Abstract
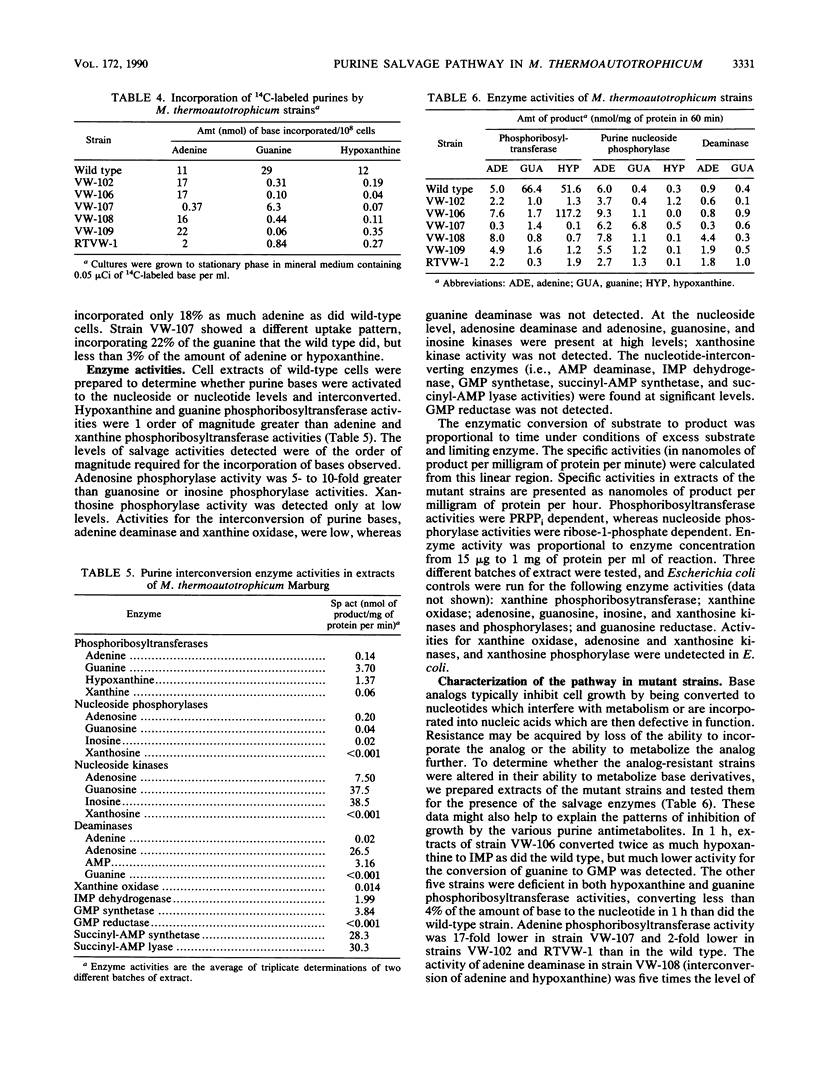
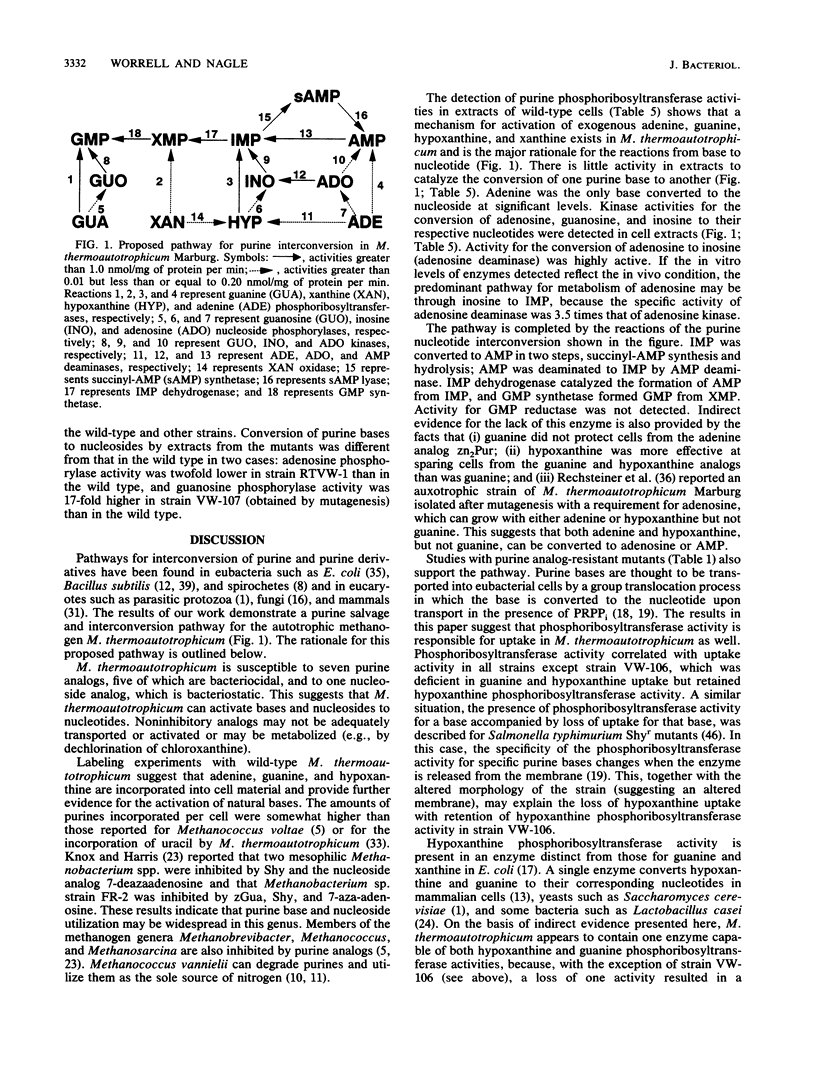
The enzymes involved in the purine interconversion pathway of wild-type and purine analog-resistant strains of Methanobacterium thermoautotrophicum Marburg were assayed by radiometric and spectrophotometric methods. Wild-type cells incorporated labeled adenine, guanine, and hypoxanthine, whereas mutant strains varied in their ability to incorporate these bases. Adenine, guanine, hypoxanthine, and xanthine were activated by phosphoribosyltransferase activities present in wild-type cell extracts. Some mutant strains simultaneously lost the ability to convert both guanine and hypoxanthine to the respective nucleotide, suggesting that the same enzyme activates both bases. Adenosine, guanosine, and inosine phosphorylase activities were detected for the conversion of base to nucleoside. Adenine deaminase activity was detected at low levels. Guanine deaminase activity was not detected. Nucleoside kinase activities for the conversion of adenosine, guanosine, and inosine to the respective nucleotides were detected by a new assay. The nucleotide-interconverting enzymes AMP deaminase, succinyl-AMP synthetase, succinyl-AMP lyase, IMP dehydrogenase, and GMP synthetase were present in extracts; GMP reductase was not detected. The results indicate that this autotrophic methanogen has a complex system for the utilization of exogenous purines.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali L. Z., Sloan D. L. Studies of the kinetic mechanism of hypoxanthine-guanine phosphoribosyltransferase from yeast. J Biol Chem. 1982 Feb 10;257(3):1149–1155. [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani G., Baresi L. Genetic transformation in the methanogen Methanococcus voltae PS. J Bacteriol. 1987 Jun;169(6):2730–2738. doi: 10.1128/jb.169.6.2730-2738.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen T. L., Whitman W. B. Incorporation of Exogenous Purines and Pyrimidines by Methanococcus voltae and Isolation of Analog-Resistant Mutants. Appl Environ Microbiol. 1987 Aug;53(8):1822–1826. doi: 10.1128/aem.53.8.1822-1826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. W., Daniels C. J., Reeve J. N. Gene structure, organization, and expression in archaebacteria. Crit Rev Microbiol. 1989;16(4):287–338. doi: 10.3109/10408418909105479. [DOI] [PubMed] [Google Scholar]
- Canale-Parola E., Kidder G. W. Enzymatic activities for interconversion of purines in spirochetes. J Bacteriol. 1982 Dec;152(3):1105–1110. doi: 10.1128/jb.152.3.1105-1110.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffee C. J. AMP deaminase from rat skeletal muscle. Methods Enzymol. 1978;51:490–497. doi: 10.1016/s0076-6879(78)51067-7. [DOI] [PubMed] [Google Scholar]
- DeMoll E., Tsai L. Conversion of purines to xanthine by Methanococcus vannielii. Arch Biochem Biophys. 1986 Nov 1;250(2):440–445. doi: 10.1016/0003-9861(86)90747-2. [DOI] [PubMed] [Google Scholar]
- DeMoll E., Tsai L. Utilization of purines or pyrimidines as the sole nitrogen source by Methanococcus vannielii. J Bacteriol. 1986 Aug;167(2):681–684. doi: 10.1128/jb.167.2.681-684.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T., Uratani B., Freese E. Purine salvage pathways of Bacillus subtilis and effect of guanine on growth of GMP reductase mutants. J Bacteriol. 1983 Jul;155(1):169–179. doi: 10.1128/jb.155.1.169-179.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H. E., Muirhead K. M., Bishop S. H. Adenylosuccinate synthetase (rabbit muscle, heart, and liver). Methods Enzymol. 1978;51:207–213. doi: 10.1016/s0076-6879(78)51029-x. [DOI] [PubMed] [Google Scholar]
- Hassan H. F., Coombs G. H. Purine and pyrimidine metabolism in parasitic protozoa. FEMS Microbiol Rev. 1988 Feb;4(1):47–83. doi: 10.1111/j.1574-6968.1988.tb02708.x-i1. [DOI] [PubMed] [Google Scholar]
- Hochstadt-Ozer J., Stadtman E. R. The regulation of purine utilization in bacteria. II. Adenine phosphoribosyltransferase in isolated membrane preparations and its role in transport of adenine across the membrane. J Biol Chem. 1971 Sep 10;246(17):5304–5311. [PubMed] [Google Scholar]
- Jackman L. E., Hochstadt J. Regulation of purine utilization in bacteria. VI. Characterization of hypoxanthine and guanine uptake into isolated membrane vesicles from Salmonella typhimurium. J Bacteriol. 1976 Apr;126(1):312–326. doi: 10.1128/jb.126.1.312-326.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. J., Nagle D. P., Jr, Whitman W. B. Methanogens and the diversity of archaebacteria. Microbiol Rev. 1987 Mar;51(1):135–177. doi: 10.1128/mr.51.1.135-177.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenitsky T. A., Neil S. M., Miller R. L. Guanine and xanthine phosphoribosyltransfer activities of Lactobacillus casei and Escherichia coli. Their relationship to hypoxanthine and adenine phosphoribosyltransfer activities. J Biol Chem. 1970 May 25;245(10):2605–2611. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGASANIK B., MOYED H. S., GEHRING L. B. Enzymes essential for the biosynthesis of nucleic acid guanine; inosine 5'-phosphate dehydrogenase of Aerobacter aerogenes. J Biol Chem. 1957 May;226(1):339–350. [PubMed] [Google Scholar]
- McElwain M. C., Pollack J. D. Synthesis of deoxyribomononucleotides in Mollicutes: dependence on deoxyribose-1-phosphate and PPi. J Bacteriol. 1987 Aug;169(8):3647–3653. doi: 10.1128/jb.169.8.3647-3653.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meile L., Kiener A., Leisinger T. A plasmid in the archaebacterium Methanobacterium thermoautotrophicum. Mol Gen Genet. 1983;191(3):480–484. doi: 10.1007/BF00425766. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Elliott D. C., Atkinson M. R. Nucleotide biosynthesis from preformed purines in mammalian cells: regulatory mechanisms and biological significance. Prog Nucleic Acid Res Mol Biol. 1970;10:87–119. doi: 10.1016/s0079-6603(08)60562-0. [DOI] [PubMed] [Google Scholar]
- Nagle D. P., Jr, Teal R., Eisenbraun A. 5-Fluorouracil-resistant strain of Methanobacterium thermoautotrophicum. J Bacteriol. 1987 Sep;169(9):4119–4123. doi: 10.1128/jb.169.9.4119-4123.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard P. Adenosine deaminase from Escherichia coli. Methods Enzymol. 1978;51:508–512. doi: 10.1016/s0076-6879(78)51070-7. [DOI] [PubMed] [Google Scholar]
- Rouvière P. E., Wolfe R. S. Novel biochemistry of methanogenesis. J Biol Chem. 1988 Jun 15;263(17):7913–7916. [PubMed] [Google Scholar]
- Sakamoto N. GMP synthetase (Escherichia coli). Methods Enzymol. 1978;51:213–218. doi: 10.1016/s0076-6879(78)51030-6. [DOI] [PubMed] [Google Scholar]
- Saxild H. H., Nygaard P. Genetic and physiological characterization of Bacillus subtilis mutants resistant to purine analogs. J Bacteriol. 1987 Jul;169(7):2977–2983. doi: 10.1128/jb.169.7.2977-2983.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryon V. V., Pollack D. Purine metabolism in Acholeplasma laidlawii B: novel PPi-dependent nucleoside kinase activity. J Bacteriol. 1984 Jul;159(1):265–270. doi: 10.1128/jb.159.1.265-270.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward D. O. Adenylosuccinate AMP-lyase (Neurospora crassa). Methods Enzymol. 1978;51:202–207. doi: 10.1016/s0076-6879(78)51028-8. [DOI] [PubMed] [Google Scholar]
- Worrell V. E., Nagle D. P., Jr, McCarthy D., Eisenbraun A. Genetic transformation system in the archaebacterium Methanobacterium thermoautotrophicum Marburg. J Bacteriol. 1988 Feb;170(2):653–656. doi: 10.1128/jb.170.2.653-656.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN E. F., MAGASANIK B. UTILIZATION AND INTERCONVERSION OF PURINE BASES AND RIBONUCLEOSIDES BY SALMONELLA TYPHIMURIUM. J Biol Chem. 1964 Jan;239:293–300. [PubMed] [Google Scholar]



