Abstract
Pseudomonas testosteroni ATCC 17410 is able to grow on testosterone. This strain was mutagenized by Tn5, and 41 mutants defective in the utilization of testosterone were isolated. One of them, called mutant 06, expressed 3-oxosteroid delta 1- and 3-oxosteroid delta 4-5 alpha-dehydrogenases only at low levels. The DNA region around the Tn5 insertion in mutant 06 was cloned into pUC19, and the 1-kbp EcoRI-BamHI segment neighbor to the Tn5 insertion was used to probe DNA from the wild-type strain. The probe hybridized to a 7.8-kbp SalI fragment. Plasmid pTES5, which is a pUC19 derivative containing this 7.8-kbp SalI fragment, was isolated after the screening by the 1-kbp EcoRI-BamHI probe. This plasmid expressed delta 1-dehydrogenase in Escherichia coli cells. The 2.2-kbp KpnI-KpnI segment of pTES5 was subcloned into pUC18, and pTEK21 was constructed. In E. coli containing the lacIq plasmid pRG1 and pTEK21, the expression of delta 1-dehydrogenase was induced by isopropyl-beta-D-thiogalactopyranoside (IPTG). The induced level was about 40 times higher than the induced level in P. testosteroni. Delta 1-Dehydrogenase synthesized in E. coli was localized in the inner membrane fraction. The minicell experiments showed that a 59-kDa polypeptide was synthesized from pTEK21, and this polypeptide was located in the inner membrane fraction. The complete nucleotide sequence of the 2.2-kbp KpnI-KpnI segment of pTEK21 was determined. An open reading frame which encodes a 62.4-kDa polypeptide and which is preceded by a Shine-Dalgarno-like sequence was identified. The first 44 amino acids of the putative product exhibited significant sequence similarity to the N-terminal sequences of lipoamide dehydrogenases.
Full text
PDF





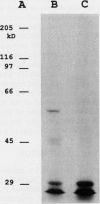
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal A. K., Monder C., Eckstein B., White P. C. Cloning and expression of rat cDNA encoding corticosteroid 11 beta-dehydrogenase. J Biol Chem. 1989 Nov 15;264(32):18939–18943. [PubMed] [Google Scholar]
- Berg D. E., Weiss A., Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Burns G., Brown T., Hatter K., Sokatch J. R. Sequence analysis of the lpdV gene for lipoamide dehydrogenase of branched-chain-oxoacid dehydrogenase of Pseudomonas putida. Eur J Biochem. 1989 Jan 15;179(1):61–69. doi: 10.1111/j.1432-1033.1989.tb14521.x. [DOI] [PubMed] [Google Scholar]
- Choi K. Y., Benisek W. F. Cloning of the gene for delta 5-3-ketosteroid isomerase from Pseudomonas testosteroni. Gene. 1987;58(2-3):257–264. doi: 10.1016/0378-1119(87)90380-5. [DOI] [PubMed] [Google Scholar]
- Choi K. Y., Benisek W. F. Nucleotide sequence of the gene for the delta 5-3-ketosteroid isomerase of Pseudomonas testosteroni. Gene. 1988 Sep 15;69(1):121–129. doi: 10.1016/0378-1119(88)90384-8. [DOI] [PubMed] [Google Scholar]
- Coulter A. W., Talalay P. Studies on the microbiological degradation of steroid ring A. J Biol Chem. 1968 Jun 25;243(12):3238–3247. [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S. J., Talalay P. Purification and mechanism of action of a steroid delta-4-5-beta-dehydrogenase. J Biol Chem. 1966 Feb 25;241(4):906–915. [PubMed] [Google Scholar]
- Griffin T. J., 4th, Kolodner R. D. Purification and preliminary characterization of the Escherichia coli K-12 recF protein. J Bacteriol. 1990 Nov;172(11):6291–6299. doi: 10.1128/jb.172.11.6291-6299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen B. W., van Kleef M. A., Duine J. A. Quinohaemoprotein alcohol dehydrogenase apoenzyme from Pseudomonas testosteroni. Biochem J. 1986 Mar 15;234(3):611–615. doi: 10.1042/bj2340611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama S., Engström P., Wolf-Watz H., Iino T., Hazelbauer G. L. Cloning of trg, a gene for a sensory transducer in Escherichia coli. J Bacteriol. 1982 Oct;152(1):372–383. doi: 10.1128/jb.152.1.372-383.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama S., Oguchi T., Iino T. Does Tn10 transpose via the cointegrate molecule? Mol Gen Genet. 1984;194(3):444–450. doi: 10.1007/BF00425556. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Sutton L., Knobel L., Mammen P. Properties of IncP-2 plasmids of Pseudomonas spp. Antimicrob Agents Chemother. 1983 Aug;24(2):168–175. doi: 10.1128/aac.24.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Kaufmann G., Schumann G., Wollweber L., Hüller E., Atrat P. Steroid-1-dehydrogenases in nocardioform bacteria studied by electrophoresis and immuno blotting techniques. J Basic Microbiol. 1990;30(6):415–423. doi: 10.1002/jobm.3620300608. [DOI] [PubMed] [Google Scholar]
- Kuliopulos A., Shortle D., Talalay P. Isolation and sequencing of the gene encoding delta 5-3-ketosteroid isomerase of Pseudomonas testosteroni: overexpression of the protein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8893–8897. doi: 10.1073/pnas.84.24.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY H. R., TALALAY P. Bacterial oxidation of steroids. I. Ring A dehydrogenations by intact cells. J Biol Chem. 1959 Aug;234(8):2009–2013. [PubMed] [Google Scholar]
- LEVY H. R., TALALAY P. Bacterial oxidation of steroids. II. Studies on the enzymatic mechanism of ring A dehydrogenation. J Biol Chem. 1959 Aug;234(8):2014–2021. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leppik R. A. Steroid catechol degradation: disecoandrostane intermediates accumulated by Pseudomonas transposon mutant strains. J Gen Microbiol. 1989 Jul;135(7):1979–1988. doi: 10.1099/00221287-135-7-1979. [DOI] [PubMed] [Google Scholar]
- Marekov L., Krook M., Jörnvall H. Prokaryotic 20 beta-hydroxysteroid dehydrogenase is an enzyme of the 'short-chain, non-metalloenzyme' alcohol dehydrogenase type. FEBS Lett. 1990 Jun 18;266(1-2):51–54. doi: 10.1016/0014-5793(90)81504-h. [DOI] [PubMed] [Google Scholar]
- Michel-Briand Y., Roux J. Role de la composition du milieu de culture dans le délai d'apparition de l'enzyme 3 alpha-hydroxystéroïde: NAD-oxydoréductase (EC I.I.I.50) de Pseudomonas testosteroni. (Phénomène de répression catabolique) Ann Inst Pasteur (Paris) 1969 Apr;116(4):448–473. [PubMed] [Google Scholar]
- Peltoketo H., Isomaa V., Mäentausta O., Vihko R. Complete amino acid sequence of human placental 17 beta-hydroxysteroid dehydrogenase deduced from cDNA. FEBS Lett. 1988 Oct 24;239(1):73–77. doi: 10.1016/0014-5793(88)80548-9. [DOI] [PubMed] [Google Scholar]
- Penasse L., Peyre M. Studies of 3-oxo steroid delta-1-oxydo reductase of Arthrobacter simplex. Steroids. 1968 Oct;12(4):525–544. doi: 10.1016/s0039-128x(68)80116-3. [DOI] [PubMed] [Google Scholar]
- Poels P. A., Groen B. W., Duine J. A. NAD(P)+-independent aldehyde dehydrogenase from Pseudomonas testosteroni. A novel type of molybdenum-containing hydroxylase. Eur J Biochem. 1987 Aug 3;166(3):575–579. doi: 10.1111/j.1432-1033.1987.tb13552.x. [DOI] [PubMed] [Google Scholar]
- RINGOLD H. J., HAYANO M., STEFANOVIC V. Concerning the stereochemistry and mechanism of the bacterial C-1,2 dehydrogenation of steroids. J Biol Chem. 1963 Jun;238:1960–1965. [PubMed] [Google Scholar]
- SIH C. J., BENNETT R. E. Steroid I-dehydrogenase of Nocardia restrictus. Biochim Biophys Acta. 1962 Jan 29;56:584–592. doi: 10.1016/0006-3002(62)90610-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skålhegg B. A. On the 3 alpha-hydroxysteroid dehydrogenase from Pseudomonas testosteroni. Purification and properties. Eur J Biochem. 1974 Jul 1;46(1):117–125. doi: 10.1111/j.1432-1033.1974.tb03603.x. [DOI] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- TALALAY P. ENZYMATIC MECHANISMS IN STEROID BIOCHEMISTRY. Annu Rev Biochem. 1965;34:347–380. doi: 10.1146/annurev.bi.34.070165.002023. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Ip K. W., Po L. Temperature-sensitive RNA synthesis during adaptive growth on testosterone of Pseudomonas testosteroni. J Steroid Biochem. 1976 Sep;7(9):691–696. doi: 10.1016/0022-4731(76)90068-6. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Lefebvre D., Lefebvre Y., Sy L. P. Membrane-bound dehydrogenases of Pseudomonas testosteroni. J Steroid Biochem. 1980 Jul;13(7):821–827. doi: 10.1016/0022-4731(80)90235-6. [DOI] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- Wovcha M. G., Brooks K. E., Kominek L. A. Evidence for two steroid 1,2-dehydrogenase activities in Mycobacterium fortuitum. Biochim Biophys Acta. 1979 Sep 28;574(3):471–479. doi: 10.1016/0005-2760(79)90243-1. [DOI] [PubMed] [Google Scholar]
- Xia Z. X., Shamala N., Bethge P. H., Lim L. W., Bellamy H. D., Xuong N. H., Lederer F., Mathews F. S. Three-dimensional structure of flavocytochrome b2 from baker's yeast at 3.0-A resolution. Proc Natl Acad Sci U S A. 1987 May;84(9):2629–2633. doi: 10.1073/pnas.84.9.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young I. G., Wallace B. J. Mutations affecting the reduced nicotinamide adenine dinucleotide dehydrogenase complex of Escherichia coli. Biochim Biophys Acta. 1976 Dec 6;449(3):376–385. doi: 10.1016/0005-2728(76)90149-3. [DOI] [PubMed] [Google Scholar]