Abstract
Neutrophils provide the first line of defense of the innate immune system by phagocytosing, killing, and digesting bacteria and fungi. Killing was previously believed to be accomplished by oxygen free radicals and other reactive oxygen species generated by the NADPH oxidase, and by oxidized halides produced by myeloperoxidase. We now know this is incorrect. The oxidase pumps electrons into the phagocytic vacuole, thereby inducing a charge across the membrane that must be compensated. The movement of compensating ions produces conditions in the vacuole conducive to microbial killing and digestion by enzymes released into the vacuole from the cytoplasmic granules.
Keywords: bacteria, protease, free radical, microbicidal, ion channel, enzyme
INTRODUCTION
Neutrophils are highly motile phagocytic cells that constitute the first line of defense of the innate immune system. They were first discovered by Elie Metchnikoff when he inserted rose thorns into starfish larvae and found that wandering mesodermal cells accumulated at the puncture site. He showed these cells to be phagocytic and described the larger cells as macrophagocytes, or macrophages, and the smaller as microphagocytes, now known as granulocytes, of which by far the most numerous are the neutrophils.
The ability of these cells to engulf and degrade bacteria was logically assumed to indicate a killing function. A microbicidal function was ascribed to the contents of their abundant cytoplasmic granules that were discharged into the phagocytic vacuole containing the microbe (1) (Figure 1). Attention was then directed toward the characterization of the granules by electron microscopy, fractionation, and biochemical analysis. Several of the purified granule proteins were shown to kill microbes.
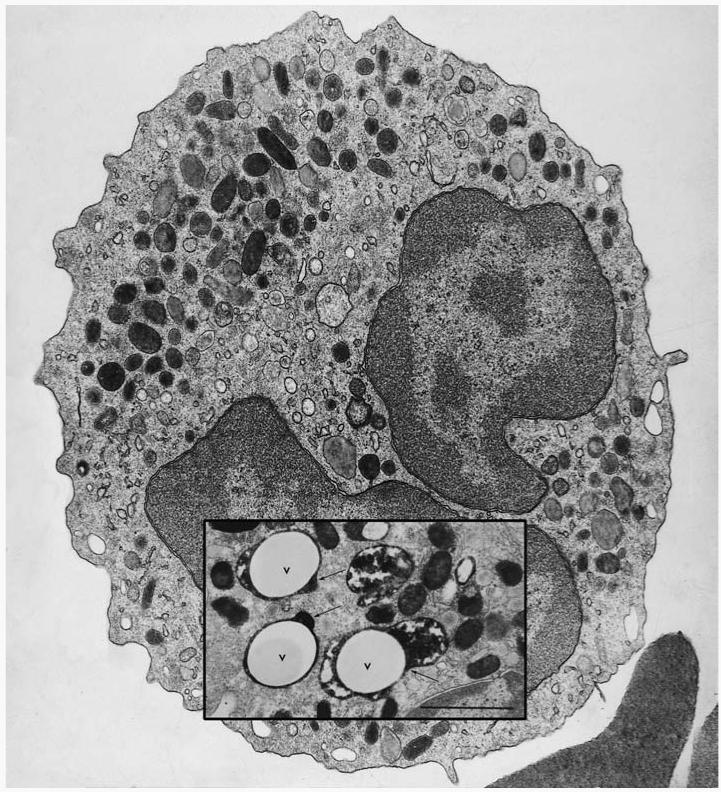
Figure 1.
Transmission electron micrograph of a human neutrophil. Inset is an image taken from a neutrophil 20 s after the phagocytosis of latex particles opsonized with IgG (V, vacuole). The section was stained for myeloperoxidase (MPO) to reveal the electron-dense product in the azurophil granules, some of which can be seen degranulating into the phagocytic vacuole (arrows). Bar = 1 μm. (Figure from 17.)
Parallel with studies into microbicidal activity of the granule contents, investigations were undertaken into the metabolism of phagocytosing neutrophils. The neutrophils demonstrated a significant “extra respiration of phagocytosis,” which was non-mitochondrial and was associated with a dramatic increase in turnover of the hexose monophosphate (HMP) shunt and the production of large amounts of H2O2 (2). These metabolic changes were shown to be essential for microbial killing.
In the late 1960s and early 1970s, a number of related discoveries cast a very different perspective on the killing process. Chronic granulomatous disease (CGD), a profound immunodeficiency to bacterial and fungal infections, was associated with failure of these metabolic changes (3). In addition, myeloperoxidase (MPO)-mediated halogenation, which is microbicidal in the test tube, was also defective in these patients (4).
Soon after its discovery in 1969, superoxide dismutase was used to show that activated neutrophils generate superoxide (5) and that this process is lacking in CGD. This important development provided a direct link between free radical chemistry and biology. At the time, most free radical chemistry was conducted by radiation biologists in test tubes, and its application to biology was purely theoretical. This new discovery was thought to prove that the production of free radical reactions in a biological process was toxic enough to kill organic structures as tough as bacteria and fungal spores. Soon these observations were extrapolated to implicate free radical reactions in a host of pathological processes involving neutrophil infiltration and tissue damage.
During the past few years, the pendulum has swung firmly back to implicating a major primary role for the granule proteins in the killing process (6), with a less direct but still facilitating and activating role for the respiratory burst through the NADPH oxidase. This review concentrates on the elucidation of these recent developments in our understanding of the relationship between the oxidase and granule enzyme activation. Because of the breadth of the subject and space limitations, references are made to authoritative reviews where available.
LIMITATIONS TO UNDERSTANDING KILLING SYSTEMS
Neutrophils are essential for resistance to bacterial and fungal infections. Severe neutropaenia invariably leads to infection by a wide range of organisms (7), most of which are not normally pathogenic, even in CGD. This, coupled with the fact that most CGD patients are able to kill most invading microbes most of the time (8), indicates that killing systems of the neutrophil are highly efficient and multilayered. Investigators once considered oxygen-dependent mechanisms essential for killing invading microbes, but such microbes can in fact be killed by other systems (9). In general, research has concentrated on determining those mechanisms involved in killing the most resistant organisms. The advent of gene-targeting technology allows researchers to determine the roles of the different antimicrobial molecules and their functional interrelationships with various microbes. Additionally, most studies have examined the killing of microbes within the phagocytic vacuole. We do not know whether neutrophils are capable of killing organisms extracellularly in vivo, nor the mechanisms involved if they are.
We have derived the bulk of our detailed information from the study of infection in CGD and the role of the oxidase in microbial killing. Because CGD patients can remain free of infection for many years (8), these methods are imprecise because they only measure some components of the lethal systems. Nonetheless, oxygen-dependent, intravacuolar killing provides a clearly defined set of processes, the examination of which has advanced knowledge of important physiological mechanisms.
THE NADPH OXIDASE
The NADPH oxidase plays a pivotal role in microbial killing because its dys-function causes CGD, characterized by a profound predisposition to bacterial and fungal infection (8, 10), and killing is compromised under anaerobic conditions (11).
Detailed reviews of the biochemistry and bioenergetics of this system have recently been undertaken (12, 13), to which I refer readers. A schematic representation of the oxidase is shown in Figure 2.
Figure 2.

Schematic representation of the NADPH oxidase. Flavocytochrome b558 is a heterodimer of gp91phox, which contains the haem- and flavin-binding sites, and p22phox. Electron transport is activated by phosphorylation and translocation to the vacuolar membrane of p47phox and p67phox. p21rac, in the GTP-bound form, is also required (12).
The Electron Transport Chain Through the Membrane
Flavocytochrome b558 is the core component of the NADPH oxidase. It is distributed between the plasma membrane and the membrane of the specific granules, and it is incorporated into the wall of the phagocytic vacuole, where it forms a conduit for electrons to be pumped from NADPH in the cytosol onto oxygen in the vacuole.
Flavocytochrome b558 is a heterodimer composed of one molecule of p22phox (α-subunit, the product of the CYBA gene) and one molecule of gp91phox (β-subunit, CYBB gene).
gp91phox
gp91phox contains the entire electron transporting machinery of the flavocytochrome b. It is composed of two major, and very different, domains.
C-Terminus: NADPH and FAD Binding
The hydrophilic C-terminal (282–570) portion of gp91phox contains the FAD- and NADPH-binding sites. These have distant, but recognizable homology to the large family of ferredoxin-NADP reductase (FNR) proteins, of which cytochrome P450 reductase, nitric oxide (NO) synthase, and yeast ferric reductase are members. This homology has allowed the construction of a model with the depiction of the FAD- and NADPH-binding sites.
N-Terminus: Haem Coordination
The hydrophobic N-terminal half of gp91phoxcontains six membrane-spanning α helices. Helices III and V each contain two histidine residues appropriately positioned (101:209 and 115:222) to coordinate two haem prosthetic groups perpendicular to the plane of the membrane. These histidine residues are completely conserved among all the NADPH OXIDASE (NOX) family members. Site-directed mutagenesis studies support the proposal that these histidine residues form the axial ligands to the haem groups. The predicted placing of the haem groups (one toward the inner face and one toward the outer face) is consistent with their function to transport electrons from the NADPH (via FAD) on the inside (cytosol) across the membrane to the interior of the phagocytic vacuole where molecular O2 is reduced to form . Biological membranes are ∼25 Å thick, and thus at least two redox centers are required to span them to allow electrons to transfer at kinetically significant rates. The haem groups are nonequivalent and have different redox potentials.
The second (120–167) and third (224–257) external loops of gp91phox contain the N-linked glycosylation sites (asparagines 132, 149, and 240).
p22phox
p22phox is a 194 amino acid (∼21 kDa) protein with a hydrophobic, membrane-spanning N-terminus (1-132). It provides high-affinity binding sites for the cytosolic NADPH oxidase subunits. p47phox binds to a proline-rich domain (151–160) in the cytoplasmic hydrophilic C-terminus and confers stability on gp91phox.
The Activating Proteins in the Cytosol
For electron transport to occur through the flavocytochrome, it must interact with a number of cytosolic proteins that translocate to the membrane of the phagocytic vacuole. This activation depends on a change in the conformation of the flavocytochrome, possibly by displacing the small helix that is predicted in the molecular model to occupy the NADPH-binding site in the inactive state (14) or through the facilitation of electron transfer between the flavin and haem.
Because of their interaction with each other, with lipids, and with phox proteins in the membranes, these cytosolic phox proteins have relatively large numbers of specific interaction domains. Targeting these molecules specifically to that region of the plasma membrane that makes up the wall of the vacuole requires specific local changes, which might include the accumulation of phosphatidylinositol phosphates (PIPs) at this site. Only a small proportion of these cytosolic proteins translocate to the membranes, and these appear to be phosphorylated, as does the flavocytochrome.
p67phox
p67phox (NOXA2 from NOX Activator) is a 59,735-Da protein (526 amino acids) with a pI of 6.12. Protein-protein interaction domains include two SH3 domains, two proline-rich regions flanking the central SH3 domain, an N-terminal TPR (tetratricopeptide repeat), and a PB1 domain C-terminal to the central SH3 domain. The TPR domains are thought to bind rac. PB1 domains are known to interact with octicosapeptide motifs, and p67phox binds to p40phox through this domain. p67phox attaches directly to flavocytochrome b558, and at high concentration, in combination with rac or in the form of a p67phox/rac chimera, p67phox is sufficient to induce electron transport.
p47phox
p47phox (NOXO2 from NOX Organizer) is a basic protein (pI = 9.6) of molecular weight 44,681 Da (390 amino acids) that is heavily phosphorylated during neutrophil activation. It contains a number of well-defined motifs, including a PX domain (involved in phosphoinositide binding), two SH3 domains (involved in protein-protein interactions), and at least one proline-rich motif (the reciprocal target for SH3 domain interactions). It appears to be an adaptor molecule forming a bridge between p22phox and p67phox, and it also binds to cytoplasmic regions of gp91phox, thereby stabilizing the attachment of p67phox to flavocytochrome b558.It might also directly influence the function of flavocytochrome b558. The N-terminal regions of p40phox and p47phox contain homologous stretches of 120–130 amino acids that form a structure called the phox homology, or PX domain, which binds to PIPs and directs these proteins to this activated membrane (reviewed in 15).
The two SH3 domains face each other to form a groove in which its C-terminal polybasic region fits. Investigators have suggested that this polybasic region is phosphorylated upon activation, releasing it from its auto-inhibitory role and making the groove accessible to bind the proline-rich tail in the C-terminal portion of p22phox.
p40phox
p40phox was discovered when it copurified with p67phox, to which it is tightly bound. It is a protein of 39,039 Da (339 amino acids), strongly homologous with p47phox, with an N-terminal PX domain, followed by an SH3 domain. Toward the C-terminus, there is an octicosapeptide repeat (also known as a PC domain) that seems to be involved in the binding of p40phox to p67phox. The protein probably functions as a shuttle partner, transporting p67phox, which does not contain a PX domain, to the membrane of the phagocytic vacuole by binding to PIPs.
p21rac
After the discovery of p47phox and p67phox, it became clear that they were not sufficient to reconstitute the active oxidase when combined with membranes. A third protein, a guanosine 5′-triphosphatase (GTP)-dependent factor, was shown to be rac1 or rac2 and was purified from cytosol. The causes of the separation of rac from its complex with guanine nucleotide dissociation inhibitors (GDI) in the cytosol are not known. Rac translocates to the membrane independently from p67phox and p47phox. Its guanosine diphosphate (GDP) is probably exchanged for GTP on the membrane through the action of P-Rex1, a 185-kDa guanine nucleotide exchange factor (GEF) that is activated by phosphatidylinositol-3,4,5-trisphosphate and by the βγ subunits of heterotrimeric G proteins.
Molecular Genetics of CGD
Defects in any one of four genes give rise to the known forms of CGD. CYBB (coding for gp91phox, NOX2) is located on the X chromosome and accounts for about 65% of cases, almost exclusively in males (except in rare female carriers in whom there is extreme lyonization). The other three genes are all autosomal, with defects in NCF1 (p47phox or NOXO2 protein), NCF2 (p67phox or NOXA2), and CYBA (p22phox), causing approximately 25%, 5%, and 5% of cases, respectively. No instances of CGD have been identified in which a lesion of p40phox is causal.
A small subgroup of CGD patients have what is known as “variant” CGD (16). In these cases there is partial loss of a protein or its function. Often as much as 10%, and up to 30% (H. Malech, personal communication), of normal oxidase activity can be measured.
PRODUCTS OF THE OXIDASE AND THEIR IMPLICATION IN MICROBIAL KILLING
Initiation of NADPH oxidase activity coincides with degranulation, with a lag phase of approximately 20 s (17). It occurs after closure of the vacuole and is limited to the plasma membrane comprising the vacuolar membrane (18). Thus, superoxide cannot be detected on the exterior of a phagocytosing cell (19, 20) unless engulfment is “frustrated” by an overwhelming excess of particles and vacuolar closure becomes impossible.
Because activity of the NADPH oxidase is essential for efficient microbial killing, investigators have focused attention on the products of the oxidase themselves as the lethal agents.
Oxygen radicals and their reaction products, collectively referred to as reactive oxygen species (ROS), are produced as a consequence of NADPH oxidase activity, which pumps superoxide () into the phagocytic vacuole. Because ROS can react with organic molecules, an enormous body of literature has developed that causally links ROS to the death of the microbe.
and H2O2
The superoxide anion radical has been recognized in chemical systems for many years. Proof of its existence in biology followed the discovery of the enzymatic function of superoxide dismutase, which accelerates the dismutation of (21). Investigators (5) soon showed that neutrophils produce large amounts of , estimated between approximately 1 (22) and 4 (6) M/l in the vacuole. The steady state concentration has been estimated to be in the μM range (22) because dismutation to H2O2 (2) is very rapid (23, pp. 60–61) under the prevailing conditions.
Experiments were performed that appeared to demonstrate the killing of microbes by generated by xanthine oxidase (24, 25). It is not clear what, if any, ROS other than and H2O2 (2) are produced in significant quantities in the vacuole.
HO•
and H2O2 can combine to generate the highly reactive hydroxyl radical (HO•) via the Haber-Weiss reaction. This requires a metal such as iron in the Fenton reaction: Fe2+ + H2O2 → Fe3+ + HO− + HO•. HO• has been measured in a broken cell preparation (26) and has been implicated as a microbicidal agent (27). These radicals are probably not found in intact cells (28) because lactoferrin, which is unsaturated in neutrophil granules (29, 30), inhibits the generation of HO• (31) and other free radical reactions (29) by binding free copper and iron. The reaction between HOCl and could produce HO• but does not appear to do so (32).
Cobalt-based radicals could be produced by the Co in cyanocobalamin (33), but a binding protein, transcobalamin 2, present in specific granules, might be there to prevent this from occurring.
Ozone
It has recently been suggested that ozone generated by an antibody-based catalysis is involved in the killing of bacteria within neutrophils (34, 35). Doubt has been subsequently raised, however, on the specificity of the indicator used for ozone, which can apparently also detect (36).
Myeloperoxidase-Mediated Halogenation
Myeloperoxidase (MPO) is a di-haem protein composed of two identical heterodimers. Each heterodimer is formed from the post-translational modification of a single polypeptide precursor. The two symmetric halves are linked by disulphide bonds between the two heavy chains. The covalently bound haem has a unique structure and exhibits unusual spectral properties that are responsible for its green color (37). MPO constitutes about 5% of the total neutrophil protein and is present in the cytoplasmic granules at very high concentrations. It makes up about 25% of the granule protein, and this achieves concentrations of about 100 mg/ml (1 mM) in the vacuole.
Investigators thought that this enzyme catalyzes the H2O2-dependent oxidation of halides that can react with and kill microbes. Experiments with the MPO-H2O2-halide system demonstrated that this enzyme can kill bacteria in the test tube (22, 38-41), and MPO-mediated halogenation has been accepted as an important antimicrobial mechanism for several decades.
A few patients were discovered whose neutrophils lacked MPO and who were also thought to be immunodeficient (42). Recently MPO knockout mice have also shown an undue susceptibility to bacterial and fungal infections (43-45).
Nitric Oxide
Although evidence suggests that neutrophils can induce the synthesis of nitric oxide (NO) synthase during sepsis (46), little evidence implicates the involvement of NO in microbial killing. Even in mice, in the neutrophils of which NO synthase is expressed at much higher levels than in humans, knocking out this molecule has little effect on the killing of microbes for which neutrophils are normally responsible. In contrast, these mice are profoundly susceptible to intracellular organisms such as S. enterica and M. tuberculosis (47), which classically proliferate within macrophages.
CYTOPLASMIC GRANULES AND THEIR CONTENTS
Researchers have known for almost a century that neutrophils phagocytose and kill microbes. Alexander Fleming discovered and named lysozyme, which he termed “a remarkable bacteriolytic element found in tissues and secretions,” including leukocytes (48). He showed that it lysed about two thirds of the bacteria he mixed with it. Researchers subsequently showed that phagocytosis was associated with discharge of the cytoplasmic granules into the vacuole (1) (Figure 1). Attention then focused on microbicidal components within these granules. The first microbicidal granule extract was called phagocytin (49), which was later shown to be composed of an array of cationic antibacterial proteins (50).
Substantial reviews have recently covered this subject (51, 52). Different subsets of granules have been characterized by electron microscopy (53), by various staining techniques, by cell fractionation (54), and by their different functions. There are two predominant types of granules, the azurophil and the specific. They are produced in the promyelocytic and myelocytic stages, and their contents depend on the proteins that are being synthesized at that time as well as on the presence of appropriate signaling peptides (51, 52). The granules also differ in their primary functions, as discussed below.
Azurophil (or Primary) Granules
The azurophils largely contain proteins and peptides directed toward microbial killing and digestion, whereas the specific granules replenish membrane components and help to limit free radical reactions. Azurophil (or primary) granules are the first to be produced. They contain MPO and three predominant neutral proteinases: cathepsin G, elastase, and proteinase 3. Bactericidal/permeability-increasing protein (BPI) was first purified as a factor that permeabilized and killed E. coli (55, 56). It has lipopolysaccharide-binding and neutralizing activities (57) and appears to be attached to the granule membrane. Defensins are peptides with molecular weights of 3000–4000 Da, and each contains six disulphide-linked cysteines (58). They exhibit antibacterial activity, but this is inhibited by physiological concentrations of salt. About one third of the total lysozyme (54) is found in these granules.
These granules contain an abundant matrix composed of strongly negatively charged sulphated proteoglycans (59). This matrix strongly binds almost all the peptides and proteins other than lysozyme, which are strongly cationic. This sequestration together with the acidic pH at which the granule interior is maintained (60) keeps these enzymes in a quiescent, inactivated state.
Specific (or Secondary) Granules
Specific granules contain unsaturated (61) lactoferrin, which binds and sequesters iron and copper; transcobalamin II, which binds cyanocobalamin; about two thirds of the lysozyme (54); neutrophil gelatinase-associated lipocalin (62); and a number of membrane proteins also present in the plasma membrane, including flavocytochrome b558 of the NADPH oxidase (63).
Gelatinase (or Tertiary) Granules
Some granules contain gelatinase in the absence of lactoferrin, although most of the lactoferrin-containing specific granules also contain gelatinase (64). The designation of granules as “gelatinase granule” refers to granules that contain gelatinase but not lactoferrin; they may represent one end of the spectrum of a single type of granule with the same contents but in differing proportions.
Lysosomes
Lysosomes contain acid hydrolases. The activity of these enzymes appears to fractionate with the azurophil granules. They are, however, released into the phagocytic vacuole much later than the azurophil contents and therefore must be in a distinct compartment (17).
Secretory Vesicles
These endocytic vesicles contain serum albumin (65) and are probably the empty vesicular structures described previously (66). They provide a valuable reservoir of membrane components. Their reassociation with the plasma membrane replenishes that which is consumed during phagocytosis, as well as its component proteins such as complement receptor (67) and flavocytochrome b558.
CONDITIONS IN THE PHAGOCYTIC VACUOLE
One must clearly understand the conditions in the phagocytic vacuole when attempting to define killing mechanisms. A heavily opsonized particle is taken up into the phagocytic vacuole within 20 s (17, 68), and killing is almost immediate (68). The apparent delay in many assays results from a low collision frequency between neutrophils and microbes, which is due to low densities of both, coupled with slow mixing (69) and suboptimal opsonization.
To determine the concentration of the vacuolar contents, one must know the volume of the space between the surface of the organism and the membrane of the phagocytic vacuole. It is certainly very small (17) (Figure 1), and possibly negligible, as has been shown in macrophages (70).
The human neutrophil has numerous granules, the contents of which are released into the vacuole and squeezed onto the surface of the organism in very high concentrations, almost like attaching a limpet mine to a target (17). Researchers have estimated that the granule protein makes up about 40% of the vacuolar volume (22), achieving protein concentrations of about 500 mg/ml (6). It was initially thought that the specific granules degranulated first, followed by the azurophils. These studies were conducted on rabbit neutrophils, and alkaline phosphatase, which we now know to be a marker for membranes, was used as the marker for the specific granules (71). In fact, both of these granule types fuse with the phagocytic vacuole with roughly similar kinetics approximately 20 s after particle uptake (17). The acid hydrolases only enter the vacuole after about 5 min, when the pH has started to fall to levels appropriate for the optimal activity of these enzymes.
Investigators had initially reported that the pH in the vacuole fell to about 6 after 3 min and to 4 after 6 min (72). However, subsequent studies have shown that the NADPH oxidase elevates the pH to about 7.8–8.0 in the first 3 min after phagocytosis, after which it gradually falls to about 7.0 after 10–15 min (68, 73, 74). The NADPH oxidase consumes 0.2 fmols of O2 when a particle the size of a bacterium is engulfed. This equates to massive amounts of , on the order of 1–4 Mols/l, that are injected into the vacuole.
NEUTRAL PROTEASES ARE ESSENTIAL FOR BACTERIAL AND FUNGAL KILLING
Although the proposal that ROS are toxic to ingested microbes was attractive, it was never adequately tested under the conditions pertaining to the phagocytic vacuole. The opportunity was provided by the development of gene targeting. This technique allowed the production of a mouse model that lacks the major neutrophil proteases: neutrophil elastase (NE) (6, 75), cathepsin G (6), or both enzymes (6, 76, 77) (Figure 3).
Figure 3.
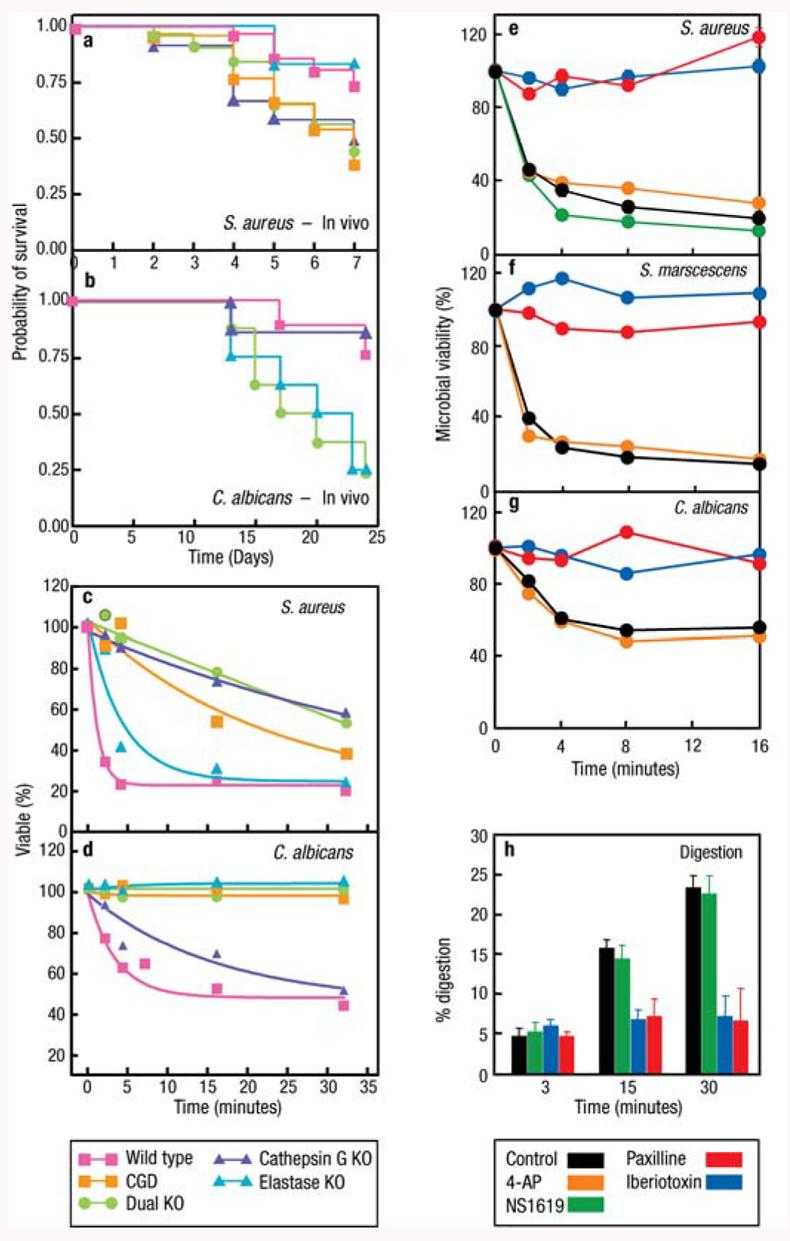
The neutral proteases elastase and cathepsin G as well as K+ flux are required for microbial killing and digestion by neutrophils. Cathepsin G, neutrophil elastase (NE), and p47phox (CGD) knockout mice are susceptible to S. aureus (a) and C. albicans (b) in vivo, and their neutrophils kill these organisms poorly in the test tube (c) and (d) (adapted from 6). Inhibition of the BKCa K+ channel with specific inhibitors paxilline (PAX) and iberiotoxin (IBTX) prevents killing of S. aureus (e), S. marscescens (f ), and C. albicans (g) by neutrophils, whereas the opener NS1619 and nonspecific inhibitor 4-aminopyridine were without effect. The BKCa K+ channel blockers also inhibited digestion of radiolabeled, killed S. aureus (h) (adapted from 74). Neither the loss of the proteases nor blockage of the BKCa channel affected phagocytosis, oxidase activity, or iodination.
NE-deficient mice were excessively susceptible to infection with Gram-negative (K. pneumoniae and E. coli) (75) but not Gram-positive (S. aureus) bacteria. NE was also necessary for protection against C. albicans (6). Both enzymes were required to kill A. fumigatus. The loss of cathepsin G alone was found by others (77) to be without effect on the killing of various of bacteria. The loss of both NE and cathepsin G conferred as profound a defect of bacterial killing as was observed with the CGD mouse model (6).
In these studies on protease-deficient mice, microbial killing was abolished despite a completely normal respiratory burst and normal levels of iodination. This established that ROS and metabolites of the action of MPO generated in the vacuole are not sufficient to kill these bacteria and fungi.
Thus, it was clear that the combination of NADPH oxidase activity and neutral protease enzymes are require for microbial killing to take place. This raises the question of the connection between these two processes.
THE RELATIONSHIP BETWEEN THE NADPH OXIDASE AND KILLING BY GRANULE CONTENTS
Activity of the NADPH Oxidase Alters the Appearance of the Contents of the Phagocytic Vacuole
The activity of the NADPH oxidase alters the appearance of the contents of phagocytic vacuoles in electron micrographs of neutrophils examined soon after they had phagocytosed bacteria (6). In normal cells, the contents of the vacuole had a diffuse, almost ground-glass appearance, with very few intact aggregates of granule contents. By contrast, in CGD cells there was little dispersion, with obvious clumping of the granular contents. This abnormal appearance was also apparent in vacuoles from a patient with variant CGD with 10% of the normal oxidase activity.
These obvious structural differences, coupled with the massive amounts of injected into the vacuole and the fact that 10% of this amount of in variant CGD (amounting to some 100–400 mMols/l) was insufficient, suggested to researchers that the oxidase was exerting some physico-chemical influence on the granule contents rather than simply producing ROS or substrate for MPO. Segal and colleagues (6) therefore turned their attention to electron transport across the membrane and its consequences for the movement of other ions.
Charge Compensation Across the Vacuolar Wall
The oxidase is electrogenic, transferring electrons, unaccompanied by protons, across the vacuolar membrane (78-81). The vacuolar volume is about 0.2 μm3, with a membrane surface area of about 1.65 μm2.In each vacuole, 0.8–2.0 fmols of are produced, and thus about 5–10 × 108 electrons pass across each μ2 of membrane. The charge on one electron is 1.6 × 10−19 coulombs, so 3–7 × 108 charges in one square micron would produce from 4.6 × 10−3 to 1.2 × 10−2 coulombs/cm2. With the capacitance of the membrane at approximately 1 microfarad/cm2 (82), this charge would depolarize the membrane potential by 4,600–11,700 volts! Depolarization of the membrane to +190 mV shuts down NADPH oxidase activity completely (83). Thus, for significant oxidase activity to occur, the charge must be compensated.
The changes in the vacuolar pH, which is elevated from that of the extracellular medium to 7.8–8.0 (68) despite the release into the vacuole of 500 mg/ml of acidic granule protein contents (6), hold the key to understanding the nature of the compensating ions (Figure 4). These granule contents are maintained at pH 5.0 in the granule by a proton pump (60) and have strong buffering powers. About 400 μmol potassium hydroxide is required per gram of granule protein to elevate the pH from 5.0 to 8.0 (6).
Figure 4.
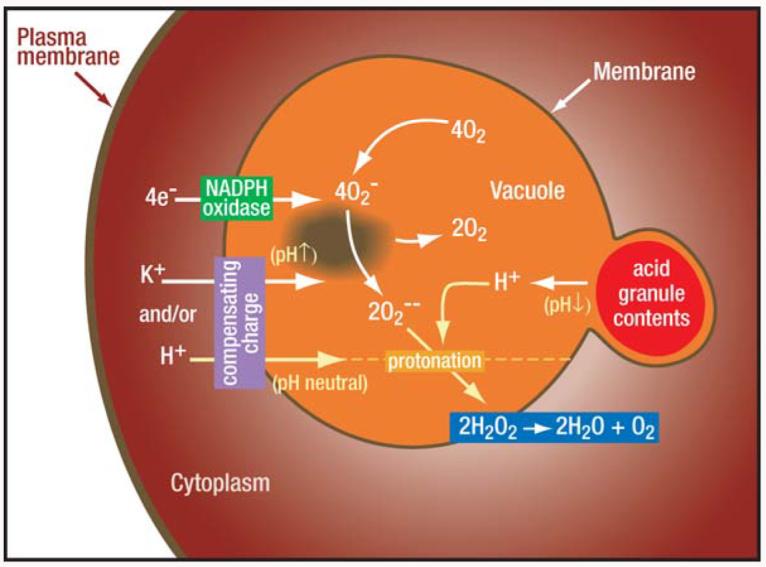
Activity of the NADPH oxidase depolarizes the membrane. The nature of the compensating charge governs the changes in vacuolar pH and tonicity. Electrons are transported across the vacuolar membrane to form , which dismutates to . and become protonated to form HO2 and H2O2, thereby consuming protons and elevating the pH in the vacuole despite the entry of acidic granule contents. This process can only occur if part of the charge is compensated by ions other than protons, which in part occurs through the passage of K+ ions (6, 74).
The vacuole becomes alkaline despite the entry of acidic granule contents, indicating that the and are consuming protons in the vacuole. This would not happen if each electron passing across the membrane was accompanied by a proton, demonstrating that compensating charges cannot be solely in the form of H+ from the cytoplasm.
The major cation in the cytoplasm is K+, which accumulates in the vacuole at concentrations of up to about 600 mM as a consequence of oxidase activity (6). Transport of K+ ions is markedly diminished when the pH rises above 8.0, indicating that the K+ channel provides an important self-regulating mechanism for elevating the vacuolar pH while also ensuring that it does not go too high.
K+ flux only accounts for about 6% of the compensating charge (6). The putative proton channel discussed below does not appear to compensate for all the rest of the charge because its inhibition with Zn2+ and Cd2+ fails to block the NADPH oxidase (74). Therefore, some other major ion flux must also be involved. As is described below, this is accomplished by the flux of chloride ions through a glycine-gated, strychnine-sensitive channel.
The K+ Enters the Phagocytic Vacuole Through BKCa Channels
K+ enters the vacuole through the large conductance Ca2+-activated K+ channel (74). Iberiotoxin (IBTX) and paxilline (PAX), both highly selective and potent inhibitors of this channel (84, 85), prevent the alkalinization of the vacuole, confirming the importance of the influx of K+ into the vacuole on alkalinization of this compartment. The IC50 values for this effect were in the region of 10 nM for IBTX and PAX, consistent with their IC50 for channel block. In addition, the BKCachannel opener, NS1619 (86), significantly augmented the rise in pH to supranormal levels. A variety of blockers and openers of other K+ channels were without effect.
86Rb+ release from activated neutrophils after stimulation with phorbol myristate acetate (PMA) was also induced by NS1619 and even further enhanced by the combination of this opener and PMA. PMA-induced and NS1619-induced efflux were both completely abrogated by IBTX and PAX. The same was found to apply to eosinophils.
BKCa channels are classically opened by the combination of membrane depolarization and elevated cytosolic Ca2+ (87). The same holds true for this channel in neutrophils and eosinophils. Neither depolarizing the membrane nor elevating the cytosolic Ca2+ was sufficient to fully open the K+ channel, whereas the combination of the two caused as much channel opening as did stimulation with PMA. Although PMA stimulation is well known to depolarize the neutrophil plasma membrane (88), it is generally thought not to elevate cytosolic Ca2+. One mechanism by which this might occur is through a drop in pH just beneath the plasma membrane as a consequence of charge separation induced by the oxidase. Corresponding elevations in Ca2+ and falls in pH were seen just beneath the plasma membrane in activated cells (74).
Charge Compensation by Protons
Protons remain in the cytoplasm as a result of charge separation, which occurs when the electrons are transported from NADPH across the wall of the phagocytic vacuole. Additional protons are produced in the cytosol by the HMP shunt, which generates NADPH (89), as well as during the production of energy by glycolysis. This proton generation by an active oxidase, estimated to be about 150 mMols/l (90), causes an initial slight fall in cytosolic pH that rapidly returns to normal.
Three mechanisms appear to be associated with the extrusion of these protons, which are extruded in roughly equimolar quantities with the that is generated (91, 92). The predominant one is a Na+/H+ antiport (93, 94). Its inhibition by the removal of extracellular Na+ or blockage with amiloride causes acidification of the cytosol upon stimulation of the cells. In addition, both Zn2+ and Cd2+-sensitive proton channels (95, 96) and vacuolar (V)-type H+ pumps, inhibited by bafilomycins (90), are also present.
Investigators generally agree that the charge induced by electron translocation (Ie) through the NADPH oxidase is compensated by proton efflux (78, 83, 97), although the identity of the proposed channel is currently highly contentious. One school of thought holds that protons pass through voltage-gated proton channels that are distinct from any NADPH oxidase component (98). The opposing view is that they pass through flavocytochrome b558 of the oxidase, gp91phox, itself (99-101).
One of the hallmarks of the assumption that Ie is largely compensated by proton fluxes is that both Zn2+ and Cd2+, known proton channel blockers (98, 102, 103), were also thought to inhibit production (83, 97). The discrepancy between the low μM concentrations of these cations that block proton channels and the mM concentrations needed to inhibit cytochrome c reduction was recently explained by the voltage dependence of Ie. Zn2+ and Cd2+ shift the threshold voltage for activating voltage-gated proton channels into the steeply voltage-dependent region of Ie, thereby attenuating production (83).
However, Zn2+ and Cd22+ inhibition of voltage-gated proton channels do not inhibit the NADPH oxidase: They have no effect on PMA-induced oxygen consumption, the true measure of oxidase activity. Zn2+ and Cd2+ interfere with the reduction of cytochrome c by accelerating the dismutation of O2− to H2O2 (74). In a system in which xanthine-xanthine oxidase generated , 3 mM concentrations of these elements induced the dismutation of to H2O2 at a rate indistinguishable from that catalyzed by superoxide dismutase (1 μg/ml). Zn2+, at concentrations three orders of magnitude greater than those causing almost complete blockage to proton channels, was also without effect on the currents measured in electrophysiological studies performed on neutrophils, eosinophils, or on PMA-induced 86Rb efflux from these cells (74). This does not mean that H+ movement through proton channels does not compensate some of the charge, but only that the justification hitherto provided is incorrect.
Charge Compensation by Cl−
We showed that K+ accounts for only about 5%–10% of the compensation of the total electron transport, and, contrary to the description in a recent critique of our work (104), we never claimed that it was the only compensating ion. More recently, we (J. Ahluwalia, G. Gabella, S. Pope, A. Warley, A. Segal, unpublished) have discovered that that Cl−, passing through strychnine-sensitive, glycine-activated homomeric channels, compensates about 90% of the charge. These channels were characterized by patch clamping whole cells and isolated phagocytic vacuoles, and by Western blotting. The removal of Cl− or the blockage of this channel abolished both the respiratory burst and microbial killing. High concentrations of Cl− and glycine required for the optimal function of these channels are contained within the cytoplasmic granules, which empty into the vacuole. NADPH oxidase activity was lost when the granules were removed and regained when Cl− was reintroduced into the vacuole. Lysozyme, cathepsin G, and elastase were inactivated by hypertonic Cl−, the removal of which would be important for their function. These Cl− fluxes provide a direct couple between the extent of degranulation and oxidase activity required to activate the released enzymes.
The Movement of K+ into the Vacuole Activates NE and Cathepsin G
The contents of the cytoplasmic azurophil granules are not freely in solution. They are almost exclusively highly cationic proteins that are strongly bound to the highly negatively charged proteoglycans heparin and chondroitin sulphate (59), in which state they are inactive. They are activated in the vacuole both by the elevation in pH described above and by the hypertonic K+. The latter breaks the charged interaction between the enzymes and the matrix, releasing them in a soluble form (6) (Figure 5). For these hypertonic conditions to develop, water must be prevented from entering the vacuole in response to the osmotic attraction of the salts. This is achieved by encasing the vacuole in a meshwork of cytoskeletal proteins, including paxillin and vinculin.
Figure 5.
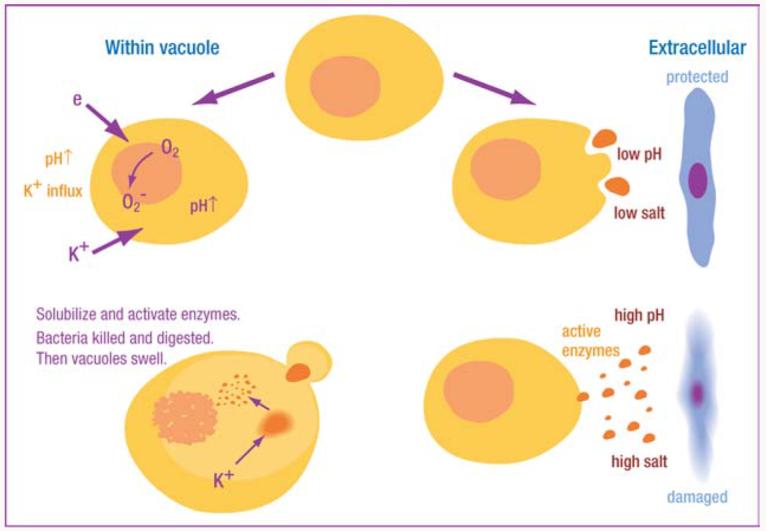
Schematic representation of interaction between NADPH oxidase and granule proteases. Electron transport through flavocytochrome b558 consumes protons in the vacuole, elevating pH to a level optimal for neutral proteases, which are also activated by K+ driven into the vacuole to compensate the charge across the membrane. The hypertonic K+ solubilizes the cationic granule proteases and peptides by displacing them from the anionic sulphated proteoglycan granule matrix. The requirement for an alkaline, hypertonic environment restricts the toxicity of these proteins to the vacuolar compartment, thereby limiting damage to normal tissues.
The importance of the accumulation of K+ in the vacuole was shown when this was diminished either with the K+ ionophore valinomycin (6), or by blocking the BKCa channel with the specific inhibitors IBTX or PAX (74). In both cases, microbial killing and digestion was almost completely prevented (Figure 3) despite the generation of normal quantities of ROS and normal levels of iodination.
Why Was the Importance of Granule Contents in the Killing Process so Overshadowed by ROS and MPO-Mediated Halogenation?
The theory that microbes are killed within the phagocytic vacuole by ROS had fertile ground on which to develop. The lack of production of and H2O2 in anaerobic cells and in CGD with impaired killing under these conditions supported this theory (3, 11), as did the concept of toxicity engendered in the name “reactive oxygen species.” Although experiments were performed in support of these ideas, the conditions under which they were performed in no way reflected the conditions pertaining in the vacuole. They were often done at the wrong pH, and never in the presence of the enormously high concentrations of protein that occur naturally.
Initial studies claimed that killing occurred by generated by the reaction of xanthine with xanthine oxidase, but in fact in those experiments the microbes were killed in the absence of the substrate xanthine, and killing was not inhibited by superoxide dismutase (24). In a similar experiment, no killing of bacteria by was observed after 15 min (25).
H2O2
H2O2, which is used as a topical antiseptic (105), is produced by neutrophils and has been thought of as capable of killing microbes within them (106, 107). Supportive evidence was provided by the finding that catalase-negative organisms rarely infect patients with CGD (108). The explanation was that these bacteria generated enough H2O2 to catalyze their own MPO-mediated halogenation within the vacuole of the neutrophil (109, 110). In vitro mutagenesis was used to generate strains of S. aureus containing varying levels of catalase, and their virulence in mice was found to be inversely proportional to their catalase content (111). Recently, however, doubts have been cast on this theory. Catalase-deficient A. nidulans (112) and S. aureus (113) are as virulent as the catalase-positive varieties in mouse models of CGD, and the bacteria could never come near to producing the relatively enormous quantities of H2O2 generated even by cells from patients with variant CGD.
When glucose oxidase was administered to CGD cells in liposomes, it appeared to correct the killing defect (114, 115). However, no explanation was provided as to how glucose would gain access to the vacuole in adequate amounts to generate sufficient quantities of H2O2, and the killing of bacteria in the extracellular medium was not excluded.
MPO
Experiments that demonstrated that the MPO-H2O2-halide system can kill bacteria in the test tube (22, 38-41) were conducted under nonphysiological conditions, with relatively low concentrations of MPO (50 μg/ml rather than 100 mgs/ml), at low pH (5.0 rather than 7.8–8.0), and, most important of all, in the absence of the high levels of proteins (approximately 500 mgs/ml) found in the vacuole. When bacteria were exposed to 100 mM H2O2 or 1 mM HOCl in the presence of 25 mg/ml granule proteins (technically much more manageable than the experimentally determined 500 mg/ml), killing was almost abolished (116).
Neutrophils clearly iodinate and chlorinate proteins when bacteria are phagocytosed, and this halogenation is dependent on an active NADPH oxidase and MPO (118). However, it is largely the proteins of the neutrophil granule rather than the microbial proteins that are iodinated (116, 119) and chlorinated (120), a highly inefficient system if its primary purpose is to halogenate bacterial proteins. Further indications as to the inefficiency of the proposed system come from the amounts of H2O2 generated. It seems highly unlikely that substrate would need to be provided at molar concentrations and that the 100 mM H2O2 produced by patients with variant CGD would be insufficient when it is effective at 50 μM in the test tube (38).
A few patients were discovered whose neutrophils lacked MPO who were also thought to be immunodeficient (42), and an MPO knockout mouse was shown to be susceptible to yeast but not bacterial infection (45). However, the advent of automated differential leukocyte counting machines, in which the identification of neutrophils depended on a peroxidase stain, revealed that about 1 in 2000 of the general population are MPO-deficient without any undue predisposition to infection (121). The neutrophils of birds also lack MPO (122).
One possible function of MPO is to protect the digestive enzymes from oxidative denaturation (123) by removing H2O2 from the phagocytic vacuole. MPO has catalase activity (124), but this only functions efficiently if the compound II that accumulates is reduced back to the native enzyme. This reduction can be achieved by the high concentrations of in the vacuole with which MPO forms an adduct to produce compound III (125). The impaired microbial killing observed in the MPO knockout mouse (126) could result from oxidative inactivation of antimicrobial proteins by the H2O2 that accumulates under these conditions (106).
MPO may also have dual functions, one as a catalase under the conditions pertaining in the vacuole, but another in a microbicidal capacity outside the cell where enzyme and substrate is much more dilute, and the pH, which is generally low at sites of infection and inflammation, is more conducive to halogenation reactions.
CONCLUDING REMARKS AND PERSPECTIVES
The complexity of the NADPH oxidase and its associated ion fluxes might seem excessive for the apparently simple purpose of activating enzymes within the phagosome. These enzymes, however, have the potential to be highly destructive to normal tissues, and yet organs housing the most exuberant inflammation and neutrophil infiltration can undergo resolution and return completely to normal a week or two later. Some of the neutrophil are removed by apoptosis, but many also necrose with the resultant release of their granules. The requirement of the combination of hypertonicity and alkalinity, neither of which occurs naturally in inflammatory foci, for the activation of these enzymes severely limits the toxicity of granules released into the tissues (Figure 5).
The demonstration that ROS and MPO-mediated halogenation are not the primary killing systems they were long believed to be has reopened many questions relating to mechanisms of innate immunity in the neutrophil. The roles of the different granule constituents in the killing and digestion of specific organisms is of interest, as are the consequences of the interaction of ROS with these granule contents on their biophysical, biochemical, and hence antimicrobial properties.
A number of problems still need to be resolved to clarify the mechanisms involved in charge compensation across the vacuolar membrane. These include the relationship between the channels conducting these charges and electron transport through flavocytochrome b558 and the mechanisms responsible for activating, regulating, and integrating the fluxes of these different ions.
ACKNOWLEDGMENTS
I thank the Wellcome Trust and CGD Research Trust for support, and Jatinder Ahluwalia, Simon Pope, and Daniel Marks for reading the manuscript. I apologize to all investigators whose work has not been cited owing to space restrictions.
LITERATURE CITED
- 1.Cohn ZA, Hirsch JG. The influence of phagocytosis on the intracellular distribution of granule-associated components of polymorphonuclear leucocytes. J. Exp. Med. 1960;112:1015–22. doi: 10.1084/jem.112.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iyer GYN, Islam DMF, Quastel JH. Biochemical aspects of phagocytosis. Nature. 1961;192:535–41. [Google Scholar]
- 3.Holmes B, Page AR, Good RA. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocyte function. J. Clin. Invest. 1967;46:1422–32. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klebanoff SJ, White LR. Iodination defect in the leukocytes of a patient with chronic granulomatous disease of childhood. N. Engl. J. Med. 1969;280:460–66. doi: 10.1056/NEJM196902272800902. [DOI] [PubMed] [Google Scholar]
- 5.Babior BM, Kipnes RS, Curnutte JT. Biological defence mechanisms: the production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest. 1973;52:741–44. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–97. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 7.Vento S, Cainelli F. Infections in patients with cancer undergoing chemotherapy: aetiology, prevention, and treatment. Lancet Oncol. 2003;4:595–604. doi: 10.1016/s1470-2045(03)01218-x. [DOI] [PubMed] [Google Scholar]
- 8.Winkelstein JA, Marino MC, Johnston RBJ, Boyle J, Curnutte J, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–69. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Segal AW, Harper AM, Garcia RC, Merzbach D. The action of cells from patients with chronic granulomatous disease on Staphylococcus aureus. J. Med. Microbiol. 1982;15:441–49. doi: 10.1099/00222615-15-4-441. [DOI] [PubMed] [Google Scholar]
- 10.Thrasher AJ, Keep NH, Wientjes F, Segal AW. Chronic granulomatous disease. Biochim. Biophys. Acta. 1994;1227:1–24. doi: 10.1016/0925-4439(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 11.Mandell GL. Bactericidal activity of aerobic and anaerobic polymorphonuclear neutrophils. Infect. Immun. 1974;9:337–41. doi: 10.1128/iai.9.2.337-341.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cross AR, Segal AW. The NADPH oxidase of professional phagocytes—prototype of the NOX electron transport chain systems. Biochem. Biophysica Acta—Bioenergetics. 2004;1657:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell. Mol. Life Sci. 2002;59:1428–59. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor WR, Jones DT, Segal AW. A structural model for the nucleotide binding domains of the flavocytochrome b−245 β-chain. Protein Sci. 1993;2:1675–85. doi: 10.1002/pro.5560021013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wientjes FB, Segal AW. PX domain takes shape. Curr. Opin. Hematol. 2003;10:2–7. doi: 10.1097/00062752-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Lew PD, Southwick FS, Stossel TP, Whitin JC, Simons E, Cohen HJ. A variant of chronic granulomatous disease: deficient oxidative metabolism due to a low-affinity NADPH oxidase. N. Engl. J. Med. 1981;305:1329–33. doi: 10.1056/NEJM198111263052207. [DOI] [PubMed] [Google Scholar]
- 17.Segal AW, Dorling J, Coade S. Kinetics of fusion of the cytoplasmic granules with phagocytic vacuoles in human polymorphonuclear leukocytes. Biochemical and morphological studies. J. Cell Biol. 1980;85:42–59. doi: 10.1083/jcb.85.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briggs RT, Robinson JM, Karnovsky ML, Karnovsky MJ. Superoxide production by polymorphonuclear leukocytes. Acytochemical approach. Histochemistry. 1986;84:371–78. doi: 10.1007/BF00482965. [DOI] [PubMed] [Google Scholar]
- 19.Segal AW, Meshulam T. Production of superoxide by neutrophils: a reappraisal. FEBS Lett. 1979;100:27–32. doi: 10.1016/0014-5793(79)81124-2. [DOI] [PubMed] [Google Scholar]
- 20.Thomas MJ, Hedrick CC, Smith S, Pang J, Jerome WG, et al. Superoxide generation by the human polymorphonuclear leukocyte in response to latex beads. J. Leukoc. Biol. 1992;51:591–96. doi: 10.1002/jlb.51.6.591. [DOI] [PubMed] [Google Scholar]
- 21.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- 22.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–17. [PubMed] [Google Scholar]
- 23.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. New York: Oxford Univ. Press; 1999. [Google Scholar]
- 24.Babior BM, Curnutte JT, Kipnes RS. Biological defense mechanisms. Evidence for the participation of superoxide in bacterial killing by xanthine oxidase. J. Lab. Clin. Med. 1975;85:235–44. [PubMed] [Google Scholar]
- 25.Rosen H, Klebanoff SJ. Bactericidal activity of a superoxide anion-generating system. A model for the polymorphonuclear leukocyte. J. Exp. Med. 1979;149:27–39. doi: 10.1084/jem.149.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambruso DR, Johnston RB., Jr Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions, and an enzymatic generating system. J. Clin. Invest. 1981;67:352–60. doi: 10.1172/JCI110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen H. Role of hydroxyl radical in polymorphonuclear leukocyte-mediated bactericidal activity. Agents Actions Suppl. 1980;7:180–84. [PubMed] [Google Scholar]
- 28.Cohen MS, Britigan BE, Pou S, Rosen GM. Application of spin trapping to human phagocytic cells: insight into conditions for formation and limitation of hydroxyl radical. Free Radic. Res. Commun. 1991;12–13(Pt. 1):17–25. doi: 10.3109/10715769109145763. [DOI] [PubMed] [Google Scholar]
- 29.Gutteridge JM, Paterson SK, Segal AW, Halliwell B. Inhibition of lipid peroxidation by the iron-binding protein lactoferrin. Biochem. J. 1981;199:259–61. doi: 10.1042/bj1990259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winterbourn CC. Lactoferrin-catalysed hydroxyl radical production. Additional requirement for a chelating agent. Biochem. J. 1983;210:15–19. doi: 10.1042/bj2100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Britigan BE, Hassett DJ, Rosen GM, Hamill DR, Cohen MS. Neutrophil degranulation inhibits potential hydroxyl-radical formation. Relative impact of myeloperoxidase and lactoferrin release on hydroxyl-radical production by iron-supplemented neutrophils assessed by spin-trapping techniques. Biochem. J. 1989;264:447–55. doi: 10.1042/bj2640447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE. Free radicals and phagocytic cells. FASEB J. 1995;9:200–9. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee R, Ragsdale SW. The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu. Rev. Biochem. 2003;72:209–47. doi: 10.1146/annurev.biochem.72.121801.161828. [DOI] [PubMed] [Google Scholar]
- 34.Wentworth P, Jr, McDunn JE, Wentworth AD, Takeuchi C, Nieva J, et al. Evidence for antibody-catalyzed ozone formation in bacterial killing and inflammation. Science. 2002;298:2195–99. doi: 10.1126/science.1077642. [DOI] [PubMed] [Google Scholar]
- 35.Babior BM, Takeuchi C, Ruedi J, Gutierrez A, Wentworth P., Jr Investigating antibody-catalyzed ozone generation by human neutrophils. Proc. Natl. Acad. Sci. USA. 2003;100:3031–34. doi: 10.1073/pnas.0530251100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kettle AJ, Clark BM, Winterbourn CC. Superoxide converts indigo carmine to isatin sulfonic acid: implications for the hypothesis that neutrophils produce ozone. J. Biol. Chem. 2004;279:18521–25. doi: 10.1074/jbc.M400334200. [DOI] [PubMed] [Google Scholar]
- 37.Fiedler TJ, Davey CA, Fenna RE. X-ray crystal structure and characterization of halide-binding sites of human myeloperoxidase at 1.8 Å resolution. J. Biol. Chem. 2000;275:11964–71. doi: 10.1074/jbc.275.16.11964. [DOI] [PubMed] [Google Scholar]
- 38.Klebanoff SJ. Myeloperoxidasehalide-hydrogen peroxide antibacterial system. J. Bacteriol. 1968;95:2131–38. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klebanoff SJ. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin. Hematol. 1975;12:117–42. [PubMed] [Google Scholar]
- 40.Klebanoff SJ. Iodination of bacteria: a bactericidal mechanism. J. Exp. Med. 1967;126:1063–78. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hampton MB, Kettle AJ, Winterbourn CC. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect. Immun. 1996;64:3512–17. doi: 10.1128/iai.64.9.3512-3517.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehrer RI, Hanifin J, Cline MJ. Defective bactericidal activity in myeloperoxidase-deficient human neutrophils. Nature. 1969;223:78–79. doi: 10.1038/223078a0. [DOI] [PubMed] [Google Scholar]
- 43.Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, et al. Differential host susceptibility to pulmonary infections with bacteria and fungi in mice deficient in myeloperoxidase. J. Infect. Dis. 2000;182:1276–79. doi: 10.1086/315843. [DOI] [PubMed] [Google Scholar]
- 44.Gaut JP, Yeh GC, Tran HD, Byun J, Henderson JP, et al. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc. Natl. Acad. Sci. USA. 2001;98:11961–66. doi: 10.1073/pnas.211190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aratani Y, Koyama H, Nyui S, Suzuki K, Kura F, Maeda N. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect. Immun. 1999;67:1828–36. doi: 10.1128/iai.67.4.1828-1836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wheeler MA, Smith SD, Garcia-Cardena G, Nathan CF, Weiss RM, Sessa WC. Bacterial infection induces nitric oxide synthase in human neutrophils. J. Clin. Invest. 1997;99:110–16. doi: 10.1172/JCI119121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chakravortty D, Hensel M. Inducible nitric oxide synthase and control of intracellular bacterial pathogens. Microbes. Infect. 2003;5:621–27. doi: 10.1016/s1286-4579(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 48.Fleming A. On a remarkable bacteriolytic element found in tissues and secretions. Proc. R. Soc. London. 1922;93:306–317. [Google Scholar]
- 49.Hirsch JG. Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J. Exp. Med. 1956;103:589–611. doi: 10.1084/jem.103.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeya HI, Spitznagel JK. Arginine-rich proteins of polymorphonuclear leukocyte lysosomes. Antimicrobial specificity and biochemical heterogeneity. J. Exp. Med. 1968;127:927–41. doi: 10.1084/jem.127.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–21. [PubMed] [Google Scholar]
- 52.Gullberg U, Bengtsson N, Bulow E, Garwicz D, Lindmark A, Olsson I. Processing and targeting of granule proteins in human neutrophils. J. Immunol. Methods. 1999;232:201–10. doi: 10.1016/s0022-1759(99)00177-5. [DOI] [PubMed] [Google Scholar]
- 53.Bainton DF. Neutrophilic leukocyte granules: from structure to function. Adv. Exp. Med. Biol. 1993;336:17–33. doi: 10.1007/978-1-4757-9182-2_3. [DOI] [PubMed] [Google Scholar]
- 54.Baggiolini M, Hirsch JG, De Duve C. Resolution of granules from rabbit heterophil leukocytes into distinct populations by zonal sedimentation. J. Cell Biol. 1969;40:529–41. doi: 10.1083/jcb.40.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiss J, Franson RC, Beckerdite S, Schmeidler K, Elsbach P. Partial characterization and purification of a rabbit granulocyte factor that increases permeability of Escherichia coli. J. Clin. Invest. 1975;55:33–42. doi: 10.1172/JCI107915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss J, Elsbach P, Olsson I, Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J. Biol. Chem. 1978;253:2664–72. [PubMed] [Google Scholar]
- 57.Ooi CE, Weiss J, Doerfler ME, Elsbach P. Endotoxin-neutralizing properties of the 25 kD N-terminal fragment and a newly isolated 30 kD C-terminal fragment of the 55–60 kD bactericidal/permeability-increasing protein of human neutrophils. J. Exp. Med. 1991;174:649–55. doi: 10.1084/jem.174.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003;3:710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 59.Kolset SO, Gallagher JT. Proteoglycans in haemopoietic cells. Biochim. Biophys. Acta. 1990;1032:191–211. doi: 10.1016/0304-419x(90)90004-k. [DOI] [PubMed] [Google Scholar]
- 60.Styrt B, Klempner MS. Internal pH of human neutrophil lysosomes. FEBS Lett. 1982;149:113–16. doi: 10.1016/0014-5793(82)81083-1. [DOI] [PubMed] [Google Scholar]
- 61.Bullen JJ, Armstrong JA. The role of lactoferrin in the bactericidal function of polymorphonuclear leucocytes. Immunology. 1979;36:781–91. [PMC free article] [PubMed] [Google Scholar]
- 62.Bundgaard JR, Sengelov H, Borregaard N, Kjeldsen L. Molecular cloning and expression of a cDNA encoding NGAL: a lipocalin expressed in human neutrophils. Biochem. Biophys. Res. Commun. 1994;202:1468–75. doi: 10.1006/bbrc.1994.2096. [DOI] [PubMed] [Google Scholar]
- 63.Segal AW, Jones OT. The subcellular distribution and some properties of the cytochrome b component of the microbicidal oxidase system of human neutrophils. Biochem. J. 1979;182:181–88. doi: 10.1042/bj1820181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hibbs MS, Bainton DF. Human neutrophil gelatinase is a component of specific granules. J. Clin. Invest. 1989;84:1395–402. doi: 10.1172/JCI114312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borregaard N, Kjeldsen L, Rygaard K, Bastholm L, Nielsen MH, et al. Stimulus-dependent secretion of plasma proteins from human neutrophils. J. Clin. Invest. 1992;90:86–96. doi: 10.1172/JCI115860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baggiolini M, Hirsch JG, De Duve C. Further biochemical and morphological studies of granule fractions from rabbit heterophil leukocytes. J. Cell Biol. 1970;45:586–97. doi: 10.1083/jcb.45.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sengelov H, Kjeldsen L, Kroeze W, Berger M, Borregaard N. Secretory vesicles are the intracellular reservoir of complement receptor 1 in human neutrophils. J. Immunol. 1994;153:804–10. [PubMed] [Google Scholar]
- 68.Segal AW, Geisow M, Garcia R, Harper A, Miller R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature. 1981;290:406–9. doi: 10.1038/290406a0. [DOI] [PubMed] [Google Scholar]
- 69.Holmes B, Quie PG, Windhorst DB, Good RA. Fatal granulomatous disease of childhood. An inborn abnormality of phagocytic function. Lancet. 1966;1:1225–28. doi: 10.1016/s0140-6736(66)90238-8. [DOI] [PubMed] [Google Scholar]
- 70.Wright SD, Silverstein SC. Phagocytosing macrophages exclude proteins from the zones of contact with opsonized targets. Nature. 1984;309:359–61. doi: 10.1038/309359a0. [DOI] [PubMed] [Google Scholar]
- 71.Bainton DF. Sequential degranulation of the two types of polymorphonuclear leukocyte granules during phagocytosis of microorganisms. J. Cell Biol. 1973;58:249–64. doi: 10.1083/jcb.58.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jensen MS, Bainton DF. Temporal changes in pH within the phagocytic vacuole of the polymorphonuclear neutrophilic leukocyte. J. Cell Biol. 1973;56:379–88. doi: 10.1083/jcb.56.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cech P, Lehrer RI. Phagolysosomal pH of human neutrophils. Blood. 1984;63:88–95. [PubMed] [Google Scholar]
- 74.Ahluwalia J, Tinker A, Clapp LH, Duchen MR, Abramov AY, et al. The large-conductance Ca2+-activated K+ channel is essential for innate immunity. Nature. 2004;427:853–58. doi: 10.1038/nature02356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley TJ, et al. Mice lacking neutrophil elastase reveal impaired host defense against Gram negative bacterial sepsis. Nat. Med. 1998;4:615–18. doi: 10.1038/nm0598-615. [DOI] [PubMed] [Google Scholar]
- 76.Tkalcevic J, Novelli M, Phylactides M, Iredale JP, Segal AW, Roes J. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity. 2000;12:201–10. doi: 10.1016/s1074-7613(00)80173-9. [DOI] [PubMed] [Google Scholar]
- 77.MacIvor DM, Shapiro SD, Pham CT, Belaaouaj A, Abraham SN, Ley TJ. Normal neutrophil function in cathepsin G-deficient mice. Blood. 1999;94:4282–93. [PubMed] [Google Scholar]
- 78.Henderson LM, Chappell JB, Jones OT. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 1987;246:325–29. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kapus A, Szaszi K, Ligeti E. Phorbol 12-myristate 13-acetate activates an electrogenic H+-conducting pathway in the membrane of neutrophils. Biochem. J. 1992;281:697–701. doi: 10.1042/bj2810697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DeCoursey TE, Cherny VV. Potential, pH, and arachidonate gate hydrogen ion currents in human neutrophils. Biophys. J. 1993;65:1590–98. doi: 10.1016/S0006-3495(93)81198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schrenzel J, Serrander L, Banfi B, Nusse O, Fouyouzi R, et al. Electron currents generated by the human phagocyte NADPH oxidase. Nature. 1998;392:734–37. doi: 10.1038/33725. [DOI] [PubMed] [Google Scholar]
- 82.Pauly H, Packer L, Schwan HP. Electrical properties of mitochondrial membranes. J. Biophys. Biochem. Cytol. 1960;7:589–601. doi: 10.1083/jcb.7.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DeCoursey TE, Morgan D, Cherny VV. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–34. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]
- 84.Sanchez M, McManus OB. Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology. 1996;35:963–68. doi: 10.1016/0028-3908(96)00137-2. [DOI] [PubMed] [Google Scholar]
- 85.Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, et al. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J. Biol. Chem. 1990;265:11083–90. [PubMed] [Google Scholar]
- 86.Lawson K. Potassium channel openers as potential therapeutic weapons in ion channel disease. Kidney Int. 2000;57:838–45. doi: 10.1046/j.1523-1755.2000.00923.x. [DOI] [PubMed] [Google Scholar]
- 87.Kaczorowski GJ, Knaus HG, Leonard RJ, McManus OB, Garcia ML. High-conductance calcium-activated potassium channels; structure, pharmacology, and function. J. Bioenerg. Biomembr. 1996;28:255–67. doi: 10.1007/BF02110699. [DOI] [PubMed] [Google Scholar]
- 88.Jankowski A, Grinstein S. A noninvasive fluorimetric procedure for measurement of membrane potential. Quantification of the NADPH oxidase-induced depolarization in activated neutrophils. J. Biol. Chem. 1999;274:26098–104. doi: 10.1074/jbc.274.37.26098. [DOI] [PubMed] [Google Scholar]
- 89.Borregaard N, Schwartz JH, Tauber AI. Proton secretion by stimulated neutrophils. Significance of hexose monophosphate shunt activity as source of electrons and protons for the respiratory burst. J. Clin. Invest. 1984;74:455–59. doi: 10.1172/JCI111442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nanda A, Gukovskaya A, Tseng J, Grinstein S. Activation of vacuolar-type proton pumps by protein kinase C. Role in neutrophil pH regulation. J. Biol. Chem. 1992;267:22740–46. [PubMed] [Google Scholar]
- 91.Takanaka K, O'Brien PJ. Proton release associated with respiratory burst of polymorphonuclear leukocytes. J. Biochem. (Tokyo) 1988;103:656–60. doi: 10.1093/oxfordjournals.jbchem.a122324. [DOI] [PubMed] [Google Scholar]
- 92.van Zwieten R, Wever R, Hamers MN, Weening RS, Roos D. Extracellular proton release by stimulated neutrophils. J. Clin. Invest. 1981;68:310–13. doi: 10.1172/JCI110250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Simchowitz L. Chemotactic factor-induced activation of Na+/H+ exchange in human neutrophils. II. Intracellular pH changes. J. Biol. Chem. 1985;260:13248–55. [PubMed] [Google Scholar]
- 94.Grinstein S, Furuya W. Cytoplasmic pH regulation in phorbol ester-activated human neutrophils. Am. J. Physiol. 1986;251(Pt. 1):C55–65. doi: 10.1152/ajpcell.1986.251.1.C55. [DOI] [PubMed] [Google Scholar]
- 95.Henderson LM, Chappell JB, Jones OT. Internal pH changes associated with the activity of NADPH oxidase of human neutrophils. Further evidence for the presence of an H+ conducting channel. Biochem. J. 1988;251:563–67. doi: 10.1042/bj2510563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nanda A, Grinstein S. Protein kinase C activates an H+ (equivalent) conductance in the plasma membrane of human neutrophils. Proc. Natl. Acad. Sci. USA. 1991;88:10816–20. doi: 10.1073/pnas.88.23.10816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Henderson LM, Chappell JB, Jones OT. Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem. J. 1988;255:285–90. [PMC free article] [PubMed] [Google Scholar]
- 98.DeCoursey TE, Morgan D, Cherny VV. The gp91phox component of NADPH oxidase is not a voltage-gated proton channel. J. Gen. Physiol. 2002;120:773–79. doi: 10.1085/jgp.20028704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Henderson LM, Meech RW. Evidence that the product of the human X-linked CGD gene, gp91-phox, is a voltage-gated H+ pathway. J. Gen. Physiol. 1999;114:771–86. doi: 10.1085/jgp.114.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maturana A, Arnaudeau S, Ryser S, Banfi B, Hossle JP, et al. Heme histidine ligands within gp91phox modulate proton conduction by the phagocyte NADPH oxidase. J. Biol. Chem. 2001;276:30277–84. doi: 10.1074/jbc.M010438200. [DOI] [PubMed] [Google Scholar]
- 101.Nanda A, Romanek R, Curnutte JT, Grinstein S. Assessment of the contribution of the cytochrome b moiety of the NADPH oxidase to the transmembrane H+ conductance of leukocytes. J. Biol. Chem. 1994;269:27280–85. [PubMed] [Google Scholar]
- 102.Thomas RC, Meech RW. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature. 1982;299:826–28. doi: 10.1038/299826a0. [DOI] [PubMed] [Google Scholar]
- 103.Henderson LM, Chappell JB, Jones OT. Internal pH changes associated with the activity of NADPH oxidase of human neutrophils. Further evidence for the presence of an H+ conducting channel. Biochem. J. 1988;251:563–67. doi: 10.1042/bj2510563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.DeCoursey TE. During the respiratory burst, do phagocytes need proton channels or potassium channels, or both? Sci. STKE. 2004:E21. doi: 10.1126/stke.2332004pe21. [DOI] [PubMed] [Google Scholar]
- 105.Miyasaki KT, Genco RJ, Wilson ME. Antimicrobial properties of hydrogen peroxide and sodium bicarbonate individually and in combination against selected oral, gram-negative, facultative bacteria. J. Dent. Res. 1986;65:1142–48. doi: 10.1177/00220345860650090601. [DOI] [PubMed] [Google Scholar]
- 106.Locksley RM, Wilson CB, Klebanoff SJ. Increased respiratory burst in myeloperoxidase-deficient monocytes. Blood. 1983;62:902–9. [PubMed] [Google Scholar]
- 107.Clifford DP, Repine JE. Hydrogen peroxide mediated killing of bacteria. Mol. Cell Biochem. 1982;49:143–49. doi: 10.1007/BF00231175. [DOI] [PubMed] [Google Scholar]
- 108.Gallin JI, Buescher ES, Seligmann BE, Nath J, Gaither T, Katz P. NIH conference. Recent advances in chronic granulomatous disease. Ann. Intern. Med. 1983;99:657–74. doi: 10.7326/0003-4819-99-5-657. [DOI] [PubMed] [Google Scholar]
- 109.Holmes B, Good RA. Laboratory models of chronic granulomatous disease. J. Reticuloendothel. Soc. 1972;12:216–37. [PubMed] [Google Scholar]
- 110.Pitt J, Bernheimer HP. Role of peroxide in phagocytic killing of pneumococci. Infect. Immun. 1974;9:48–52. doi: 10.1128/iai.9.1.48-52.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mandell GL. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcalleukocyte interaction. J. Clin. Invest. 1975;55:561–66. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chang YC. Virulence of catalasedeficient Aspergillus nidulans in p47phox−/− mice. Implications for fungal pathogenicity and host defense in chronic granulomatous disease. J. Clin. Invest. 1998;101:1843–50. doi: 10.1172/JCI2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Messina CG, Reeves EP, Roes J, Segal AW. Catalase negative Staphylococcus aureus retain virulence in mouse model of chronic granulomatous disease. FEBS Lett. 2002;518:107–10. doi: 10.1016/s0014-5793(02)02658-3. [DOI] [PubMed] [Google Scholar]
- 114.Ismail G, Boxer LA, Baehner RL. Utilization of liposomes for correction of the metabolic and bactericidal deficiencies in chronic granulomatous disease. Pediatr. Res. 1979;13:769–73. doi: 10.1203/00006450-197906000-00010. [DOI] [PubMed] [Google Scholar]
- 115.Gerber CE, Bruchelt G, Falk UB, Kimpfler A, Hauschild O, et al. Reconstitution of bactericidal activity in chronic granulomatous disease cells by glucose-oxidase-containing liposomes. Blood. 2001;98:3097–105. doi: 10.1182/blood.v98.10.3097. [DOI] [PubMed] [Google Scholar]
- 116.Reeves EP, Nagl M, Godovac-Zimmermann J, Segal AW. Reassessment of the microbicidal activity of reactive oxygen species and hypochlorous acid with reference to the phagocytic vacuole of the neutrophil granulocyte. J. Med. Microbiol. 2003;52:643–51. doi: 10.1099/jmm.0.05181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Deleted in proof.
- 118.Klebanoff SJ, Clark RA. Iodination by human polymorphonuclear leukocytes: a re-evaluation. J. Lab. Clin. Med. 1977;89:675–86. [PubMed] [Google Scholar]
- 119.Segal AW, Garcia RC, Harper AM, Banga JP. Iodination by stimulated human neutrophils. Studies on its stoichiometry, subcellular localization and relevance to microbial killing. Biochem. J. 1983;210:215–25. doi: 10.1042/bj2100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chapman AL, Hampton MB, Senthilmohan R, Winterbourn CC, Kettle AJ. Chlorination of bacterial and neutrophil proteins during phagocytosis and killing of Staphylococcus aureus. J. Biol. Chem. 2002;277:9757–62. doi: 10.1074/jbc.M106134200. [DOI] [PubMed] [Google Scholar]
- 121.Nauseef WM. Myeloperoxidase deficiency. Hematol. Oncol. Clin. N. Am. 1988;2:135–58. [PubMed] [Google Scholar]
- 122.Penniall R, Spitznagel JK. Chicken neutrophils: oxidative metabolism in phagocytic cells devoid of myeloperoxidase. Proc. Natl. Acad. Sci. USA. 1975;72:5012–15. doi: 10.1073/pnas.72.12.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kobayashi M, Tanaka T, Usui T. Inactivation of lysosomal enzymes by the respiratory burst of polymorphonuclear leukocytes. Possible involvement of myeloperoxidase-H2O2-halide system. J. Lab. Clin. Med. 1982;100:896–907. [PubMed] [Google Scholar]
- 124.Kettle AJ, Winterbourn CC. A kinetic analysis of the catalase activity of myeloperoxidase. Biochemistry. 2001;40:10204–12. doi: 10.1021/bi010940b. [DOI] [PubMed] [Google Scholar]
- 125.Winterbourn CC, Garcia RC, Segal AW. Production of the superoxide adduct of myeloperoxidase (compound III) by stimulated human neutrophils and its reactivity with hydrogen peroxide and chloride. Biochem. J. 1985;228:583–92. doi: 10.1042/bj2280583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, et al. Critical role of myeloperoxidase and nicotinamide adenine dinucleotide phosphate-oxidase in high-burden systemic infection of mice with Candida albicans. J. Infect. Dis. 2002;185:1833–37. doi: 10.1086/340635. [DOI] [PubMed] [Google Scholar]