Abstract
To examine the effect of reinforcer density in prize-based abstinence reinforcement, heroin/cocaine users (N = 116) in methadone maintenance (100 mg/day) were randomly assigned to a noncontingent control group (NonC) or to 1 of 3 groups that earned prize draws for abstinence: manual drawing with standard prize density (MS) or computerized drawing with standard (CS) or high (CH) density. Probabilities (prizes/draw) were standard (50%) and high (78%); prize density was double blind. Mean prize values were CH, $286; CS, $167; MS, $139; and NonC, $171. Outcomes were % opioid/cocaine-negative urines during the 12-week intervention and then 8 weeks postintervention as well as diagnosis of dependence up to 6 months poststudy. CH had significantly more negative specimens than did NonC during intervention and had more than all groups during postintervention treatment: Mean % negative (95% confidence interval) during postintervention treatment adjusted for baseline drug use and dropout were CH, 55% (14%–90%); CS, 7% (1%–27%); MS, 4% (1%–12%); and NonC, 3% (1%–10%). Current cocaine dependence diagnoses after treatment were significantly lower in contingent compared with noncontingent groups. Computerized drawing with higher-density prizes enhanced reduction of cocaine use; abstinence reinforcement had long-term therapeutic benefits.
Keywords: contingency management, opiate, cocaine dependence, opiate dependence, methadone maintenance
One of the most robust means of inducing behavioral change in patients with heroin and cocaine dependence is a set of behavioral techniques called contingency management (CM; Higgins et al., 1991; Higgins & Silverman, 1999). An especially powerful component of CM is a voucher-based escalating-reinforcement schedule developed by Higgins et al. (1991), in which reinforcement increases with each consecutive drug-negative urine sample. This procedure has repeatedly been shown effective in the treatment of cocaine abuse and dependence, with or without concurrent dependence on heroin (Higgins et al., 1991; Silverman et al., 1996). Compared with a control procedure in which equivalent values of vouchers are given independent of cocaine use, the escalating-reinforcement procedure reduces the frequency of cocaine use, increases the mean duration of abstinence, reduces patients’ ratings of their desire for cocaine, and increases the frequency with which abstainers report engaging in coping behaviors (Silverman et al., 1998).
The voucher reinforcers used in the escalating-reinforcement procedure are certificates presented immediately on provision of a drug-negative urine sample; monetary values indicated on the certificates accrue and are redeemable later for a variety of goods and services. The use of vouchers obviates the use of cash while enabling each patient’s reinforcers to be tailored to his or her preferences—preferences that can vary substantially among patients (Chutuape, Silverman, & Stitzer, 1998). However, community treatment programs have been slow to adopt voucher-based CM, in its empirically tested form, into standard care, with its cost being the most frequently cited barrier to implementation (Amass & Kamien, 2004; Kirby, Benishek, Dugosh, & Kerwin, 2006).
Cocaine and heroin use by patients in methadone maintenance has also been successfully treated with a lower-cost prize-based CM procedure in which the reinforcers are prize draws (Petry & Martin, 2002; Petry, Martin, & Simcic, 2005). In a typical implementation of this procedure, participants draw from 250 slips or tokens in a bowl; 125 of the tokens say “Sorry, try again,” 109 are redeemable for a small prize (e.g., a $1 restaurant coupon), 15 are redeemable for a large prize worth up to $20 (e.g., portable stereos, watches, clothing, gift certificates), and 1 is redeemable for a jumbo prize worth up to $100 (e.g., a VCR). Prizes are kept on site, enabling tangible reinforcement to be given immediately.
To optimize the beneficial effects of prize-based CM, additional research is needed to evaluate the manner in which varying the density of reinforcement may influence abstinence from multiple drug use by using this procedure (Petry & Martin, 2002). Studies of reinforcement parameters have important clinical implications for the cost-versus-efficacy issue of CM, in that an ideal arrangement would be to identify a procedure with the lowest cost that also preserves the efficacy of the treatment intervention. For example, in a study examining the effect of reinforcer size, higher-value prizes ($240 expected maximum total) were more effective than lower-value prizes ($80 expected maximum total; Petry et al., 2004).
To further address this question, in the present study we tested whether outcomes in a prize-based procedure would improve with a higher density of reinforcement—even if participants were not explicitly made aware of it. We use the term density instead of probability when referring to reinforcement manipulation in the present study because both the probability of receiving prizes as well as the total magnitude of the prizes given varied on average between groups receiving standard versus high-density reinforcement.
In pilot work, we had implemented a manual drawing procedure with marked wooden balls drawn from a drum, and results of drawings (both number and prize size) were recorded manually and then entered into a spreadsheet for analysis. For the present study, we developed a computerized drawing procedure—automated contingency management (ACM; Vahabzadeh et al., 2007)—which allowed us to manipulate prize density in a manner that was not likely to be perceived by patients and staff and to automatically electronically record drawing results and prize selection. The computerized drawing procedure reduced both the possibility of error and the resources expended to track the participants’ progress, prize-selection history, and total winnings. This automated system also eliminated the possibility of any manipulation of prize-drawing outcome by the participants. A potential drawback of the automated procedure was that computerized drawings might not generate the same level of enthusiasm as manual drawings. Therefore, we randomly assigned participants to the manual drawing procedure or to the computerized drawing procedure, and within the computerized drawing condition, we manipulated the likelihood of winning a prize (unbeknownst to participants). Our hypothesis was that an increase in the likelihood of winning a prize in our drawing procedure would increase abstinence from cocaine.
Method
Participants
The study was conducted at the Intramural Research Program of the National Institute on Drug Abuse, Baltimore, Maryland, between May 2003 and August 2005. This study was approved by the local institutional review board for human research. Participants (N = 116) were recruited through advertisements in a variety of local newspapers and television stations selected to ensure exposure to both sexes and all ethnicities. They gave informed written consent prior to participation. Screening included medical, psychiatric, and drug-use histories; physical examination; standard laboratory screens; and a battery of assessment instruments, including the Addiction Severity Index (McLellan et al., 1985) and Diagnostic Interview Schedule (Version IV; Robins, Cottler, Bucholz, & Compton, 1995). Eligibility criteria were age 18–65 years, cocaine and heroin use (by self-report and urine screen), and physical dependence on heroin. Exclusion criteria were current psychotic, bipolar, or major depressive disorders; current physical dependence on alcohol or sedatives; unstable serious medical illness; estimated IQ below 80 as assessed by the Shipley Institute of Living Scale (Zachary, 1986); and urologic conditions that precluded urine collection. The inclusion and exclusion criteria were designed to maximize generalizability (external validity) while minimizing risk to patients.
On enrolling in the study, participants began methadone-maintenance therapy (described below). Participants were eligible for randomization if at least 4 of 15 urine specimens tested positive for heroin and cocaine (not necessarily on the same days) during the first 5 weeks of treatment (baseline). Stratification of randomization was made by race, sex, employment status, probation status, and frequency of opiate- and cocaine-positive urine specimens during baseline. Among the 211 individuals who qualified for the study, 10 lost contact with the program and failed to initiate treatment and 48 dropped out or were discontinued from the study before being randomized; of those, 4 were incarcerated, 6 were discharged for noncompliance with study/clinic rules, 4 were discharged for medical reasons, and 34 missed three visits in a row. An additional 33 participants completed baseline but failed to meet drug-use criteria for randomization. Finally, 4 completed baseline but, because of a technician error, were counted as having failed to meet drug-use criteria for randomization. The remaining 116 were randomized and were included in the analyses. Baseline demographic and clinical characteristics of each group of randomized participants are listed in Table 1. Figure 1 shows participants’ flow through the study and the percentage of participants who completed the study.
Table 1.
Demographic Characteristics and Retention Rates by Group
| Groupa |
||||
|---|---|---|---|---|
| Variable | NC | MS | CS | CH |
| Age (in years; M ± SD) | 38.7 ± 8.7 | 38.2 ± 6.8 | 37.6 ± 8.8 | 33.9 ± 8.8 |
| Gender | ||||
| Female | 45% | 30% | 47% | 55% |
| Male | 55% | 70% | 53% | 45% |
| Race | ||||
| African American | 40% | 45% | 47% | 60% |
| Other | 60% | 55% | 53% | 40% |
| Heroin use (in years; M ± SD) | 10.3 ± 6.9 | 10.1 ± 6.5 | 9.4 ± 6.9 | 8.5 ± 8.3 |
| Cocaine use (in years; M ± SD) | 9.5 ± 6.9 | 8.2 ± 7.1 | 8.7 ± 7.5 | 7.3 ± 7.2 |
| Days heroin useb (M ± SD) | 29.1 ± 4.9 | 30 ± 0 | 29.6 ± 1.7 | 28.6 ± 6.2 |
| Days cocaine useb (M ± SD) | 15.9 ± 10.5 | 17.7 ± 8.8 | 14.1 ± 9.6 | 16.5 ± 9.9 |
| IQ (M ± SD) | 93 ± 8.1 | 97 ± 8.4 | 93 ± 7.6 | 95 ± 9.2 |
| Years of education (M ± SD) | 11.5 ± 1.5 | 11.6 ± 1.2 | 11.1 ± 1.4 | 11.7 ± 1.7 |
| Legal incomeb (M ± SD) | 521 ± 752.9 | 514 ± 798.9 | 502 ± 757 | 542 ± 790.4 |
| Study retention (weeks; M ± SD) | 22.0 ± 5.7 | 20.9 ± 7.1 | 20.5 ± 7.3 | 22.2 ± 5.3 |
Note. NC = noncontingent; MS = manual (contingent) group; CS = computerized standard-density (contingent) group; CH = computerized high-density (contingent) group.
There were no significant differences between groups on the measures listed (p > .05).
In the last 30 days before admission.
Figure 1.
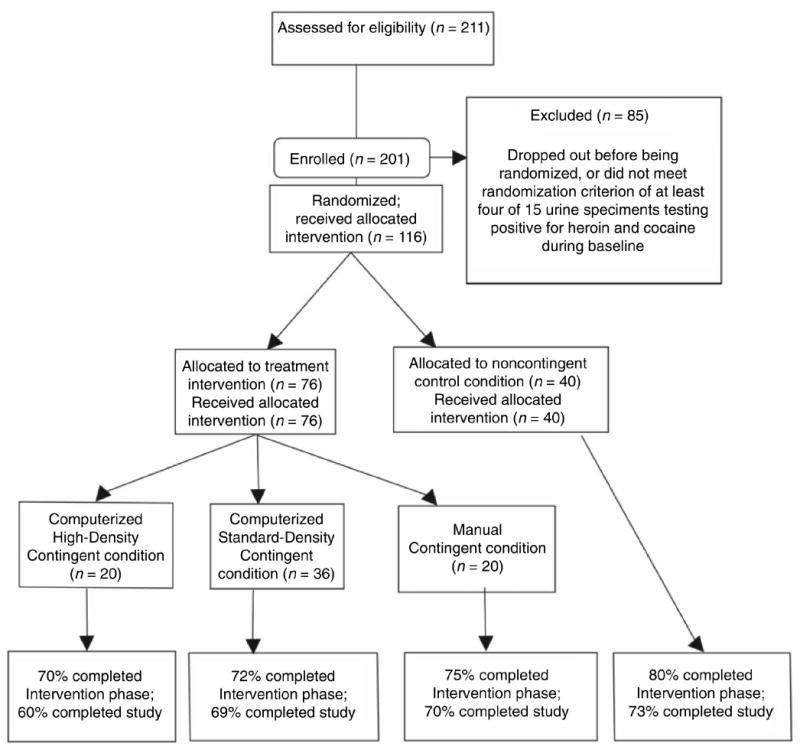
CONSORT flowchart of participants’ progress through the study.
Standard Treatment
All participants received, without charge, daily methadone and weekly individual counseling throughout the study. Methadone HCl (Mallinckrodt, Inc., St. Louis, MO) was administered orally in 35 ml of a cherry-flavored solution and was increased to a target of 100 mg/day within the first 2 weeks. Because the purpose of the study was to investigate the effect of the behavioral intervention on heroin and cocaine use, methadone doses remained constant for up to 25 weeks. However, in cases of participants reporting side effects from methadone, doses were adjusted on the basis of feedback from the participant and the clinical judgment of the clinical staff and medically responsible physician to minimize these events. Methadone dose was not blinded. During screening, participants were informed that the maximum methadone dose that would be offered during the study was 100 mg/day; before entering and during the study, anyone who indicated that this dose was not adequate was offered assistance in transferring to a community treatment program. Four patients did not tolerate the 100 mg/day methadone dose. Of these 4 patients, 2 received 80 mg/day, and 2 received 90 mg/day. None requested transfer because 100 mg/day was too low. The mean stabilized dose (99.5 mg) was approximately the same as the maximum dose (100 mg) because of the low percentage (3%) of patients receiving a dose other than 100 mg. During counseling, counselors completed a semistructured psychosocial assessment and followed a treatment plan for each participant. Reduction of substance use was the primary goal. Individual counseling sessions were devoted to discussion of cessation of all illicit drugs and to addressing problem areas of psychosocial functioning (e.g., vocational, medical, educational, and emotional).
Data Collection
Mondays, Wednesdays, and Fridays, urine specimens were collected under the observation of laboratory technicians and tested for cocaine and opiates (Enzyme Multiplied Immunoassay Technique; cutoffs for positive 300 ng/ml). Urine results were the primary outcome measures. Repeated outcome measures in the random-effects mixed-regression models (see Data Analysis section) were urines simultaneously negative for opiates and cocaine.
Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM–IV; American Psychiatric Association, 1994) diagnoses of heroin or cocaine dependence at study exit and during 3 and 6 months postdischarge follow-up visits, the secondary outcome measures, were collected with the Substance Dependence Severity Scale, a semistructured clinical interview consisting of substance-severity scales with items keyed to every criterion of DSM–IV dependence and abuse (Miele et al., 2000). Participants endorsing three or more symptoms of dependence on a substance were classified as dependent on that substance.
Study Timeline and Groups
The study consisted of a 5-week baseline of standard treatment during which eligibility for randomization was determined (see above), a 12-week experimental intervention plus standard treatment phase, and an 8-week maintenance/postintervention phase. During the maintenance/postintervention phase, prize-based reinforcement was discontinued, but standard treatment continued. Thereafter, participants transferred to other treatment programs or underwent a 10-week methadone taper.
Each participant was randomly assigned to one of three contingent conditions (manual/standard, n = 20; computerized/standard, n = 36; or computerized/high prize density, n = 20) or to a noncontingent (yoked) control group (n = 40; the disparate group sizes resulted from an error in a computerized randomization algorithm). Sample sizes were based on a priori power analyses so that, in pairwise comparisons using two-tailed tests, we would have 80% power to detect a large difference in proportions of negative urine specimens (h = .90) and 60% power to detect a medium-large difference (h =.70; Cohen, 1988).
Participants were randomized to the conditions by a study technician who used a Microsoft Excel macro that stratified randomization by race, sex, employment status, probation status, and frequency of opiate- and cocaine-positive urine specimens during baseline. Participants were not told that they could be randomized to a standard- or high-density group; rather, they were simply given the ranges of probabilities of winning; the consent form stated “The likelihood of winning may be higher for some participants than for other participants, but it will always be within the ranges listed above.” Thus, prize density was blind to participants (and staff). Other aspects of the study (manual vs. computerized and contingent vs. noncontingent) were unblinded because of the nature of the intervention.
Rules for earning draws were the same for all three contingent groups. Each urine specimen negative for either opiates or cocaine earned 1 draw; each specimen negative for both drugs earned 4 draws. Missed specimens counted as positive. Weekly bonus draws were earned if all specimens that week tested negative for both drugs. The number of bonus draws increased with each consecutive week of abstinence: 5 the 1st week, 6 the 2nd week, and up to 16 for the 12th week. Positive or missed urine specimens reset the bonus draws to 5. The maximum possible number of draws was 270.
Participants in the noncontingent control groups were randomized to manual (n = 10), computerized (n = 17), and high-density computerized draws (n = 13), and received opportunities to draw for prizes according to a schedule yoked to a participant in a contingent group. In this manner, the present study differs from previous studies of prize-based CM that have used control groups that did not receive any opportunity to draw for prizes (Petry & Martin, 2002; Petry et al., 2005). The noncontingent condition is useful because it controls for the potential therapeutic benefit of monetary support in an economically disadvantaged population as well as the additional attention received by participants in CM. It is important to note that administering CM reinforcers noncontingently does not increase drug use; previous work using CM systematically tested whether noncontingent vouchers increase use of either the target drug or other drugs and found that they do not (Schroeder, Gupman, Epstein, Umbricht, & Preston, 2003).
The following are the parameters of the schedules of reinforcement: For the manual and standard-density computerized conditions, probabilities of winning a prize were 50% no prize ($0), 44% small prize ($1–$5), 6% large prize ($20), and 0.4% jumbo prize ($100). For the high-density computerized condition, probabilities were 22% no prize, 65% small prize, 12% large prize, and 0.8% jumbo prize. Twelve weeks of continuous abstinence from both cocaine and opiate use would enable a participant in the high-density condition to receive on average a maximum possible total of $1,391 in prizes. The same duration of continuous abstinence would enable a participant in the standard-density condition to receive on average a maximum possible total of $788 in prizes.
The prize-based reinforcement procedure was modeled after one that was used by Petry and Martin (2002). Participants drew from a rotating drum that contained 250 wooden balls. Each ball was marked with a symbol that indicated the prize type—none, small, large, or jumbo. Participants drew the number of balls they had earned, and the number of prizes received was recorded in a logbook. Balls were returned to the drum after each participant made his or her draws. The computerized version of the drawings (ACM) provided a user-friendly graphical interface. When participants entered the computerized drawing procedure, a screen displayed the words “Hello (first name of participant). Today you have earned a total of [N] draws! Good luck.” Participants clicked on an icon that stated “Hit draw” to draw for a prize N times corresponding to N number of prize draws they had earned. Each time they drew, participants received a prompt indicating the outcome of the draw. A panel displayed a running tally of the day’s draw results for that participant, including total draws earned; draws remaining; number of small, large, and jumbo prizes won; and total number of prizes won. After completing all draws for a given day, participants selected prizes from a cabinet in the same room in which the drawing occurred or from a category-specific list (small, large, jumbo) shown on the computer. The system recorded the details of the process, including tracking the history of the winnings and the prizes the participants selected (i.e., date, description of the prize, method of delivery, identity of the staff person who disbursed the prize, and the size of the prize, i.e., small, large, jumbo).
Data Analysis
Preliminary analyses showed no significant differences among the three noncontingent groups; therefore, their data were pooled, leaving four groups in all. Intake measures were analyzed by analysis of variance or Pearson χ2 to test comparability among groups. To address the issue of attrition, study retention as a function of drawing procedure and contingency was analyzed with a log-rank test (SAS LIFETEST procedure; Version 9.1 for all software used in the study) of time until provision of the final urine sample.
Urine results from intervention and maintenance (postintervention), our primary outcome measures, were analyzed by random-effects mixed-regression models (SAS GLIMMIX macro). Random-effects mixed-regression models have been widely accepted in the CM literature as appropriate analytical tools for longitudinal data since they were introduced in the late 1980s. They have been shown to compare favorably with traditional repeated-measures approaches (Nich & Carroll, 1997). These likelihood-based models use iterative methods that utilize all of the existing data, both on an individual and on a group level, to estimate treatment outcomes over time. They facilitate intent-to-treat analyses by interpolating missing values (with appropriate penalties reflected in larger standard errors) rather than deleting participants with missing values or coding all missing values identically. They also allow correlations between repeated measurements to be specified; in our case, a first-order autoregressive covariance structure was used. This covariance structure allows the correlations of measurements taken further apart to be less than those taken closer to one another, a reasonable assumption for most clinical trials. The repeated outcome measures in our models were urines simultaneously negative for opiates and cocaine. We also conducted analyses to assess whether groups differed in terms of urines negative for cocaine and urines negative for opiates. The independent variables were group (manual standard density, computerized standard density, computerized high density, or noncontingent control), a covariate for dropout (continuous variable: the number of the last urine specimen collected during the maintenance/postintervention phase of the study), and covariates for baseline drug use (expressed three ways: percent urine specimens negative for both opiates and cocaine, percent negative for cocaine, and percent negative for opiates; all percentages were arcsine transformed to correct for heterogeneity of variance; Hogg & Craig, 1995). Because of their conceptual similarity, the three measures of baseline drug use were checked for multicollinearity via the diagnostic output of PROC REG procedure in SAS; their condition indices and variance inflation factors were low enough to justify their simultaneous inclusion. Baseline drug use was included as a covariate because although, as noted below, baseline drug use was not significantly different across the groups, earlier work has shown that baseline drug use is a major predictor of treatment response (Preston et al., 1998). The term for dropout was included on the basis of the pattern-mixture approach to controlling for the nonrandom nature of missing data—that is, for the possibility that dropouts differed in some systematic way from study completers (Hedeker & Gibbons, 1997). Post hoc analyses of pairwise differences between least-squares means were analyzed by Tukey–Kramer tests (via the PDIFF option in the LSMEANS statement of the SAS GLIMMIX macro); t values for these post hoc tests are presented. These tests maintained familywise Type I error rate at an alpha level of .05.
DSM–IV substance dependence at study exit and at 3 and 6 months postdischarge, our secondary outcome measure, was also analyzed by random-effects mixed regression (SAS GLIMMIX macro) in a model identical to that described above. To examine the effect of methadone treatment status during the follow-up, random-effects mixed regressions were conducted in which the dependent variable was current DSM–IV cocaine or heroin dependence and the independent variable was participation in methadone-maintenance treatment at the time of follow-up, coded dichotomously.
Alpha level for all analyses was .05 (two-tailed). Analyses were conducted on an intent-to-treat basis. We restricted our analyses to assessing our primary and secondary outcome measures and did not conduct ancillary exploratory analyses.
Results
Demographics and Study Retention
Overall, participants were the following: age in years (M = 37, SD = 8.4); 46% African-American (most non-Caucasians in the study were African-American), 52% White, 1% Hispanic, and 1% Asian; 44% women, 56% men; a mean of 9.7 (SD = 7.4) and 8.8 (SD = 7.3) lifetime years of heroin and cocaine use, respectively; and a mean of 29 (SD = 4.3) and 17 (SD = 10) days of heroin and cocaine use in the last 30 days before admission, respectively. On the basis of Diagnostic Interview Schedule, 97% met DSM–IV criteria for current heroin dependence. Out of 116 participants, 52 (45%) met DSM–IV criteria for current cocaine dependence; another 20 (17%) met criteria for remitted cocaine dependence. The remaining 44 participants (38%) were negative for cocaine dependence; of them, 5 (11%) met criteria for current cocaine abuse, 7 (16%) met criteria for remitted cocaine abuse, and the remaining 32 (73%) were negative for cocaine abuse. However, all 116 participants tested positive for cocaine during the week before treatment started and at least four times during baseline. Pearson χ2 analyses and analyses of variance revealed that demographic, Addiction Severity Index, and Diagnostic Interview Schedule characteristics at intake did not differ significantly across groups (Table 1). Participants who were randomized to an intervention group did not differ from those who dropped out before randomization on baseline characteristics such as gender, age, race, years of heroin use, years of cocaine use, days of heroin use in the last 30 days prior to admission, days of cocaine use in the last 30 days prior to admission, IQ, years of education, or income.
Length of time in treatment, a variable that may be associated with treatment outcome, did not differ across groups. Mean (SD) study retention was 21.3 (6.4) weeks and did not differ across groups, log-rank χ2(3) =4.3, p = .23 (Table 1; ns for the four groups: noncontingent [yoked] control = 40; manual/standard = 20; computerized/standard = 36; computerized/high prize density = 20). Nonetheless, our GLIMMIX analyses controlled for length of time in treatment by including a covariate term for dropout.
Urinalysis and Prizes
Across the four treatment groups, the percentages of urine specimens negative for opioids were 23% in baseline, 58% in intervention, and 62% in maintenance. The percentages of urine specimens negative for cocaine were 15% in baseline, 26% in intervention, and 25% in maintenance. The percentages of urine specimens negative for both were 6% in baseline, 22% in intervention, and 21% in maintenance.
Group mean percentages of urine specimens simultaneously negative for opiates and cocaine at each urine collection point across time are shown in Figure 2A. The manual contingent group appeared to have a lower baseline rate of abstinence than had the other groups. Although drug use during baseline among groups was not significantly different, F(3, 112) = 1.06, p = .369, baseline drug use was included as a covariate term in GLIMMIX analyses of intervention and maintenance data. Comparison of Panels A and B in Figure 2 shows the effects of controlling for baseline drug use; the values in Panel B are μ values, which are percentages of drug-negative urine specimens after adjustment for all covariates in the GLIMMIX model. In the intervention phase, the percentage of urine specimens negative for both cocaine and opiates was significantly greater in the computerized high-density contingent group, t(108) = −2.44, p < .05, than in the noncontingent control group. The manual contingent and computer standard-density contingent groups did not differ from the noncontingent control group in the intervention phase. The mean adjusted percentage of urine specimens negative for both cocaine and opiates during intervention and the corresponding 95% confidence intervals (CIs) were the following: noncontingent control group, 3% (CI = 1, 7); manual contingent, 5% (CI = 2, 11); computerized standard-density contingent, 7% (CI = 2, 20); computerized high-density contingent, 19% (CI = 6, 45).
Figure 2.
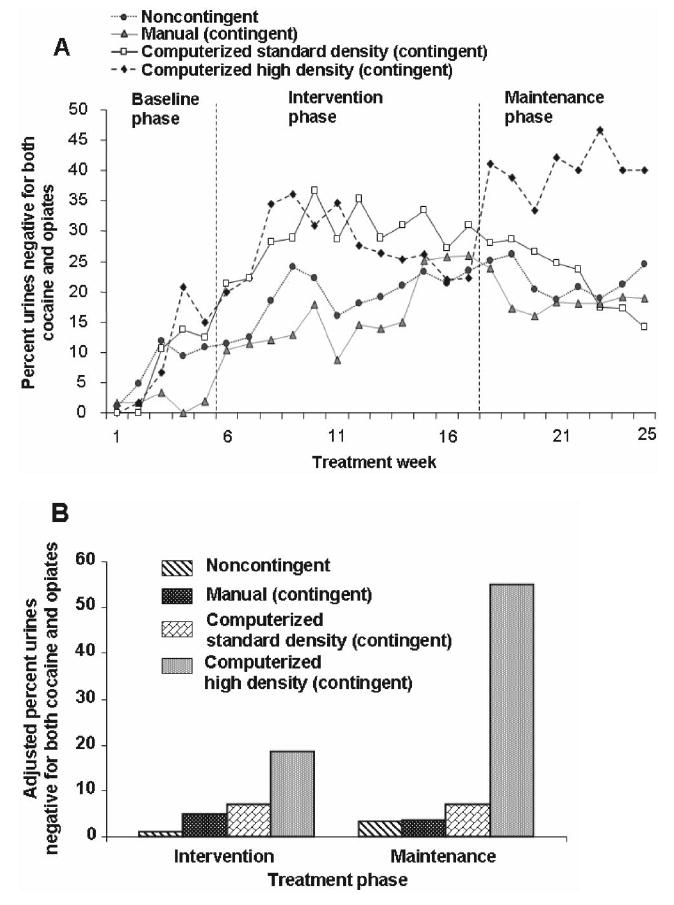
A: Mean raw percentages of urine specimens by treatment group that were negative from both cocaine and opiate use in the baseline phase (first 5 weeks), prize-drawing intervention phase (next 12 weeks), and maintenance/posttreatment (return-to-baseline) phase (final 8 weeks). The seeming group difference in baseline drug use is not statistically significant but is controlled for in the adjusted percentages shown in Panel B. B: Adjusted percentages of urine specimens by treatment group that were negative from both cocaine and opiate use in the prize-drawing intervention phase (12 weeks), and maintenance/posttreatment (return-to-baseline) phase (final 8 weeks). Each adjusted percentage is a μ value from a random-effects mixed-regression model (SAS GLIMMIX macro) controlling for baseline drug use—expressed as (a) the percentage of urine specimens negative for both opiates and cocaine, arcsine transformed; (b) negative for cocaine, arcsine transformed; and (c) negative for opiates, arcsine transformed—and for treatment duration (continuous variable: the number of the last urine specimen collected during the maintenance/postintervention phase of the study).
In the maintenance phase, the percentage of urine specimens negative for both cocaine and opiates was significantly greater in the computerized high-density contingent group than in all other groups: noncontingent t(68) = −2.98, p < .01; computerized standard contingent t(68) = −2.14 p < .05; and manual contingent t(68) = −2.84, p < .01. The manual and computerized contingent groups did not differ from the noncontingent control group in the maintenance phase. The mean adjusted percentages of urine specimens negative for both cocaine and opiates during the maintenance phase and the corresponding 95% CIs were the following: noncontingent control, 3% (CI = 1, 10); manual contingent, 4% (CI = 1, 12); computerized standard-density contingent, 7% (CI = 1, 27); computerized high-density contingent, 55% (CI = 14, 90).
These differences among groups were primarily due to group differences in cocaine use but not opiate use. The percentage of urine specimens negative for opiates did not significantly differ among groups in the intervention and maintenance phases, whereas in both phases, the percentage of urine specimens negative for cocaine was significantly greater in the computerized high-density group than in the noncontingent control group: intervention t(108) = −2.47, p < .01; maintenance t(68) = −2.18, p < .05. The manual and computer standard-density contingent groups did not differ from the noncontingent group in either phase. During intervention, the mean adjusted percentages of urine specimens negative for cocaine and the corresponding 95% CIs were the following: noncontingent control, 6% (CI = 3, 13); manual contingent, 8% (CI = 4, 17); computerized standard-density contingent, 18% (CI = 7, 40); and computerized high-density contingent, 27% (CI = 11, 55). During the maintenance phase, the mean adjusted percentage of urine specimens negative for cocaine and the corresponding 95% CI were the following: noncontingent control, 5% (CI = 1, 28); manual contingent, 7% (CI = 1, 40); computerized standard-density contingent, 17% (CI = 1, 77); and computerized high-density contingent, 78% (CI = 4, 100).
Both the high-density contingent group and the noncontingent control group yoked to high-density contingent group participants received more prizes per prize draw (78% and 76% of draws were winners, respectively) than did the other groups (51%, 59%, 51%, and 60% of draws were winners for the computerized standard contingent, manual contingent, and noncontingent control group yoked to computerized standard and manual contingent groups, respectively). The mean total prize amounts received per group over the 12-week intervention period were computerized high-density contingent, $286; computerized standard-density contingent, $167; manual standard-density contingent, $139; noncontingent control group, $171. Noncontingent control participants yoked to the computerized high-density group received $270 in prizes, whereas those yoked to the standard-density computerized and manual groups received $183 and $122, respectively.
DSM–IV Cocaine and Heroin Dependence at Study Exit and Follow-Up Visits
Table 2 lists the adjusted percentages with corresponding 95% CIs of participants meeting criteria for current DSM–IV cocaine dependence and heroin dependence at study exit and follow-up visits. Each adjusted percentage is a μ value from a random-effects mixed-regression model (SAS GLIMMIX macro), controlling for baseline drug use and for days in treatment. In the contingent-reinforcement groups, there were significantly more patients in remission of DSM–IV diagnoses of cocaine dependence at the end of treatment and during the 3 and 6 months postdischarge follow-up periods compared with those in the noncontingent control group: noncontingent versus computerized high-density group t(56) = 2.03, p < .05; noncontingent versus computerized standard-density group t(56) = 2.54, p < .05; and noncontingent versus manual contingent group t(56) = 2.04, p < .05. There were no significant differences among the contingent-reinforcement groups. For DSM–IV heroin dependence at study exit and follow-up visits, there was no main effect of group, F(3, 56) = 0.38, p = .769, and there were no significant differences among contingent-reinforcement groups.
Table 2.
Adjusted Percentages With 95% Confidence Intervals (CIs) of Participants Meeting DSM–IV Criteria for Current Cocaine or Heroin Dependence at Study Exit and at 3- and 6-Month Follow-Up Visits (fv)
| Group
|
||||||||
|---|---|---|---|---|---|---|---|---|
| NC
|
MS
|
CS
|
CH
|
|||||
| Variable | M | CI | M | CI | M | CI | M | CI |
| % cocaine dependent | ||||||||
| At exit | 61 | 36, 81 | 31 | 9, 69 | 27 | 12, 52 | 28 | 8, 64 |
| 3-month fv | 70 | 44, 88 | 25 | 5, 67 | 22 | 7, 51 | 20 | 5, 57 |
| 6-month fv | 68 | 32, 91 | 8 | 1, 45 | 18 | 4, 51 | 22 | 5, 60 |
|
| ||||||||
| % heroin dependent | ||||||||
| At exit | 28 | 13, 52 | 46 | 15, 80 | 32 | 14, 57 | 20 | 5, 51 |
| 3-month fv | 46 | 22, 72 | 16 | 3, 52 | 36 | 13, 68 | 22 | 5, 59 |
| 6-month fv | 31 | 9, 68 | 11 | 1, 54 | 46 | 15, 81 | 24 | 6, 61 |
Note. NC = noncontingent; MS = manual (contingent) group; CS = computerized standard-density (contingent) group; CH = computerized high-density (contingent) group; DSM–IV = Diagnostic and Statistical Manual of Mental Disorders, 4th ed.
Patients in methadone-maintenance treatment at follow-up did not differ from those not in treatment with respect to DSM–IV cocaine dependence, t(13) = 1.45, p > .05; but were less likely than those not in treatment to meet DSM–IV criteria for heroin dependence, t(13) = 8.28, p < .05.
Discussion
As we hypothesized, higher density of reinforcement led to higher abstinence rates from cocaine. Contingent reinforcement of abstinence was accompanied by decreases in cocaine-associated problems as indicated by lower rates of current diagnoses of cocaine dependence after treatment. These findings are of clinical importance as the study demonstrated the long-lasting benefits of prize-based abstinence reinforcement and should guide its implementation in community settings. It is especially noteworthy that in the group receiving the higher density of prizes, enhanced abstinence was observed even after the contingent prize drawing was discontinued (Figure 2). This result accords well with general principles of operant behavior, which indicate that intermittent spacing of primary reinforcers with sufficiently high density to sustain the target behavior, in this case the choice of abstinence over drug use, may allow for a relatively high persistence of the target behavior once the reinforcers are discontinued (Ferster & Skinner, 1957). Previous CM work has shown similar benefits after the intervention has ended compared with more modest effects during the intervention itself (Preston, Umbricht, Wong, & Epstein, 2001). The possibility that prize-based reinforcement intervention could have long-lasting effects has been suggested by Petry and Martin (2002).
At the standard prize density, the computerized drawing procedure was at least as effective as the manual drawing. This suggests that the computerized systems retain the therapeutic efficacy of the prize-based reinforcement procedure with the added benefits of the capacity for electronic data capture and prize-density manipulations not likely to be perceived by participants. The ACM system used here also provides an accurate, tamper-proof method for implementation of the prize-based drawing procedure in a manner that would not be possible with a manual prize-drawing procedure. The ACM also allowed us to examine prize-density manipulations blinded to both participants and staff members.
Unexpectedly, the standard prize density was not especially effective in our hands, regardless of whether implemented manually or electronically, despite having been shown to be effective in reducing heroin/cocaine use in a similar population (Petry & Martin, 2002). The reason for this is not clear, though several possibilities exist. One difference is that our study used a noncontingent group to control for nonspecific effects of prize delivery not used in the earlier study. It is possible that some participants in the noncontingent control group did not believe that the awarding of draws was really independent of their drug use and so decreased their drug use, effectively decreasing the difference between the intended active and control interventions. A second possibility is that our population was somewhat more treatment resistant, and the higher density of reinforcement was needed to produce a significant effect. Baseline rates of abstinence were lower in our study (23% for opioids and 15% for cocaine) compared with those in the Petry and Martin (2002) study, with abstinence rates at intake in the ranges of 70%–74% for opioids and 53%–61% for cocaine in the study groups. Other research has shown that drug use early in treatment or at intake is a significant predictor of treatment outcome in substance abuse treatment (Higgins, Badger, & Budney, 2000; Moore & Budney, 2002; Preston et al., 1998), with lower rates of abstinence associated with poorer outcome.
The results of the present study are consistent with prior work showing that lower reinforcement magnitude in both prize- and voucher-based CM is associated with lower efficacy in reducing drug use (Dallery, Silverman, Chutuape, Bigelow, & Stitzer, 2001; Petry et al., 2004). Results from the Petry et al. (2004) study demonstrated that reduced prize size, with no change in the probability of winning any prize, is less efficacious in decreasing cocaine use. The computerized high-density contingent group received prizes in 78% of draws worth $286 over the 12-week intervention. In contrast, the computerized standard-density contingent group received prizes in only 51% of draws worth on average only $167 and the manual contingent group in 59% of draws worth only $139. It is interesting to note that, in our methadone-maintenance patients, the impact of the higher-density prize-based reinforcement was in reducing cocaine use, whereas opiate use was unaffected. Petry and Martin (2002) found a similar effect. Thus, the beneficial effects of the abstinence reinforcement schedule targeting opioid and cocaine use in the context of methadone maintenance were produced through reducing cocaine use rather than reducing opiate use. These present results suggest that valuable resources in methadone-maintenance outpatient clinics may be best targeted toward establishing a CM intervention for those clients that are also cocaine dependent.
In operant terms, the prize-based procedure is a second-order schedule of reinforcement: Prize draws are conditioned reinforcers that are available on a continuous reinforcement (fixed-ratio 1) schedule, although the prizes are delivered following only a fraction of the draws. In preclinical studies, second-order schedules of reinforcement have been shown to support robust and persistent operant responding for both natural and drug reinforcers (Alderson, Robbins, & Everitt, 2000; Goldberg, Kelleher, & Goldberg, 1981; Goldberg & Tang, 1977; Katz, 1979, 1980; Mello et al., 1995). In addition, laboratory studies suggest that humans will learn to respond to a variety of reinforcers under second-order schedules (Lamb et al., 1991; Mello, Mendelson, Palmieri, Lex, & Teoh, 1990; Panlilio et al., 2005).
In applying an operant schedule that has reliably been demonstrated to produce robust and prolonged responding with relatively low-cost prizes, the procedure used here may have important clinical benefits, reinforcing abstinence in a cost-effective manner. Our patients in the high-density contingent group received prizes worth on average only $286 during the 12-week intervention, less than the cost of commonly studied voucher-based CM procedures (Downey, Helmus, & Schuster, 2000; Higgins, Badger, & Budney, 2000; Piotrowski et al., 1999; Robles et al., 2000; Silverman et al., 1996). Thus, our procedure may be more amenable to implementation in community-based settings (Peirce et al., 2006; Willenbring, Hagedorn, Postier, & Kenny, 2004).
The clinical significance of our results is underscored by the finding that, in the contingent-reinforcement groups, there were significantly more patients in remission of DSM–IV diagnoses of cocaine dependence at the end of treatment and during the 3 and 6 months postdischarge follow-up periods compared with yoked control participants receiving prize draws irrespective of drug abstinence. This suggests that decreases in problems associated with cocaine use persisted after treatment ended in the contingent groups. Taken together, these data may have important clinical implications because they demonstrate broad treatment benefits from decreasing cocaine use through our drawing procedure that reinforces cocaine abstinence.
The study had some limitations. Baseline drug use appeared unexpectedly greater in one of the experimental groups (though this difference was not statistically significant and was controlled for in our analyses). We did not assess participants’ awareness of the reinforcement-density manipulation, so we cannot categorically state that participants were unaware of being in the higher- or lower-prize-winning groups, though we believe that the blind was maintained, especially as participants did not watch each other draw. Generalizability may be limited by our having excluded participants with alcohol dependence and major psychiatric disorders. These disorders are likely to be prevalent in a nonresearch treatment population. Another difference from community practice that might limit generalizability is the fact that we did not individualize methadone dose, except to adjust downward for side effects. However, the 100 mg/day dose is generally considered adequate for most patients, and it is higher than that used in other trials of CM in methadone-maintained populations. For example, the approximate mean methadone doses were 70 mg/day in Petry and Martin (2002), 86 mg/day in Peirce et al. (2006), 50–70 mg/day in Epstein, Hawkins, Covi, Umbricht, and Preston (2003), and 65–85 mg/day in Schottenfeld et al. (2005). These studies showed significant decreases in cocaine and/or opiate use at the doses tested. Lastly, there was a relatively high attrition rate before randomization. However, it should be noted that the attrition rate in the present study is comparable with that of previous studies in methadone-maintained patients receiving CM (Preston, Umbricht, & Epstein, 2000; Preston et al., 2001; Silverman et al., 1998).
In summary, our findings suggest that a computer-automated prize-based reinforcement procedure with sufficiently high density of reinforcement may effectively produce persistent reductions in the use of cocaine, as well as long-lasting decreases in cocaine-use-associated problems, in methadone-maintenance patients who continue to use while in treatment. The results of this study are likely to generalize to other cocaine or opiate treatment trials in different settings. Participants were recruited through advertisements in a variety of local newspapers and television stations selected to ensure exposure to both sexes and all ethnicities to maximize generalizability (external validity). Because we did not tell participants about the enhancement of reinforcement density, this effect may reflect a relatively pure application of operant principles, not requiring patients’ full awareness of the probability of winning a prize; follow-up studies should directly assess whether participants’ responses are related to awareness of the reinforcement density.
Acknowledgments
This work was supported by the IRP of the NIH, NIDA.
References
- Alderson HL, Robbins TW, Everitt BJ. Heroin self-administration under a second-order schedule of reinforcement: Acquisition and maintenance of heroin-seeking behaviour in rats. Psychopharmacology. 2000;153:120–133. doi: 10.1007/s002130000429. [DOI] [PubMed] [Google Scholar]
- Amass L, Kamien J. A tale of two cities: Financing two voucher programs for substance abusers through community donations. Experimental and Clinical Psychopharmacology. 2004;12:147–155. doi: 10.1037/1064-1297.12.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Chutuape MA, Silverman K, Stitzer ML. Survey assessment of methadone treatment services as reinforcers. American Journal of Drug and Alcohol Abuse. 1998;24:1–16. doi: 10.3109/00952999809001695. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Dallery J, Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of opiate plus cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcer magnitude. Experimental and Clinical Psychopharmacology. 2001;9:317–325. doi: 10.1037//1064-1297.9.3.317. [DOI] [PubMed] [Google Scholar]
- Downey KK, Helmus TC, Schuster CR. Treatment of heroin-dependent poly-drug abusers with contingency management and buprenorphine maintenance. Experimental and Clinical Psychopharmacology. 2000;8:176–184. doi: 10.1037//1064-1297.8.2.176. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Hawkins WE, Covi L, Umbricht A, Preston KL. Cognitive-behavioral therapy plus contingency management for cocaine use: Findings during treatment and across 12-month follow-up. Psychology of Addictive Behaviors. 2003;17:73–82. doi: 10.1037/0893-164X.17.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster CB, Skinner BF. Schedules of reinforcement. New York: Appleton-Century-Crofts; 1957. [Google Scholar]
- Goldberg SR, Kelleher RT, Goldberg DM. Fixed-ratio responding under second-order schedules of food presentation or cocaine injection. Journal of Pharmacology and Experimental Therapeutics. 1981;218:271–281. [PubMed] [Google Scholar]
- Goldberg SR, Tang AH. Behavior maintained under second-order schedules of intravenous morphine injection in squirrel and rhesus monkeys. Psychopharmacology (Berlin) 1977;51:235–242. doi: 10.1007/BF00431630. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychological Methods. 1997;2:64–78. [Google Scholar]
- Higgins ST, Badger GJ, Budney AJ. Initial abstinence and success in achieving longer term cocaine abstinence. Experimental and Clinical Psychopharmacology. 2000;8:377–386. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, et al. A behavioral approach to achieving initial cocaine abstinence. American Journal of Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Silverman K, editors. Motivating behavior change among illicit-drug abusers: Research on contingency management interventions. Washington, DC: American Psychological Association; 1999. [Google Scholar]
- Hogg RV, Craig AT. Introduction to mathematical statistics. 5. New York: Macmillan; 1995. [Google Scholar]
- Katz JL. A comparison of responding maintained under second-order schedules of intramuscular cocaine injection or food presentation in squirrel monkeys. Journal of the Experimental Analysis of Behavior. 1979;32:419–431. doi: 10.1901/jeab.1979.32-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL. Second-order schedules of intramuscular cocaine injection in the squirrel monkey: Comparisons with food presentation and effects of d-amphetamine and promazine. Journal of Pharmacology and Experimental Therapeutics. 1980;212:405–411. [PubMed] [Google Scholar]
- Kirby KC, Benishek LA, Dugosh KL, Kerwin ME. Substance abuse treatment providers’ beliefs and objections regarding contingency management: Implications for dissemination. Drug and Alcohol Dependence. 2006;85:19–27. doi: 10.1016/j.drugalcdep.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Preston KL, Schindler CW, Meisch RA, Davis F, Katz JL, et al. The reinforcing and subjective effects of morphine in post-addicts: A dose–response study. Journal of Pharmacology and Experimental Therapeutics. 1991;259:1165–1173. [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, et al. New data from the Addiction Severity Index: Reliability and validity in three centers. Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Palmieri S, Lex BW, Teoh SK. Operant acquisition of alcohol by women. Journal of Pharmacology and Experimental Therapeutics. 1990;253:237–245. [PubMed] [Google Scholar]
- Mello NK, Negus SS, Lukas SE, Mendelson JH, Sholar JW, Drieze JJ. A primate model of polydrug abuse: Cocaine and heroin combinations. Journal of Pharmacology and Experimental Therapeutics. 1995;274:1325–1337. [PubMed] [Google Scholar]
- Miele GM, Carpenter KM, Smith Cockerham M, Trautman KD, Blaine J, Hasin DS. Substance Dependence Severity Scale (SDSS): Reliability and validity of a clinician-administered interview for DSM–IV substance use disorders. Drug and Alcohol Dependence. 2000;59:63–75. doi: 10.1016/s0376-8716(99)00111-8. [DOI] [PubMed] [Google Scholar]
- Moore BA, Budney AJ. Abstinence at intake for marijuana dependence treatment predicts response. Drug and Alcohol Dependence. 2002;67:249–257. doi: 10.1016/s0376-8716(02)00079-0. [DOI] [PubMed] [Google Scholar]
- Nich C, Carroll K. Now you see it, now you don’t: A comparison of traditional versus random-effects regression models in the analysis of longitudinal follow-up data from a clinical trial. Journal of Consulting and Clinical Psychology. 1997;65:252–261. doi: 10.1037//0022-006x.65.2.252. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Yasar S, Nemeth-Coslett R, Katz JL, Henningfield JE, Solinas M, et al. Human cocaine-seeking behavior and its control by drug-associated stimuli in the laboratory. Neuropsychopharmacology. 2005;30:433–443. doi: 10.1038/sj.npp.1300599. [DOI] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satter-field F, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: A National Drug Abuse Treatment Clinical Trials Network study. Archives of General Psychiatry. 2006;63:201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. Journal of Consulting and Clinical Psychology. 2002;70:398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Simcic F., Jr Prize reinforcement contingency management for cocaine dependence: Integration with group therapy in a methadone clinic. Journal of Consulting and Clinical Psychology. 2005;73:354–359. doi: 10.1037/0022-006X.73.2.354. [DOI] [PubMed] [Google Scholar]
- Petry NM, Telford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treating cocaine users: How low can we go, and with whom? Addiction. 2004;99:349–360. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski NA, Tusel DJ, Sees KL, Reilly PM, Banys P, Meek P, et al. Contingency contracting with monetary reinforcers for abstinence from multiple drugs in a methadone program. Experimental and Clinical Psychopharmacology. 1999;7:399–411. doi: 10.1037//1064-1297.7.4.399. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Higgins ST, Brooner RK, Montoya I, Schuster CR, et al. Cocaine use early in treatment predicts outcome in a behavioral treatment program. Journal of Consulting and Clinical Psychology. 1998;66:691–696. doi: 10.1037//0022-006x.66.4.691. [DOI] [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Epstein DH. Methadone dose increase and abstinence reinforcement for treatment of continued heroin use during methadone maintenance. Archives of General Psychiatry. 2000;57:395–404. doi: 10.1001/archpsyc.57.4.395. [DOI] [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Wong CJ, Epstein DH. Shaping cocaine abstinence by successive approximation. Journal of Consulting and Clinical Psychology. 2001;69:643–654. doi: 10.1037//0022-006x.69.4.643. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM., III . The Diagnostic Interview Schedule (Version IV) St Louis, MO: Washington University; 1995. [Google Scholar]
- Robles E, Silverman K, Preston KL, Cone EJ, Katz E, Bigelow GE, et al. The brief abstinence test: Voucher-based reinforcement of cocaine abstinence. Drug and Alcohol Dependence. 2000;58:205–212. doi: 10.1016/s0376-8716(99)00090-3. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Chawarski MC, Pakes JR, Pantalon MV, Carroll KM, Kosten TR. Methadone versus buprenorphine with contingency management or performance feedback for cocaine and opioid dependence. American Journal of Psychiatry. 2005;162:340–349. doi: 10.1176/appi.ajp.162.2.340. [DOI] [PubMed] [Google Scholar]
- Schroeder JR, Gupman AE, Epstein DH, Umbricht A, Preston KL. Do noncontingent vouchers increase drug use? Experimental and Clinical Psychopharmacology. 2003;11:195–201. doi: 10.1037/1064-1297.11.3.195. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Higgins ST, Brooner RK, Montoya ID, Contoreggi C, et al. Increasing opiate abstinence through voucher-based reinforcement therapy. Drug and Alcohol Dependence. 1996;41:157–165. doi: 10.1016/0376-8716(96)01246-x. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Umbricht-Schneiter A, Montoya ID, Schuster CR, Preston KL. Broad beneficial effects of cocaine abstinence reinforcement among methadone patients. Journal of Consulting and Clinical Psychology. 1998;66:811–824. doi: 10.1037//0022-006x.66.5.811. [DOI] [PubMed] [Google Scholar]
- Vahabzadeh M, Lin J-L, Epstein DH, Mezghanni M, Schmittner J, Preston KL. Computerized contingency management for motivating behavior change: Automated tracking and dynamic reward reinforcement management. Proceedings of the 20th IEEE International Symposium on Computer-Based Medical Systems (CBMS 2007); 2007. pp. 85–90. [Google Scholar]
- Willenbring ML, Hagedorn HJ, Postier AC, Kenny M. Variations in evidence-based clinical practices in nine United States Veterans Administration opioid agonist therapy clinics. Drug and Alcohol Dependence. 2004;75:97–106. doi: 10.1016/j.drugalcdep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Zachary R. Shipley Institute of Living Scale, revised manual. Los Angeles: Western Psychological Services; 1986. [Google Scholar]


