Abstract
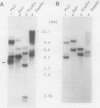
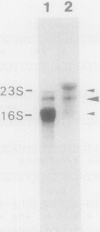
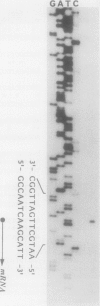
Previously, we reported the cloning of the ribulose-1,5-bisphosphate carboxylase genes (rbcL1-rbcS1) of Thiobacillus ferrooxidans Fe1 (T. Kusano, K. Sugawara, C. Inoue, and N. Suzuki, Curr. Microbiol. 22:35-41, 1991). With these genes as probes, a second set of ribulose-1,5-bisphosphate carboxylase genes (rbcL2-rbcS2) was identified in the same strain and cloned. rbcL1 and rbcL2 encode the large subunits, and rbcS1 and rbcS2 encode the small subunits. Similar restriction patterns between these gene sets suggested a high level of sequence homology. In fact, sequence analysis showed that a 2.2-kb region, including the entire large and small subunit structural genes, was totally conserved in rbcL1-rbcS1 and rbcL2-rbcS2. The rbcL1 (rbcL2) and rbcS1 (rbcS2) genes were 1,422 and 333 bp in length and encoded 473- and 110-amino-acid proteins, respectively. The genes were separated by a 90-bp spacer sequence and were preceded by possible ribosome-binding sites. The N-terminal amino acid sequences of the subunit proteins, synthesized in Escherichia coli, were determined by Edman degradation and found to agree with the deduced amino acid sequences, except for the N-terminal methionine residue. The transcriptional start site of the rbc genes was determined by primer extension, and the size of the rbc transcript was estimated to be about 2.1 kb, suggestive of the cotranscription of rbcL1-rbcS1 and/or rbcL2-rbcS2 mRNAs. Comparisons of amino acid sequences of both subunits with those of other organisms revealed that the ribulose-1,5-bisphosphate carboxylase of T. ferrooxidans, a chemoautotrophic bacterium, is phylogenetically closer to the photosynthetic bacterium Chromatium vinosum than to another chemoautotrophic bacterium, Alcaligenes eutrophus.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K., Caton J. Sequence analysis of the Alcaligenes eutrophus chromosomally encoded ribulose bisphosphate carboxylase large and small subunit genes and their gene products. J Bacteriol. 1987 Oct;169(10):4547–4558. doi: 10.1128/jb.169.10.4547-4558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K., Wilke-Douglas M. Genetic and physical mapping and expression in Pseudomonas aeruginosa of the chromosomally encoded ribulose bisphosphate carboxylase genes of Alcaligenes eutrophus. J Bacteriol. 1987 May;169(5):1997–2004. doi: 10.1128/jb.169.5.1997-2004.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avni A., Edelman M., Rachailovich I., Aviv D., Fluhr R. A point mutation in the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase/oxygenase affects holoenzyme assembly in Nicotiana tabacum. EMBO J. 1989 Jul;8(7):1915–1918. doi: 10.1002/j.1460-2075.1989.tb03594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boczar B. A., Delaney T. P., Cattolico R. A. Gene for the ribulose-1,5-bisphosphate carboxylase small subunit protein of the marine chromophyte Olisthodiscus luteus is similar to that of a chemoautotrophic bacterium. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4996–4999. doi: 10.1073/pnas.86.13.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj D. K., Snyder K. M., Abeles A. L. P1 plasmid replication: multiple functions of RepA protein at the origin. Proc Natl Acad Sci U S A. 1985 May;82(9):2588–2592. doi: 10.1073/pnas.82.9.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. E., Haselkorn R. Isolation and sequence of the gene for the large subunit of ribulose-1,5-bisphosphate carboxylase from the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1835–1839. doi: 10.1073/pnas.80.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaton M. A., Bock S. E., Zwick H., Freeman J. A. Common properties shared by growth-associated proteins of the regenerating optic nerve of goldfish (C. auratus). Neurosci Lett. 1988 Feb 29;85(2):267–271. doi: 10.1016/0304-3940(88)90363-1. [DOI] [PubMed] [Google Scholar]
- Dron M., Rahire M., Rochaix J. D. Sequence of the chloroplast DNA region of Chlamydomonas reinhardii containing the gene of the large subunit of ribulose bisphosphate carboxylase and parts of its flanking genes. J Mol Biol. 1982 Dec 25;162(4):775–793. doi: 10.1016/0022-2836(82)90547-2. [DOI] [PubMed] [Google Scholar]
- Estelle M., Hanks J., McIntosh L., Somerville C. Site-specific mutagenesis of ribulose-1,5-bisphosphate carboxylase/oxygenase. Evidence that carbamate formation at Lys 191 is required for catalytic activity. J Biol Chem. 1985 Aug 15;260(17):9523–9526. [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Localization and mapping of CO2 fixation genes within two gene clusters in Rhodobacter sphaeroides. J Bacteriol. 1988 May;170(5):2153–2158. doi: 10.1128/jb.170.5.2153-2158.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M., Rahire M. Sequence, evolution and differential expression of the two genes encoding variant small subunits of ribulose bisphosphate carboxylase/oxygenase in Chlamydomonas reinhardtii. J Mol Biol. 1986 Oct 5;191(3):421–432. doi: 10.1016/0022-2836(86)90137-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge S., Sigal I., Thomas B., Arentzen R., Cordova A., Lorimer G. A site-specific mutation within the active site of ribulose-1,5-bisphosphate carboxylase of Rhodospirillum rubrum. EMBO J. 1984 Dec 1;3(12):2737–2743. doi: 10.1002/j.1460-2075.1984.tb02204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hartman F. C., Larimer F. W., Mural R. J., Machanoff R., Soper T. S. Essentiality of Glu-48 of ribulose bisphosphate carboxylase/oxygenase as demonstrated by site-directed mutagenesis. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1158–1163. doi: 10.1016/0006-291x(87)91558-0. [DOI] [PubMed] [Google Scholar]
- Hartman F. C., Soper T. S., Niyogi S. K., Mural R. J., Foote R. S., Mitra S., Lee E. H., Machanoff R., Larimer F. W. Function of Lys-166 of Rhodospirillum rubrum ribulosebisphosphate carboxylase/oxygenase as examined by site-directed mutagenesis. J Biol Chem. 1987 Mar 15;262(8):3496–3501. [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Igarashi Y., McFadden B. A., el-Gul T. Active site histidine in spinach ribulosebisphosphate carboxylase/oxygenase modified by diethyl pyrocarbonate. Biochemistry. 1985 Jul 16;24(15):3957–3962. doi: 10.1021/bi00336a024. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J. Thiobacillus ferrooxidans. The bioenergetics of an acidophilic chemolithotroph. Biochim Biophys Acta. 1982 Nov 30;683(2):89–117. doi: 10.1016/0304-4173(82)90007-6. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintworth R., Husemann M., Salnikow J., Bowien B. Chromosomal and plasmid locations for phosphoribulokinase genes in Alcaligenes eutrophus. J Bacteriol. 1985 Nov;164(2):954–956. doi: 10.1128/jb.164.2.954-956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Viale A. M., Takabe T., Akazawa T., Wada K., Shinozaki K., Kobayashi K., Sugiura M. Sequence and expression of genes encoding the large and small subunits of ribulose 1,5-bisphosphate carboxylase/oxygenase from Chromatium vinosum. Gene. 1991 Jan 2;97(1):55–62. doi: 10.1016/0378-1119(91)90009-z. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane D. J., Stahl D. A., Olsen G. J., Heller D. J., Pace N. R. Phylogenetic analysis of the genera Thiobacillus and Thiomicrospira by 5S rRNA sequences. J Bacteriol. 1985 Jul;163(1):75–81. doi: 10.1128/jb.163.1.75-81.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H. Ribulosebisphosphate carboxylase: amino acid sequence of a peptide bearing the activator carbon dioxide. Biochemistry. 1981 Mar 3;20(5):1236–1240. doi: 10.1021/bi00508a028. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of a genomic DNA clone for the small subunit of ribulose bis-phosphate carboxylase-oxygenase from tobacco. Nucleic Acids Res. 1985 Apr 11;13(7):2373–2386. doi: 10.1093/nar/13.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Nierzwicki-Bauer S. A., Curtis S. E., Haselkorn R. Cotranscription of genes encoding the small and large subunits of ribulose-1,5-bisphosphate carboxylase in the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5961–5965. doi: 10.1073/pnas.81.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison P. D., Tabita F. R. Modification of ribulose bisphosphate carboxylase from Rhodospirillum rubrum with tetranitromethane. Biochem Biophys Res Commun. 1979 May 14;88(1):85–91. doi: 10.1016/0006-291x(79)91699-1. [DOI] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959 May;77(5):642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss J. V., Stringer C. D., Hartman F. C. Identification of essential lysyl and cysteinyl residues in spinach ribulosebisphosphate carboxylase/oxygenase modified by the affinity label N-bromoacetylethanolamine phosphate. J Biol Chem. 1978 Aug 25;253(16):5707–5711. [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. The gene for the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase is located close to the gene for the large subunit in the cyanobacterium Anacystis nidulans 6301. Nucleic Acids Res. 1983 Oct 25;11(20):6957–6964. doi: 10.1093/nar/11.20.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. The nucleotide sequence of the tobacco chloroplast gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Gene. 1982 Nov;20(1):91–102. doi: 10.1016/0378-1119(82)90090-7. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Yamada C., Takahata N., Sugiura M. Molecular cloning and sequence analysis of the cyanobacterial gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4050–4054. doi: 10.1073/pnas.80.13.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori T., Inoue C., Sugawara K., Kusano T., Kitagawa Y. Cloning and expression of Thiobacillus ferrooxidans mercury ion resistance genes in Escherichia coli. J Bacteriol. 1989 Jun;171(6):3458–3464. doi: 10.1128/jb.171.6.3458-3464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer C. D., Hartman F. C. Sequences of two active-site peptides from spinach ribulosebisphosphate carboxylase/oxygenase. Biochem Biophys Res Commun. 1978 Feb 28;80(4):1043–1048. doi: 10.1016/0006-291x(78)91351-7. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A. D-ribulose 1,5-diphosphate carboxylase from Rhodospirillum rubrum. II. Quaternary structure, composition, catalytic, and immunological properties. J Biol Chem. 1974 Jun 10;249(11):3459–3464. [PubMed] [Google Scholar]
- Tabita F. R. Molecular and cellular regulation of autotrophic carbon dioxide fixation in microorganisms. Microbiol Rev. 1988 Jun;52(2):155–189. doi: 10.1128/mr.52.2.155-189.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle E., Kobayashi H., Akazawa T. Transcriptional regulation of genes for plant-type ribulose-1,5-bisphosphate carboxylase/oxygenase in the photosynthetic bacterium, Chromatium vinosum. Eur J Biochem. 1988 May 2;173(3):483–489. doi: 10.1111/j.1432-1033.1988.tb14024.x. [DOI] [PubMed] [Google Scholar]
- Viale A. M., Kobayashi H., Akazawa T. Distinct properties of Escherichia coli products of plant-type ribulose-1,5-bisphosphate carboxylase/oxygenase directed by two sets of genes from the photosynthetic bacterium Chromatium vinosum. J Biol Chem. 1990 Oct 25;265(30):18386–18392. [PubMed] [Google Scholar]
- Viale A. M., Kobayashi H., Akazawa T. Expressed genes for plant-type ribulose 1,5-bisphosphate carboxylase/oxygenase in the photosynthetic bacterium Chromatium vinosum, which possesses two complete sets of the genes. J Bacteriol. 1989 May;171(5):2391–2400. doi: 10.1128/jb.171.5.2391-2400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmann C. C., Ramage R. T., Bohnert H. J., Ostrem J. A. Identification of an assembly domain in the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1198–1202. doi: 10.1073/pnas.86.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yates J. R., Cunningham R. P., Holmes D. S. IST2: an insertion sequence from Thiobacillus ferrooxidans. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7284–7287. doi: 10.1073/pnas.85.19.7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. R., Holmes D. S. Two families of repeated DNA sequences in Thiobacillus ferrooxidans. J Bacteriol. 1987 May;169(5):1861–1870. doi: 10.1128/jb.169.5.1861-1870.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]