Abstract
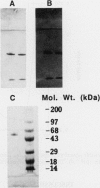
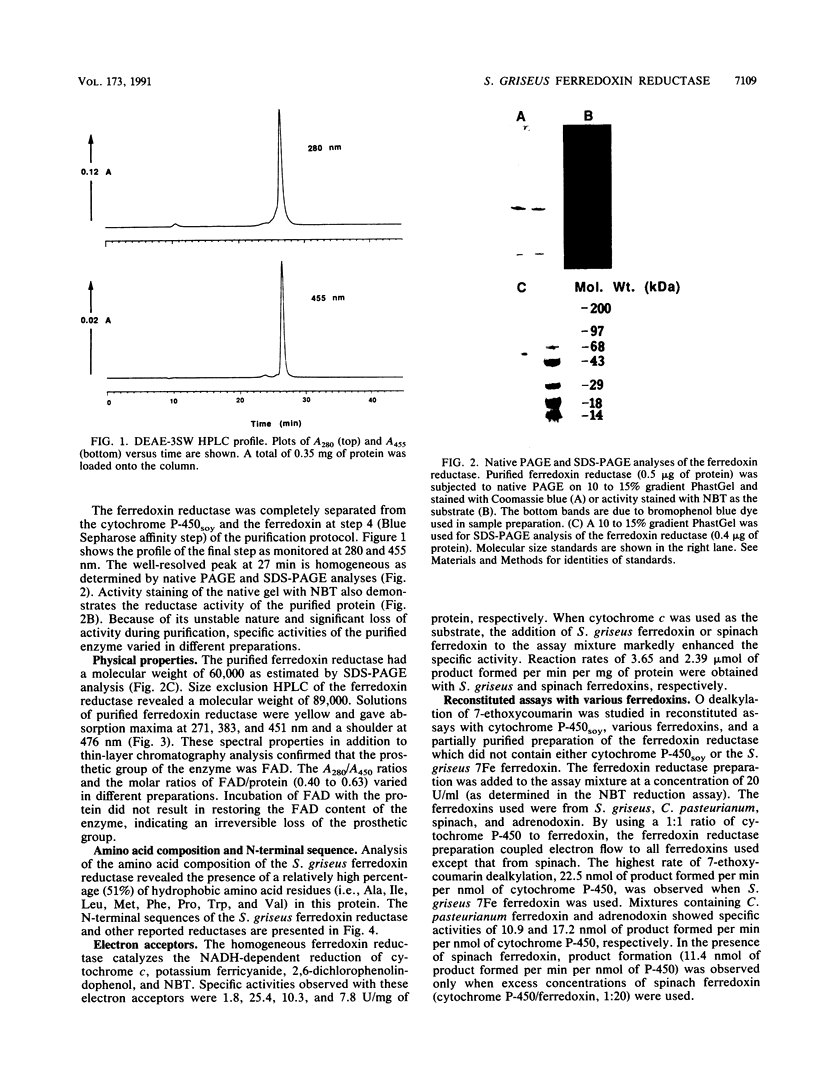
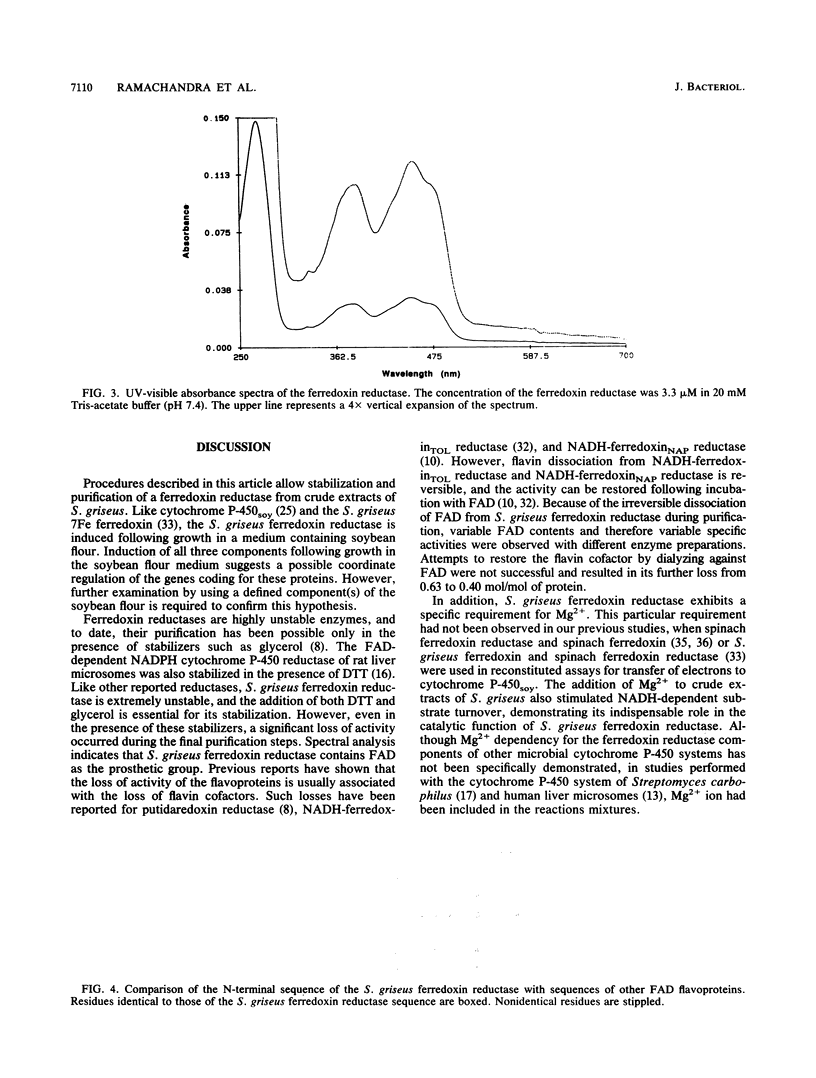
We have purified an NADH-dependent ferredoxin reductase from crude extracts of Streptomyces griseus cells grown in soybean flour-enriched medium. The purified protein has a molecular weight of 60,000 as determined by sodium dodecyl sulfate gel electrophoresis. The enzyme requires Mg2+ ion for catalytic activity in reconstituted assays, and its spectral properties resemble those of many other flavin adenine dinucleotide-containing flavoproteins. A relatively large number of hydrophobic amino acid residues are found by amino acid analysis, and beginning with residue 7, a consensus flavin adenine dinucleotide binding sequence, GXGXXGXXXA, is revealed in this protein. In the presence of NADH, the ferredoxin reductase reduces various electron acceptors such as cytochrome c, potassium ferricyanide, dichlorophenolindophenol, and nitroblue tetrazolium. However, only cytochrome c reduction by the ferredoxin reductase is enhanced by the addition of ferredoxin. In the presence of NADH, S. griseus ferredoxin and cytochrome P-450soy, the ferredoxin reductase mediates O dealkylation of 7-ethoxycoumarin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benen J. A., Van Berkel W. J., Van Dongen W. M., Müller F., De Kok A. Molecular cloning and sequence determination of the lpd gene encoding lipoamide dehydrogenase from Pseudomonas fluorescens. J Gen Microbiol. 1989 Jul;135(7):1787–1797. doi: 10.1099/00221287-135-7-1787. [DOI] [PubMed] [Google Scholar]
- Berg A., Gustafsson J. A., Ingelman-Sundberg M. Characterization of a cytochrome P-450-dependent steroid hydroxylase system present in Bacillus megaterium. J Biol Chem. 1976 May 10;251(9):2831–2838. [PubMed] [Google Scholar]
- Bonomi F., Long R. C., Kurtz D. M., Jr Purification and properties of a membrane-bound NADH-cytochrome-b5 reductase from erythrocytes of the sipunculid worm, Phascolopsis gouldii. Biochim Biophys Acta. 1989 Nov 30;999(2):147–156. doi: 10.1016/0167-4838(89)90211-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broadbent D. A., Cartwright N. J. Bacterial attack on phenolic ethers. Electron acceptor-substrate binding proteins in bacterial O-dealkylases: purification and characterization of cytochrome P-450npd of Nocardia. Microbios. 1974;9(35):119–130. [PubMed] [Google Scholar]
- Chu J. W., Kimura T. Studies on adrenal steroid hydroxylases. Molecular and catalytic properties of adrenodoxin reductase (a flavoprotein). J Biol Chem. 1973 Mar 25;248(6):2089–2094. [PubMed] [Google Scholar]
- Colby J., Dalton H. Resolution of the methane mono-oxygenase of Methylococcus capsulatus (Bath) into three components. Purification and properties of component C, a flavoprotein. Biochem J. 1978 May 1;171(2):461–468. doi: 10.1042/bj1710461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus I. C., Wagner G. C. Bacterial P-450cam methylene monooxygenase components: cytochrome m, putidaredoxin, and putidaredoxin reductase. Methods Enzymol. 1978;52:166–188. doi: 10.1016/s0076-6879(78)52019-3. [DOI] [PubMed] [Google Scholar]
- Haigler B. E., Gibson D. T. Purification and properties of NADH-ferredoxinNAP reductase, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990 Jan;172(1):457–464. doi: 10.1128/jb.172.1.457-464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanukoglu I., Gutfinger T. cDNA sequence of adrenodoxin reductase. Identification of NADP-binding sites in oxidoreductases. Eur J Biochem. 1989 Mar 15;180(2):479–484. doi: 10.1111/j.1432-1033.1989.tb14671.x. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Kamataki T., Sugiura M., Yamazoe Y., Kato R. Purification and properties of cytochrome P-450 and NADPH-cytochrome c (P-450) reductase from human liver microsomes. Biochem Pharmacol. 1979 Jul 1;28(13):1993–2000. doi: 10.1016/0006-2952(79)90214-4. [DOI] [PubMed] [Google Scholar]
- Katagiri M., Ganguli B. N., Gunsalus I. C. A soluble cytochrome P-450 functional in methylene hydroxylation. J Biol Chem. 1968 Jun 25;243(12):3543–3546. [PubMed] [Google Scholar]
- Krauth-Siegel R. L., Blatterspiel R., Saleh M., Schiltz E., Schirmer R. H., Untucht-Grau R. Glutathione reductase from human erythrocytes. The sequences of the NADPH domain and of the interface domain. Eur J Biochem. 1982 Jan;121(2):259–267. doi: 10.1111/j.1432-1033.1982.tb05780.x. [DOI] [PubMed] [Google Scholar]
- Kurzban G. P., Strobel H. W. Preparation and characterization of FAD-dependent NADPH-cytochrome P-450 reductase. J Biol Chem. 1986 Jun 15;261(17):7824–7830. [PubMed] [Google Scholar]
- Matsuoka T., Miyakoshi S., Tanzawa K., Nakahara K., Hosobuchi M., Serizawa N. Purification and characterization of cytochrome P-450sca from Streptomyces carbophilus. ML-236B (compactin) induces a cytochrome P-450sca in Streptomyces carbophilus that hydroxylates ML-236B to pravastatin sodium (CS-514), a tissue-selective inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme-A reductase. Eur J Biochem. 1989 Oct 1;184(3):707–713. doi: 10.1111/j.1432-1033.1989.tb15070.x. [DOI] [PubMed] [Google Scholar]
- Narhi L. O., Fulco A. J. Characterization of a catalytically self-sufficient 119,000-dalton cytochrome P-450 monooxygenase induced by barbiturates in Bacillus megaterium. J Biol Chem. 1986 Jun 5;261(16):7160–7169. [PubMed] [Google Scholar]
- Peterson J. A., Lorence M. C., Amarneh B. Putidaredoxin reductase and putidaredoxin. Cloning, sequence determination, and heterologous expression of the proteins. J Biol Chem. 1990 Apr 15;265(11):6066–6073. [PubMed] [Google Scholar]
- RAJAGOPALAN K. V., HANDLER P. THE ABSORPTION SPECTRA OF IRON-FLAVOPROTEINS. J Biol Chem. 1964 May;239:1509–1514. [PubMed] [Google Scholar]
- Roome P. W., Jr, Philley J. C., Peterson J. A. Purification and properties of putidaredoxin reductase. J Biol Chem. 1983 Feb 25;258(4):2593–2598. [PubMed] [Google Scholar]
- Roome P. W., Peterson J. A. The reduction of putidaredoxin reductase by reduced pyridine nucleotides. Arch Biochem Biophys. 1988 Oct;266(1):32–40. doi: 10.1016/0003-9861(88)90233-0. [DOI] [PubMed] [Google Scholar]
- Sagara Y., Takata Y., Miyata T., Hara T., Horiuchi T. Cloning and sequence analysis of adrenodoxin reductase cDNA from bovine adrenal cortex. J Biochem. 1987 Dec;102(6):1333–1336. doi: 10.1093/oxfordjournals.jbchem.a122178. [DOI] [PubMed] [Google Scholar]
- Sariaslani F. S., Kunz D. A. Induction of cytochrome P-450 in Streptomyces griseus by soybean flour. Biochem Biophys Res Commun. 1986 Dec 15;141(2):405–410. doi: 10.1016/s0006-291x(86)80187-5. [DOI] [PubMed] [Google Scholar]
- Sariaslani F. S. Microbial enzymes for oxidation of organic molecules. Crit Rev Biotechnol. 1989;9(3):171–257. doi: 10.3109/07388558909036736. [DOI] [PubMed] [Google Scholar]
- Sariaslani F. S., Rosazza J. P. Novel Biotransformations of 7-Ethoxycoumarin by Streptomyces griseus. Appl Environ Microbiol. 1983 Aug;46(2):468–474. doi: 10.1128/aem.46.2.468-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariaslani F. S., Stahl R. G., Jr Activation of promutagenic chemicals by Streptomyces griseus containing cytochrome P-450soy. Biochem Biophys Res Commun. 1990 Jan 30;166(2):743–749. doi: 10.1016/0006-291x(90)90872-k. [DOI] [PubMed] [Google Scholar]
- Shafiee A., Hutchinson C. R. Purification and reconstitution of the electron transport components for 6-deoxyerythronolide B hydroxylase, a cytochrome P-450 enzyme of macrolide antibiotic (erythromycin) biosynthesis. J Bacteriol. 1988 Apr;170(4):1548–1553. doi: 10.1128/jb.170.4.1548-1553.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian V., Liu T. N., Yeh W. K., Narro M., Gibson D. T. Purification and properties of NADH-ferredoxinTOL reductase. A component of toluene dioxygenase from Pseudomonas putida. J Biol Chem. 1981 Mar 25;256(6):2723–2730. [PubMed] [Google Scholar]
- Trower M. K., Emptage M. H., Sariaslani F. S. Purification and characterization of a 7Fe ferredoxin from Streptomyces griseus. Biochim Biophys Acta. 1990 Mar 1;1037(3):281–289. doi: 10.1016/0167-4838(90)90026-c. [DOI] [PubMed] [Google Scholar]
- Trower M. K., Marshall J. E., Doleman M. S., Emptage M. H., Sariaslani F. S. Primary structure of a 7Fe ferredoxin from Streptomyces griseus. Biochim Biophys Acta. 1990 Mar 1;1037(3):290–296. doi: 10.1016/0167-4838(90)90027-d. [DOI] [PubMed] [Google Scholar]
- Trower M. K., Sariaslani F. S., Kitson F. G. Xenobiotic oxidation by cytochrome P-450-enriched extracts of Streptomyces griseus. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1417–1422. doi: 10.1016/s0006-291x(88)81033-7. [DOI] [PubMed] [Google Scholar]
- Trower M. K., Sariaslani F. S., O'Keefe D. P. Purification and characterization of a soybean flour-induced cytochrome P-450 from Streptomyces griseus. J Bacteriol. 1989 Apr;171(4):1781–1787. doi: 10.1128/jb.171.4.1781-1787.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai R. L., Gunsalus I. C., Dus K. Composition and structure of camphor hydroxylase components and homology between putidaredoxin and adrenodoxin. Biochem Biophys Res Commun. 1971 Dec 3;45(5):1300–1306. doi: 10.1016/0006-291x(71)90160-4. [DOI] [PubMed] [Google Scholar]
- Ullah A. J., Murray R. I., Bhattacharyya P. K., Wagner G. C., Gunsalus I. C. Protein components of a cytochrome P-450 linalool 8-methyl hydroxylase. J Biol Chem. 1990 Jan 25;265(3):1345–1351. [PubMed] [Google Scholar]