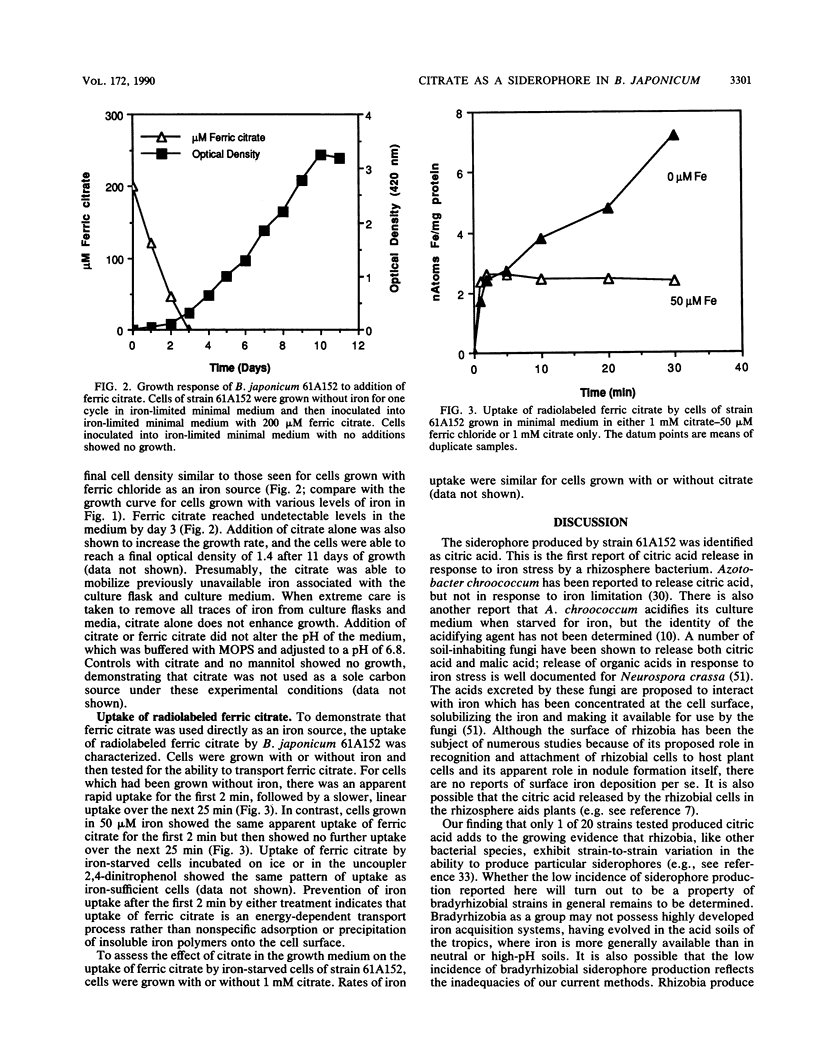
Abstract
Under iron-limiting conditions, many bacteria secrete ferric iron-specific ligands, generically termed siderophores, to aid in the sequestering and transport of iron. One strain of the nitrogen-fixing soybean symbiont Bradyrhizobium japonicum, 61A152, was shown to produce a siderophore when 20 B. japonicum strains were screened with all six chemical assays commonly used to detect such production. Production by strain 61A152 was detected via the chrome azurol S assay, a general test for siderophores which is independent of siderophore structure. The iron-chelating compound was neither a catechol nor a hydroxamate and was ninhydrin negative. It was determined to be citric acid via a combination of thin-layer chromatography and high-voltage paper electrophoresis; this identification was verified by a specific enzymatic assay for citric acid. The inverse correlation which was observed between citric acid release and the iron content of the medium suggested that ferric citrate could serve as an iron source. This was confirmed via growth and transport assays. Exogenously added ferric citrate could be used to overcome iron starvation, and iron-deficient cells actively transported radiolabeled ferric citrate. These results, taken together, indicate a role for ferric citrate in the iron nutrition of this strain, which has been shown to be an efficient nitrogen-fixing strain on a variety of soybean cultivars.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames-Gottfred N. P., Christie B. R., Jordan D. C. Use of the Chrome Azurol S Agar Plate Technique To Differentiate Strains and Field Isolates of Rhizobium leguminosarum biovar trifolii. Appl Environ Microbiol. 1989 Mar;55(3):707–710. doi: 10.1128/aem.55.3.707-710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., DeVoe I. W. Iron acquisition by Neisseria meningitidis in vitro. Infect Immun. 1980 Feb;27(2):322–334. doi: 10.1128/iai.27.2.322-334.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin C. L., Neilands J. B., Phaff H. J. Rhodotorulic acid from species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a new alanine-containing ferrichrome from Cryptococcus melibiosum. J Bacteriol. 1970 Sep;103(3):722–733. doi: 10.1128/jb.103.3.722-733.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J Bacteriol. 1980 May;142(2):581–587. doi: 10.1128/jb.142.2.581-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enard C., Diolez A., Expert D. Systemic virulence of Erwinia chrysanthemi 3937 requires a functional iron assimilation system. J Bacteriol. 1988 Jun;170(6):2419–2426. doi: 10.1128/jb.170.6.2419-2426.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H. M., Bruderer T., Hennecke H. Essential and non-essential domains in the Bradyrhizobium japonicum NifA protein: identification of indispensable cysteine residues potentially involved in redox reactivity and/or metal binding. Nucleic Acids Res. 1988 Mar 25;16(5):2207–2224. doi: 10.1093/nar/16.5.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. The inducible citrate-dependent iron transport system in Escherichia coli K12. Biochim Biophys Acta. 1973 Nov 30;330(1):90–101. doi: 10.1016/0005-2736(73)90287-3. [DOI] [PubMed] [Google Scholar]
- Gill P. R., Jr, Neilands J. B. Cloning a genomic region required for a high-affinity iron-uptake system in Rhizobium meliloti 1021. Mol Microbiol. 1989 Sep;3(9):1183–1189. doi: 10.1111/j.1365-2958.1989.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Holzberg M., Artis W. M. Hydroxamate siderophore production by opportunistic and systemic fungal pathogens. Infect Immun. 1983 Jun;40(3):1134–1139. doi: 10.1128/iai.40.3.1134-1139.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knosp O., von Tigerstrom M., Page W. J. Siderophore-mediated uptake of iron in Azotobacter vinelandii. J Bacteriol. 1984 Jul;159(1):341–347. doi: 10.1128/jb.159.1.341-347.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langman L., Young I. G., Frost G. E., Rosenberg H., Gibson F. Enterochelin system of iron transport in Escherichia coli: mutations affecting ferric-enterochelin esterase. J Bacteriol. 1972 Dec;112(3):1142–1149. doi: 10.1128/jb.112.3.1142-1149.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong S. A., Neilands J. B. Relationship of siderophore-mediated iron assimilation to virulence in crown gall disease. J Bacteriol. 1981 Aug;147(2):482–491. doi: 10.1128/jb.147.2.482-491.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger A. J., Ratledge C. Iron transport in Mycobacterium smegmatis: Uptake of iron from ferric citrate. J Bacteriol. 1982 Jan;149(1):131–135. doi: 10.1128/jb.149.1.131-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering H., Gruber W. Determination of citrate with citrate lyase. Anal Biochem. 1966 Dec;17(3):369–376. doi: 10.1016/0003-2697(66)90172-2. [DOI] [PubMed] [Google Scholar]
- Moody M. D., Dailey H. A. Siderophore utilization and iron uptake by Rhodopseudomonas sphaeroides. Arch Biochem Biophys. 1984 Oct;234(1):178–186. doi: 10.1016/0003-9861(84)90339-4. [DOI] [PubMed] [Google Scholar]
- Nadler K. D., Johnston A. W., Chen J. W., John T. R. A Rhizobium leguminosarum mutant defective in symbiotic iron acquisition. J Bacteriol. 1990 Feb;172(2):670–677. doi: 10.1128/jb.172.2.670-677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Iron absorption and transport in microorganisms. Annu Rev Nutr. 1981;1:27–46. doi: 10.1146/annurev.nu.01.070181.000331. [DOI] [PubMed] [Google Scholar]
- Page W. J. Iron-Dependent Production of Hydroxamate by Sodium-Dependent Azotobacter chroococcum. Appl Environ Microbiol. 1987 Jul;53(7):1418–1424. doi: 10.1128/aem.53.7.1418-1424.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler U., Staudenmaier H., Zimmermann L., Braun V. Genetics of the iron dicitrate transport system of Escherichia coli. J Bacteriol. 1988 Jun;170(6):2716–2724. doi: 10.1128/jb.170.6.2716-2724.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux C. R., Jordan D. C., Rattray J. B. Iron requirement of Rhizobium leguminosarum and secretion of anthranilic acid during growth on an iron-deficient medium. Arch Biochem Biophys. 1986 Jul;248(1):175–182. doi: 10.1016/0003-9861(86)90414-5. [DOI] [PubMed] [Google Scholar]
- Rioux C., Jordan D. C., Rattray J. B. Colorimetric determination of catechol siderophores in microbial cultures. Anal Biochem. 1983 Aug;133(1):163–169. doi: 10.1016/0003-2697(83)90238-5. [DOI] [PubMed] [Google Scholar]
- Roessler P. G., Nadler K. D. Effects of iron deficiency on heme biosynthesis in Rhizobium japonicum. J Bacteriol. 1982 Mar;149(3):1021–1026. doi: 10.1128/jb.149.3.1021-1026.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Skorupska A., Choma A., Deryło M., Lorkiewicz Z. Siderophore containing 2,3-dihydroxybenzoic acid and threonine formed by Rhizobium trifolli. Acta Biochim Pol. 1988;35(2):119–130. [PubMed] [Google Scholar]
- Staudenmaier H., Van Hove B., Yaraghi Z., Braun V. Nucleotide sequences of the fecBCDE genes and locations of the proteins suggest a periplasmic-binding-protein-dependent transport mechanism for iron(III) dicitrate in Escherichia coli. J Bacteriol. 1989 May;171(5):2626–2633. doi: 10.1128/jb.171.5.2626-2633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin L. O. Translocation of iron citrate and phosphorus in xylem exudate of soybean. Plant Physiol. 1970 Mar;45(3):280–283. doi: 10.1104/pp.45.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]


