Abstract
The tight coupling between load (L) and grip (G) forces during voluntary manipulation of a hand-held object is well established. The current study is to examine grip-load force coupling when motion of the hand with an object was either self-generated (voluntary) or externally generated. Subjects performed similar cyclic movements of different loads at various frequencies with three types of manipulation: (a) voluntary oscillation, (b) oscillating the right arm via the pulley system by the left leg (self-driven oscillation), (c) oscillating the arm via the pulley system by another person (other-driven oscillation). During the self-generated movements: (a) the grip forces were larger and (b) grip-load force modulation was more pronounced than in the externally generated movements. The G-L adjustments are not completely determined by the mechanics of object motion; non-mechanical factors related to movement performance, for instance perceptual factors, may affect the G-L coupling. Potentially the results of study can be used to provide hand therapists with another way for administering the Rapid Exchange Gripping Test.
Keywords: grip force, load force, manipulation, voluntary, passive, feed-forward control
1. Introduction
Motor control of grip is essential in daily life and the measurement of grip force is commonly used by hand therapists as an index of the hand function. However, the testing is usually limited to static measurements that do not tell much about the ability of the patients to coordinate the grasping with the object manipulation. In daily activity, grasping and transporting object is a frequently encountered task and its dysfunction can severely impact the patient’s normal living.
When people manipulate hand-held objects they change the grip force during the performance [1–5]. The precise patterns of the grip force adjustments during manipulation are not well known, for instance it is not known how the grasping forces change during the golf swing and tennis stroke. However, one manipulation task is studied in detail, this is the vertical movement of a vertically oriented object. The task is similar to lifting a glass filled with liquid. In such a task, people modulate the grasping force according to the lifted load and inertial forces arising due to the object acceleration. To prevent slipping the employed grip force should be larger than the minimal required force; the difference between the actual grip force and the minimal force necessary to prevent slipping was coined the safety margin [6]. The tight coupling between load (L) and grip (G) forces during voluntary movement of a hand-held object in vertical direction is well established [1–5, 7, 8]. It was also shown that the grip force is affected by physical properties of the object, such as the shape of object, texture of contact surface, as well as task properties, such as predictability or unpredictability [5, 9–13]. It is interesting to note that the grip force modulation in people is in sharp contrast to the grasp control realized in contemporary robotic hands where—to simplify the control—the grasp force is fixed during the performance [14].
Examination of grip-load force coupling has direct clinical relevance and can provide recommendations for hand therapy. Studies have shown that the coupling of the grip forces with the object motion can be affected by both ages and neurological conditions. A study by Kinoshita and Francis [15] has shown that during lifting an object elderly subjects had larger number of fluctuations in the grip force-time curve and longer force application time than the younger subjects. Other studies also demonstrated that precision grip force control capacity became degraded with advancing age [10, 15, 16]. Patients with some neurological disorders also experience difficulties in precise adjustments of the grip force to the task demands [9, 11, 17–23]. For instance, patients with cerebral stroke have deficits in sensory input and therefore demonstrated an exaggerated grip force compared to normal control [41]. By studying the ability of children with hemiplegic CP to scale the fingertip force output before lifting a small object whose texture and weight were varied, Gordon and Duff [11] found that children with hemiplegia can use both cues to anticipate the grip force but required more trials than normal control children. The study suggested that the lack of initial anticipatory control can be due to the imprecise representation of the object. The degraded representation might result from disturbed sensory mechanisms.
Grip force adjustments to the movement is a very robust mechanism established at an earlier age [24]: when people lift an object they increase the grasping force. This coupling by itself can be used for rehabilitation (e.g. stroke patients); when patients cannot exert a sufficiently large gripping force voluntarily it is worth trying to alleviate the grasp force increase by exerting the force during cyclic vertical arm movements. However, prior to performing such attempts some basic studies have to be done.
Potentially the grip-load force coupling can be used to provide hand therapists a simple and alternate way to administer Rapid Exchange Gripping Test (REG). The REG was first reported by Hildreth et al. (1989) [25] and used to determine whether the grip strengths are over or under the initial maximal static strength. The test assumes that if a maximal effort was exerted initially it is unlikely that the REG results will exceed this level due to the speed of gripping and fatigue.
When people move up or down a hand-held object the load force (L) equals L=W+ma, where W is the static weight, m is mass of the object and a is the acceleration of its center of mass. The product ma is an inertia force. As the load force increases, the grip force (G) also increases [1–5, 11, 26–30] (see also [31] for a review), even when a performer purposefully grasps the object before the lifting with a force substantially above the slipping threshold [5] (minimum grip force necessary to prevent slipping). G is modulated during shaking and point-to-point arm movements [2, 3, 5, 27] as well as during locomotion [4]. Local skin anesthesia and digit cooling make the coordination of L and G less precise but does not change the general pattern of coordination [8, 32, 33]. When people lift an object from a support, they initially exert sufficiently large G force and only after that generate the force in vertical direction necessary to lift the object. Based on these observations, it has been concluded that the G-L coupling is mainly controlled by a feed-forward mechanism (a central controller regulates the grip force according to the expected load force) [5, 28] aimed at preventing dropping the object. In general, changes in grip force with load force that occur without a time delay or at a time delay that does not allow the action of feedback loops from proprioceptors are termed feed-forward. A feed-forward mechanism can be justified by observing the in-phase modulation and perfect synchronization of grip and load forces: if the feed-forward control is used the time lag between these two forces is negligible.
Other than the voluntary, or self-generated task, the effect of externally generated motion (when someone else is moving the object) on the grip-load force control also draws attention. For instance, it has been shown that when the motion of the object is externally generated the grip force changes fall behind the load force changes [12, 34]. Several studies analyzed reactive and predictive grip force control by systematically varying the predictability of the physical properties of object [28, 35]. Anticipatory force control relies on the predictability of forces that have to be exerted to manipulate objects [34, 36]. Afferent signals from the flexed arm contribute to the grip force response under unpredictable perturbation and prior knowledge and experience play a role in grip force modulation [37]. However, visual feedback did not affect grip force performance during externally guided movements [13].
The grip force during object manipulation can be expanded into three fractions that reflect different effects of gravity and inertia forces on the grip force control [38]: (A) Static fraction Gst reflects grip force related to holding a load statically. To put it simply, people exert larger G forces when they hold heavier objects. The relation is linear [6]. (B) Stato-dynamic fraction Gsd reflects a steady change in the grip force when the same load is moved in a cyclic fashion. Its existence was attributed to a non-specific reaction of the central nervous system (CNS) to the perceived risk of dropping the object [38]. (C) Dynamic fraction Gdy is due to the acceleration-related adjustments of the grip force. Load force is related to movement of the object by the laws of mechanics and the tight coupling between the load and grip forces might suggest that for the given friction conditions the grip force is also determined solely by the load forces acting at the digit tips. It has been reported that skin receptors play a major role in adjusting the G force to the L force and friction conditions at the finger-object interfaces [28]. Hence, we may expect that if an object is being moved in the same way, i.e. along the same path, at the same speed and acceleration, the grasping force should change in the same manner because the information from the skin receptors did not change. However, evidences of non-mechanical factors, such as confidence in grip safety during cyclic arm movement have been also reported [1, 5].
In this study we compared the effects of three patterns of manipulation—(a) voluntary, (b) self-driven (when the right arm is moved via a pulley by the left leg) and (c) other-driven (when the arm is moved by another person)—on the Gsd and Gdy fractions. We expected that to find one of two outcomes: (a) if the G-L relation is defined completely by movement mechanics and by the sensory information from the fingertip contacts, the G-L coupling and all the above mentioned fractions should be the same in all three tasks, or (b) if the G force is influenced by the perceived risk of dropping the object, and the perceived risk is smaller during voluntary arm movements (due to their complete predictability) the grasping force during the voluntary movements should be smaller than during arm movements induced by the contralateral leg or another person. Specifically, we hypothesized that during the self-driven and other-driven arm movements: (1) G would increase (to secure the object from dropping) and (2) the percentage contribution of the Gsd and Gdy fractions into G will change, in particular (2a) Gsd —i.e. an overall increase in the grip force that is not associated with the load force fluctuations—will increase (because of the increased uncertainty of the situation and a larger risk of dropping the object) and (2b) the Gdy contribution will decrease.
The study was motivated not only by academic interests but also by desire to explore a potential for using the manipulation of the hand-held objects for rehabilitation of patients with decreased hand coordination, for instance the stroke survivors. To start a study on patients the benchmark data obtained on healthy subjects are necessary. Obtaining such data was a secondary goal of this study.
2. Method
Participants
Six healthy right-handed male subjects (27±6 yrs) participated voluntarily in this study. Subjects had no previous history of neurological conditions or trauma to the upper extremities. All subjects gave informed consent according to the procedures approved by the Office for Regulatory Compliance of The Pennsylvania State University.
Apparatus & Procedure
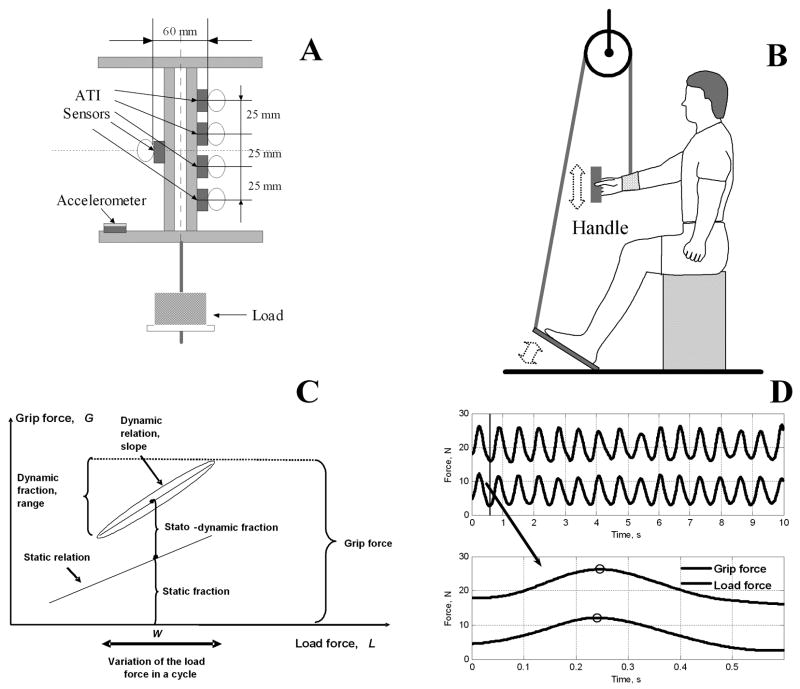
The subjects grasped a handle instrumented with five six-component force/moment transducers (Nano-17, ATI Industrial Automation, Garner, NC, USA) and a tri-axial accelerometer (EGA 3, Entran, USA, range ±5g, weight 7 gram). The handle was built of two horizontal bars and two vertical pillars (Figure 1, Panel A). The center points of the index and middle finger sensors were located 37.5 and 12.5 mm, respectively, above the midpoint of the handle. The center points of the ring and little finger sensors were located 12.5 and 37.5 mm, respectively, below the midpoint. The grip width (the horizontal distance between the surfaces of the thumb contact and the finger contacts) was 60 mm. The surfaces of the transducers were covered with 100-grit sandpaper. In different subjects, the coefficient of friction ranged between 1.4 and 1.5. (for the detailed description of the instrumented handle see [38]).
Figure 1.
Panel A. Schematic drawing of the handle. Panel B. Schematic drawing of the experimental setup. A pedal connected to a pulley system was used to pull the forearm. Panel C. Decomposition of the grasping force into the static, stato-dynamic and dynamic fractions. W is the object weight. The static relation is represented by a straight line. The dynamic relation is represented by an ellipse. Panel D. A typical grip force and load force (N) time histories with zoom-in of the upper panel. The peak values of the curves are labeled with circles and the time lag can be determined by calculating the time interval between the two peaks.
The output signals of the ATI sensors were fed into two 32-channel 12-bit AD converters (PCI-6033E; National Instruments, Austin, TX, USA). The signals of the EGA accelerometer were fed into SCXI-1520, a signal-conditioning module (National Instruments) and then into a 12-bit AD converter (PCI-6052E; National Instruments). The digital signals were processed by a Dell computer (Dimension 8200). The sampling frequency was set at 200 Hz and data were recorded by a customized program written in LabView 6.1 (National Instruments).
At the beginning of each test, the subjects were asked to hold the handle in equilibrium vertically with minimum effort and the static finger forces were recorded for 30 seconds and the averaged values were used for further analyses. Two horizontal ropes, ten cm apart in the vertical direction, were used to mark the target amplitude of the movements. The task was to move the handle along a straight line in the vertical direction and to keep its orientation constant throughout the test. Three types of manipulation were compared: (a) voluntary oscillation, (b) oscillating the right arm via the pulley system by the left leg (self-driven oscillation, Figure 1, Panel B), (c) oscillating the arm via the pulley system by another person (other-driven oscillation, for consistency the task was performed by the same person throughout the test). In manipulation type (a) and (b) the forearm of the subject was supported by a brace and connected to the pulley system. The brace was made of thermoplastic and fitted the forearm tightly. Note that in all three tasks the object moved cyclically with similar amplitude and frequency; hence the object velocity and acceleration were similar. According to basic mechanics, in a cyclic motion for which the displacement is given by y = yosinωt, where yo is the oscillation amplitude and ω is the circular frequency (rad/s), the velocity equals dy/dt = yoωcosωt and the acceleration is d2y/dt2 = −yoω2 sinωt. Hence in the present experiment the maximum acceleration at fastest movement frequency was 0.05×(2×π×2)2 = 7.88 m/s2 ≈ 0.804 g, i.e. it was always less than the acceleration due to gravity (g = 9.81 m/s2). Because during the downward motion the acceleration was always smaller than the acceleration due to gravity the object was never in a free fall and the force exerted by the subject on the object was always positive, i.e. directed upward. The vertical motion of the handle was confirmed by the small acceleration signals in the horizontal plane.
In tasks (b) and (c) the subjects were instructed not to resist and not to help the oscillating force acting on the forearm, and follow the external drive passively. Three movement frequencies, 1 Hz, 1.5 Hz and 2 Hz provided via a metronome and five different weights —3.8 N, 6.3 N, 8.8 N, 11.3 N and 13.8 N were used. Each test lasted 15 s; 20-s breaks were given between consecutive trials. The order of tasks was randomized to reduce the effect of test sequence. To prevent the analysis of initial transients, subjects and/or experimenter practiced each condition until they could follow the metronome and perform the task adequately. During the test, the subjects were allowed to see the movement of their forearm; in the previous research visual feedback was found not to affect the grip force modulation [19]. We restricted the analysis to the planar case. We present here only the results for the thumb forces. In the studied tasks that were limited to strictly vertical movements the thumb normal force equals the total force of the four fingers. The thumb force and the four-finger total force cancel each other thus assuring that the object does not accelerate in the horizontal acceleration. Hence, when recording the thumb normal force we also know the total force of the four fingers. Raw data were low-pass filtered at 5 Hz with a fourth-order zero-lag Butterworth filter.
The static, stato-dynamic and dynamic (range and the G-L slope, see Figure 1, Panel C) fractions of G were computed for each trial; for the details of the analyses see [38]. The stato-dynamic fraction of the grip force was determined as the difference between the projection of the geometric center of the dynamic force-force ellipse recorded during an oscillation test on the grip force axis and the grip force in static condition (Figure 1, Panel C). Note that this difference corresponds to the instances when the handle acceleration is zero. Hence the inertial force ma at these instances is also zero and the load force L momentarily equals the object weight. In spite of this similarity, the grip force during oscillation test is higher than the force at rest. The stato-dynamic fraction of G represents this difference. A repeated measure 3-way ANOVA with the MANIPULATION (three levels: Voluntary, Self-driven, Other-driven), WEIGHT (five levels) and FREQUENCY (three levels) as the factors was employed. The data were tested for sphericity. The Mauchly’s test of sphericity was used. If sphericity was violated, the degrees of freedom were adjusted as necessary with the Greenhouse-Geisser method. The statistical analysis was done using the SPSS package (SPSS Inc., Chicago, IL USA).
We also tested an assumption that the manipulation type affects the G-L synchronization. To illustrate the concept of synchronization an example of the G-L curves in a single oscillation cycle is presented in Figure 1, Panel D. To obtain the data on the synchronization in the entire trials that include many cycles the phase analysis of the relations between the G force and vertical acceleration in the individual trials was performed (for the methods see [14]). Fast Fourier transform (FFT) analysis of the handle acceleration and the digit forces was performed for each data time series over each test period. The phase angles at which the power spectrum magnitude attains the maximum were defined. The phase differences (relative phase) between the signals were then computed.
To determine the effect of the MANIPULATION (3 levels) on the phase angles and their variability in the trials (i.e. the standard deviations) a 1-way ANOVA and non-parametric Kruskal-Wallis test were used, respectively. Also, the 30-s tasks were divided into halves (15-s long) or triples (10-s long) and the phase angles were calculated for each part. The differences were used as a measure of possible changes in the phase angles during the trials. Furthermore, cross-correlation analysis of the acceleration- and G-time histories in individual trials has been performed.
3. Results
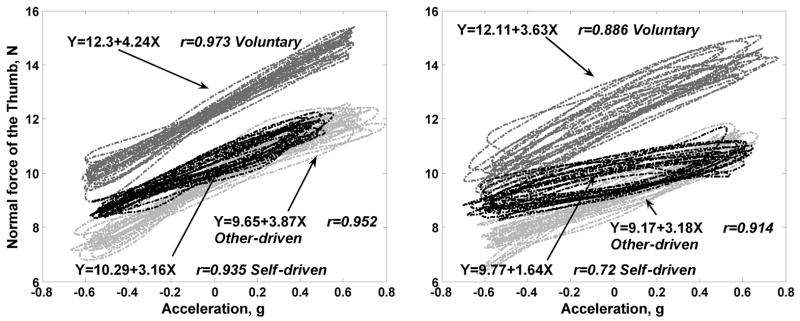
Typical results of the grip force-acceleration relations in the cycles are presented in Figure 2.
Figure 2.
Relations between the grasp force G (Newtons) and vertical acceleration of the handle (measured in g=9.82 m/s2) in two subjects during voluntary, self-driven and other-driven manipulation. Frequency 1.5 Hz, weight of the handle 8.8 N.
It follows from Figure 2 that: (a) G changes with the acceleration in all the tasks and (b) forces are larger during voluntary manipulation. The group averages and the mean SDs of the phase angles across all 15 tasks were 1.28±2.35, 0.89±13.02 and 3.55±10.78 degree for voluntary, other-driven and self-driven manipulation, respectively. The relationship between the time lag (τ) and phase lag (θ) is τ = θ/2πf. Therefore, the time lag between grip force and acceleration/load force was in general less than 5 ms with grip force leading in most of the cases. The small phase lag/time lag indicates that the grip force and load force were modulated in phase.
The 3-way ANOVA results are presented in Table 1. To save space only the p values are given. The high level interactions did not reach the level of statistical significance (p=0.05); they are not included in the table.
Table 1.
Effect of the experimental conditions on the digit forces, p values. Statistically significant effects (p< 0.05) are printed in bold italics.
| Experimental variables (factors) | Static forces (component) | Statodynamic component | Dynamic component: slope | Dynamic component: range |
|---|---|---|---|---|
| Manipulation, M (3 levels) | 0.086 | 0.163 | 0.002* | 0.014* |
| Weight, W (5 levels) | 0.004* | 0.000* | 0.000* | 0.000* |
| Frequency, F (3 levels) | - | 0.000* | 0.000* | 0.000* |
| M ×W | 0.289 | 0.101 | 0.170 | 0.000* |
| M ×F | - | 0.310 | 0.652 | 0.117 |
| W×F | - | 0.000* | 0.004* | 0.000* |
MANIPULATION affected statistically significantly only the dynamic fraction of the grip force, both the range and the slope. The other two factors, WEIGHT and FREQUENCY, affected statistically significantly both Gsd and Gdy fractions (with the exception of the effect of FREQUENCY on the static force). The Dynamic fraction, range and Dynamic fraction, slope were both affected by the FREQUENCY.
The effects of MANIPULATION pattern on the dynamic force component are illustrated in Figure 3. In contrast to our expectations, the largest dynamic effects were observed during the voluntary movements.
Figure 3.
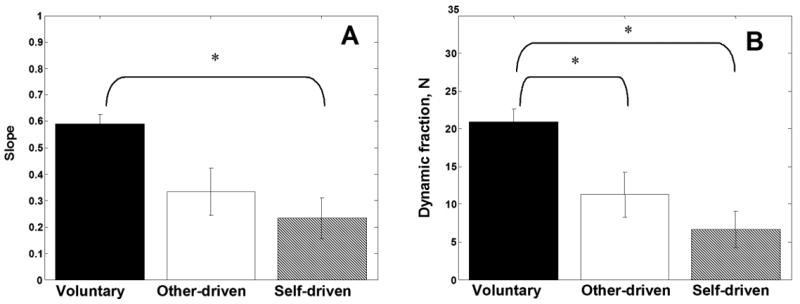
Effects of MANIPULATION on the dynamic force components. (A) Slope of G-L regression. (B) Range of Gdy changes. Group average and standard errors. Symbols * indicate statistically significant differences.
The slope decreased with higher frequency in all three studied tasks, voluntary, self-driven and other-driven. It increased with the weight at frequencies of 1 Hz and 1.5 Hz but not at 2 Hz where systematic changes have not been observed (Figure 4). Hence the slopes of the Gdy-L relations are affected by the type of manipulation, its frequency and the weight magnitude (at the low frequencies).
Figure 4.
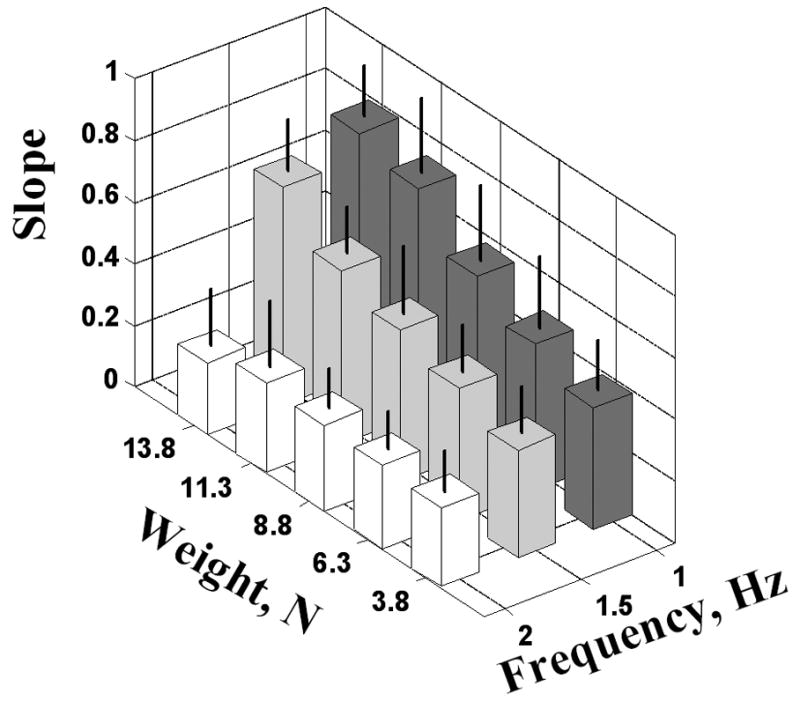
The slopes of the Gdy-L relations at different frequencies and weights, the self-driven manipulation (mean±SD).
While the effects of the MANIPULATION on the stato-dynamic fraction did not reach the level of statistical significance (p=0.16, see Table 1), the tendency was opposite to our hypothesis: the highest values were observed during the voluntary manipulation (20.56±2.07 N) followed by the other-driven (19.49±2.18 N) and self-driven manipulation (18.4±.43 N).
The grip force changed in synchrony with L in all investigated tasks, with some irregular deviations. The group averages and the mean SDs of the phase angles across all 15 tasks were 1.28±2.35, 0.89±13.02 and 3.55±10.78 degree for voluntary, other-driven and self-driven manipulation, respectively. There were no statistically significant differences in the average values of the phase angles across the manipulation types (the results of the 1-way ANOVAs). The variability of the phase angles in the trials was smaller during the voluntary manipulation in most of the tasks (when compared for individual subjects in 60 trials out of 90). However, the Kruskal-Wallis test confirmed statistically significant effects of the MANIPULATION on the intra-trial standard deviations only in 6 trials out of 15 (four of them for the 1.5-Hz frequency). To find whether systematic changes in the phase angle during the trials exist we tried the following techniques: (a) plotted the phase angle by cycles, (b) computed the cross-correlation functions, (c) split the individual trials into two or three parts and compared the phase angles in each part, e.g. the first and second half. These techniques were used to analyze each of the 270 trials. We were not able to find any consistent pattern of the phase angle changes in the trials. Such changes if they occurred would support the idea on increasing role of the feed-forward control during the trial.
4. Discussion
In some previous research [39, 40] the subjects performed similar motion against different resulting loads on the object (elastic, viscous, or inertial). At different load conditions the subjects modulated their grip force differently. In the present study not only the object kinematics but also the load forces perceived by the performer were similar. Hence, all the differences in the G-L coupling were to the manner in which the task was performed. It has been previously reported [37] that during voluntary object manipulation the slope of the Gdy-L relation decreased with frequency and as a rule increased with the weight of the object. The results of this study suggest that this conclusion is valid not only for voluntary manipulation. An increase in the acceleration/frequency resulted in a decrease in slope while an increase in the object mass made the slope larger. Therefore, the grip force was adjusted not only to the magnitude of the load force but also to the differential contributions of the mass and acceleration to the ma product. It is not presently clear why the CNS prefers this type of force adjustments.
In summary, (a) the pattern of MANIPULATION (voluntary, other-driven, self-driven) affects mainly the dynamic component of the grasping force with the highest influence manifested during the active manipulation; (b) the slopes of the Gdy-L relations are similarly affected by the oscillation frequency and the weight magnitude in the voluntary and passive object manipulation.
In all three conditions, the in-phase modulation and perfect synchronization of grip and load forces suggests a feed-forward control of the grip force. In the other-driven tasks, the subjects apparently timed their grip changes to metronome beats. So, grip force can be well timed to an internal clock (as in voluntary movements without any metronome [1, 5], and to an external clock. While we did not find differences in the G-L synchronization among the tasks, we did find the differences in the components of the grasping force.
The overall higher forces in the voluntary condition are counter-intuitive. If grip forces were adjusted based on internal representation of the movement dynamics predicting load forces plus a safety margin [1, 5, 11], other-driven condition is expected to lead to higher forces, not lower. A tentative explanation is that in conditions of poor predictability, humans prefer to live dangerously close to losing the grip, which may improve sensitivity of receptors to partial slips (as in [38]). This can be viewed as a feed-forward control mechanism supplemented with a sensitive feedback loop, for example in case the other person suddenly moves the load differently. This interpretation also fits the observations of lower slope and smaller range of the dynamic component in other-driven manipulation. Another explanation is that during the self-driven and other-driven manipulation external force applied to the subjects’ arm provides additional sensory input. It has been recently shown [7] that when humans lifted and transported an object with one hand touching that wrist lightly with a finger of the contralateral hand the grip forces decreased. These results corroborate the hypothesis that additional sensory input can result in lower grip forces, or equivalently a better grip force control. Therefore, the lower dynamic fraction in the self-driven task might be attributed to additional sensory input. Finally, one of possible explanations is that during other-driven and self-driven movements when the arm was supported by an external mechanism de-recruitment of muscle force throughout the entire limb, including the digit flexors, occurs [we thank an anonymous reviewer for suggesting this explanation]. It should also be noted that the subjects were instructed to not actively follow the movement during self-driven and other-driven manipulation. However, the subjects might have predicted magnitude of the minimal required force through a feed-forward manner in which an internal representation of the dynamics was established. The anticipatory control results in reduced effort, or equivalently, a lower grip force.
A general conclusion is that the G-L adjustment is not completely determined by the object motion; other non-mechanical factors related to movement performance, for instance perceived magnitude of the minimal required force and additional sensory information, may affect the G-L coupling. Potentially, the methodology described in this study can be transferred into clinical setting and applied to evaluate the function of grip force control, especially the performance of grip-load force modulation under different task conditions. As it was already mentioned, subjects with neurological disorders showed deficits in sensory input, exaggerated safety margin and less precision of grip force control so the methodology can be applied to quantify the variables involved in the grip force control. Innovatively, the study differentiates the effect of self-initiated and externally generated tasks and this methodology can be used to investigate whether sensory input in patients with neurological disorders has been degraded and how the sensory deficit is associated with the changes in grip force control. Since the control is realized in a feed-forward manner the effects of the neurological degradation are expected to be indirect, via the distorted representation of the object properties. Another immediate application of this study will be to provide a complimentary test of REG which is used to examine the sincerity of maximum grip efforts. In particular, we agree with the anonymous journal reviewer who suggested to explore the following questions (we thank the reviewer both for the suggestions and for the permission to reproduce them here): 1) is there an optimal frequency for alternating hand placement on a dynamometer? and 2a) should the patient voluntarily pass a dynamometer between the hands, in time with an audio cue (analogous to self-driven/voluntary oscillation?), 2b) should the clinician alternately pass the dynamometer to the patient’s hands, again, in time with an audio cue (analogous to other-driven?), and 2c) should the clinician hold the dynamometer while the patient grips it, or permit the patient to hold the instrument (alters load force)?
Acknowledgments
This work was supported in part by grants AR-048563, AG-018751 and NS35032 from the National Institutes of Health, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flanagan JR, Tresilian J, Wing AM. Coupling of grip force and load force during arm movements with grasped objects. Neuroscience Letters. 1993;152(1–2):53–56. doi: 10.1016/0304-3940(93)90481-y. [DOI] [PubMed] [Google Scholar]
- 2.Flanagan JR, Tresilian JR. Grip-load force coupling: a general control strategy for transporting objects. J Exp Psychol Hum Percept Perform. 1994;20(5):944–57. doi: 10.1037//0096-1523.20.5.944. [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita H, et al. Individual finger forces acting on a grasped object during shaking actions. Ergonomics. 1996;39(2):243–56. doi: 10.1080/00140139608964455. [DOI] [PubMed] [Google Scholar]
- 4.Gysin P, Kaminski TR, Gordon AM. Coordination of fingertip forces in object transport during locomotion. Exp Brain Res. 2003;149(3):371–9. doi: 10.1007/s00221-003-1380-1. [DOI] [PubMed] [Google Scholar]
- 5.Flanagan JR, Wing AM. The stability of precision grip forces during cyclic arm movements with a hand-held load. Exp Brain Res. 1995;105(3):455–64. doi: 10.1007/BF00233045. [DOI] [PubMed] [Google Scholar]
- 6.Westling G, Johansson RS. Factors influencing the force control during precision grip. Exp Brain Res. 1984;53(2):277–84. doi: 10.1007/BF00238156. [DOI] [PubMed] [Google Scholar]
- 7.Aruin AS. Support-specific modulation of grip force in individuals with hemiparesis. Arch Phys Med Rehabil. 2005;86(4):768–75. doi: 10.1016/j.apmr.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 8.Nowak DA, et al. The effects of digital anaesthesia on predictive grip force adjustments during vertical movements of a grasped object. Eur J Neurosci. 2001;14(4):756–62. doi: 10.1046/j.0953-816x.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- 9.Babin-Ratte S, et al. Impaired anticipatory finger grip-force adjustments in a case of cerebellar degeneration. Exp Brain Res. 1999;128(1–2):81–5. doi: 10.1007/s002210050821. [DOI] [PubMed] [Google Scholar]
- 10.Cole K, Rotella D. Old age impairs the use of arbitrary visual cues for predictive control of fingertip forces during grasp. Exp Brain Res. 2002;143(1):35–41. doi: 10.1007/s00221-001-0965-9. [DOI] [PubMed] [Google Scholar]
- 11.Gordon AM, Duff SV. Fingertip forces during object manipulation in children with hemiplegic cerebral palsy. I: anticipatory scaling. Dev Med Child Neurol. 1999;41(3):166–75. doi: 10.1017/s0012162299000353. [DOI] [PubMed] [Google Scholar]
- 12.Johansson RS, et al. Somatosensory control of precision grip during unpredictable pulling loads. I. Changes in load force amplitude. Exp Brain Res. 1992;89(1):181–91. doi: 10.1007/BF00229015. [DOI] [PubMed] [Google Scholar]
- 13.Nowak DA. Different modes of grip force control: voluntary and externally guided arm movements with a hand-held load. Clin Neurophysiol. 2004;115(4):839–48. doi: 10.1016/j.clinph.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 14.Gao F, Latash ML, Zatsiorsky VM. Internal forces during object manipulation. Exp Brain Res. 2005;165(1):69–83. doi: 10.1007/s00221-005-2282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinoshita H, Francis PR. A comparison of prehension force control in young and elderly individuals. Eur J Appl Physiol. 1996;74:450–460. doi: 10.1007/BF02337726. [DOI] [PubMed] [Google Scholar]
- 16.Vandervoort AA, Hayes KC, Belanger AY. Strength and endurance of skeletal muscle in the elderly. Physiotherapy (Can) 1986;38:167–175. [Google Scholar]
- 17.Fellows SJ, et al. Precision grip deficits in cerebellar disorders in man. Clinical Neurophysiology: Official Journal of The International Federation of Clinical Neurophysiology. 2001;112(10):1793–1802. doi: 10.1016/s1388-2457(01)00623-x. [DOI] [PubMed] [Google Scholar]
- 18.Fellows SJ, Noth J. Grip force abnormalities in de novo Parkinson’s disease. Mov Disord. 2004;19(5):560–5. doi: 10.1002/mds.10710. [DOI] [PubMed] [Google Scholar]
- 19.Fellows SJ, Noth J, Schwarz M. Precision grip and Parkinson’s disease. Brain. 1998;121(Pt 9):1771–84. doi: 10.1093/brain/121.9.1771. [DOI] [PubMed] [Google Scholar]
- 20.Hermsdorfer J, et al. Prehension with the ipsilesional hand after unilateral brain damage. Cortex. 1999;35(2):139–61. doi: 10.1016/s0010-9452(08)70791-3. [DOI] [PubMed] [Google Scholar]
- 21.Serrien DJ, et al. Grip force adjustments induced by predictable load perturbations during a manipulative task. Exp Brain Res. 1999;124(1):100–6. doi: 10.1007/s002210050604. [DOI] [PubMed] [Google Scholar]
- 22.Serrien DJ, Wiesendanger M. Grip-load force coordination in cerebellar patients. Exp Brain Res. 1999;128(1–2):76–80. doi: 10.1007/s002210050820. [DOI] [PubMed] [Google Scholar]
- 23.Marwaha R, et al. Load and grip force coordination in static bimanual manipulation tasks in multiple sclerosis. Motor Control. 2006;10(2):160–77. doi: 10.1123/mcj.10.2.160. [DOI] [PubMed] [Google Scholar]
- 24.Forssberg H, et al. Development of human precision grip. I: Basic coordination of force. Exp Brain Res. 1991;85(2):451–7. doi: 10.1007/BF00229422. [DOI] [PubMed] [Google Scholar]
- 25.Hildreth DH, et al. Detection of submaximal effort by use of the rapid exchange grip. J Hand Surg [Am] 1989;14(4):742–5. doi: 10.1016/0363-5023(89)90204-9. [DOI] [PubMed] [Google Scholar]
- 26.Danion F. How dependent are grip force and arm actions during holding an object? Exp Brain Res. 2004;158(1):109–19. doi: 10.1007/s00221-004-1882-5. [DOI] [PubMed] [Google Scholar]
- 27.Flanagan JR, Wing AM. Modulation of grip force with load force during point-to-point arm movements. Exp Brain Res. 1993;95(1):131–43. doi: 10.1007/BF00229662. [DOI] [PubMed] [Google Scholar]
- 28.Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res. 1984;56(3):550–64. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- 29.Nakazawa N, et al. Force control of a robot hand emulating human’s grasping motion. Systems, Man, and Cybernetics, 1999. IEEE SMC ‘99 Conference Proceedings. 1999 IEEE International Conference on. 1999: Theoretical or Mathematical Experimental. [Google Scholar]
- 30.Nakazawa N, et al. Experimental study on human’s grasping force. Robot and Human Communication, 1996., 5th IEEE International Workshop on; 1996. [Google Scholar]
- 31.Flanagan J, Johansson RS. Hand Movements. In: Ramashandran V, editor. Encyclopedia of the Human Brain. Academic Press; San Deigo: 2002. pp. 399–414. [Google Scholar]
- 32.Monzee J, Lamarre Y, Smith AM. The effects of digital anesthesia on force control using a precision grip. J Neurophysiol. 2003;89(2):672–83. doi: 10.1152/jn.00434.2001. [DOI] [PubMed] [Google Scholar]
- 33.Nowak DA, Hermsdorfer J. Digit cooling influences grasp efficiency during manipulative tasks. Eur J Appl Physiol. 2003;89(2):127–33. doi: 10.1007/s00421-002-0759-1. [DOI] [PubMed] [Google Scholar]
- 34.Cole KJ, Abbs JH. Grip force adjustments evoked by load force perturbations of a grasped object. J Neurophysiol. 1988;60(4):1513–22. doi: 10.1152/jn.1988.60.4.1513. [DOI] [PubMed] [Google Scholar]
- 35.Blakemore SJ, Goodbody SJ, Wolpert DM. Predicting the consequences of our own actions: the role of sensorimotor context estimation. J Neurosci. 1998;18(18):7511–8. doi: 10.1523/JNEUROSCI.18-18-07511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scholz JP, Latash ML. A study of a bimanual synergy associated with holding an object. Human Movement Science. 1998;17(6):753. [Google Scholar]
- 37.Danion F. The contribution of non-digital afferent signals to grip force adjustments evoked by brisk unloading of the arm or the held object. Clin Neurophysiol. 2007;118(1):146–54. doi: 10.1016/j.clinph.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Zatsiorsky V, Gao F, Latash ML. Motor control goes beyond physics: differential effects of gravity and inertia on finger forces during manipulation of hand-held objects. Exp Brain Res. 2005;162:300–308. doi: 10.1007/s00221-004-2152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Descoins M, Danion F, Bootsma RJ. Predictive control of grip force when moving object with an elastic load applied on the arm. Exp Brain Res. 2006;172(3):331–42. doi: 10.1007/s00221-005-0340-3. [DOI] [PubMed] [Google Scholar]
- 40.Flanagan JR, Wing AM. The role of internal models in motion planning and control: evidence from grip force adjustments during movements of hand-held loads. J Neurosci. 1997;17(4):1519–28. doi: 10.1523/JNEUROSCI.17-04-01519.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hermsdorfer J, et al. Grip force control during object manipulation in cerebral stroke. Clin Neurophysiol. 2003;114(5):915–29. doi: 10.1016/s1388-2457(03)00042-7. [DOI] [PubMed] [Google Scholar]