Abstract
Aims
Chronic pelvic pain disorders often overlap. We have shown that acute colonic irritation can produce acute irritative micturition patterns and acutely sensitize bladder afferent responses to mechanical and chemical stimuli. We hypothesize that with time, colonic irritation can lead to neurogenic changes in the bladder and the development of chronic bladder sensitization.
Methods
Micturition patterns were measured in rats 60–90 days after the induction of trinitrobenzenesulfonic acid (TNBS) colitis in the resolution phase of this model. Total and activated mast cells (MCs) were quantified in the bladder, while mRNA levels of stem cell factor (SCF) (a.k.a. MC growth factor) and nerve growth factor (NGF) (a MC and nociceptive C-fiber stimulator) were quantified in the bladder and L6-S1 dorsal root ganglia (DRG).
Results
Following intra-rectal TNBS, voiding volume was reduced (p<0.005), while voiding frequency was increased (p<0.05), both by ~50%. Furthermore, both the percentage and density of activated bladder MCs were significantly elevated (p<0.05), although total MC counts were not statistically increased. At the molecular level, urinary bladder SCF expression increased 2-fold (p<0.005), as did NGF (p<0.01), while L6-S1 DRG levels were not significantly elevated.
Conclusions
Chronic cystitis in the rat as evidenced by changes in micturition patterns and the recruitment of activated MCs can occur during the resolution phase of TNBS colitis. These changes, of which MCs may play an important role, appear to be maintained over time and may occur via stimulation of convergent pelvic afferent input resulting in the upregulation of neurotrophic factors in the target organ.
Keywords: Stem cell factor, Bowel, Neurogenic Cystitis, Micturition, Degranulation
INTRODUCTION
Because the colorectum and urinary bladder are two pelvic organs whose functions are an integral part of daily, conscious, physiologic pelvic activity, it’s not surprising that irritable bowel syndrome (IBS) and interstitial cystitis (IC), analogous disorders of pelvic visceral pain and urgency, are the two commonest chronic pelvic pain (CPP) disorders that collectively affect as many as 15% of men and women [Mathias et al., 1996; Moldwin, 2002; Wesselmann, 2001; Zondervan et al., 2001]. IBS, an intestinal disorder characterized by chronic or recurrent lower abdominal pain or discomfort associated with altered stool consistency and frequency [Thompson et al., 2000], is the most common gastrointestinal cause of CPP, affecting 50% of such women presenting to gynecologic clinics [Hogston, 1987; Prior et al., 1989; Walker et al., 1991; Walker et al., 1996]. IC or painful bladder syndrome is characterized by unpleasant urinary symptoms such as urinary frequency, urgency, nocturia, and, most notably, pain related to bladder filling in the absence of active infection or organic disease [Koziol, 1994; Ratner, 2001; Moldwin, 2002].
As many as 40–60% of patients diagnosed with IBS were also found to exhibit symptoms and fulfill diagnostic criteria for IC [Prior et al., 1989; Whorwell et al., 1986], while correspondingly, as many as 50% of patients diagnosed with IC were also found to have symptoms and fulfill diagnostic criteria for IBS [Alagiri et al., 1997; Novi et al., 2005]. Perhaps not surprisingly, frank inflammatory conditions of the colon such as diverticulitis and inflammatory bowel disease are also associated with dysuria and IC [Gibson, 1969; Alagiri et al., 1997]. Moreover, this phenomenon is not limited to the colon and bladder as other pelvic regions and organs with shared dermatomes also exhibit significant clinical overlap. For example, 26% of patients with IC had concurrent pain of the vulva or vulvodynia [Fitzpatrick et al., 1993], while 45% of males with chronic prostatitis or male CPP exhibited pain with bladder filling, a classic feature of IC [Moldwin, 2002]. The high concurrence rate of IBS, IC, and other CPP disorders suggests a common predisposition, a shared etiologic factor, or possible cross-sensitization of pelvic organs [Pezzone et al., 2005].
Recently, in a novel experimental model of acute pelvic organ irritation and neural crosstalk between pelvic organs, we demonstrated that acute cystitis can lower colorectal sensory thresholds to balloon distension and that acute colitis can produce acute irritative micturition patterns [Pezzone et al., 2005]. The development of pelvic organ cross-sensitization in the acute setting suggested a role for and subsequent modulation of pre-existing afferent pathways in the pelvis. Fittingly, follow-up studies performed in our laboratory employing single unit C-fiber bladder afferent recording revealed that acute colonic irritation is capable of sensitizing urinary bladder afferents to mechanical and chemical stimuli. Interruption of the neural input to the bladder ameliorated this effect, suggesting a direct afferent pathway from the colon [Ustinova et al., 2006].
The long-term effects of colonic irritation on physiologic function of the urinary bladder and vice versa have not been previously studied. Using chemical, immune, and mechanical irritation of the bladder, Dupont and colleagues [Dupont et al., 2001] found chronic morphologic changes in sensory and motor neurons innervating the bladder and alterations in the number and distribution of bladder mast cells. Although cross-organ sensitization was not assessed by design, it was hypothesized that the neuroplasticity observed in these models of direct bladder irritation could explain the long-term symptoms and pain seen even after resolution of bladder inflammation [Dupont et al., 2001]. In these direct models of bladder irritation, one may hypothesize that the associated neuroplasticity may not necessarily be limited to the bladder itself and may involve other pelvic organs as acute cross-sensitization studies suggest [Giamberardino, 1999; Dmitrieva and Berkley, 2002; Pezzone et al., 2005; Ustinova et al., 2006]. Thus, acute irritation of one pelvic organ may influence physiologic (both sensory and motor) function in another via direct neural circuits or perhaps convergent sensory input. We hypothesize that with continued stimulation of cross-organ neural circuitry, physiologic changes could develop in not only the affected pelvic organ but also in other target organs in the pelvis with convergent afferent input. This chronic neurogenic stimulation and consequential cross-organ sensitization by release of inflammatory mediators or growth factors could lead to the development and overlap of CPP disorders [Pezzone et al., 2005].
To investigate this hypothesis, we evaluated the physiologic effects of colonic irritation on urinary bladder function by measuring micturition patterns in awake female rats 60–90 days after the induction of TNBS colitis during the resolution phase. To begin to identify the histologic and biochemical changes in the afferent and end-organ pathways that may be responsible for neurogenic inflammation and afferent sensitization in the pelvis following colonic irritation, bladder mast cell numbers and percent activation were quantified. In a parallel series of animals, RT-PCR was used to quantify mRNA levels of stem cell factor (SCF, a.k.a. mast cell growth factor) and nerve growth factor (NGF, a neurotrophic factor and also a mast cell stimulatory factor that acts synergistically in this regard with SCF [Hirata et al., 1993; Carnahan et al., 1994; Lourenssen et al., 2000] in urinary bladders and L6-S1 dorsal root ganglia (DRG) (corresponding to lumbosacral afferent input to the urinary bladder and distal colorectum of the rat).
MATERIALS and METHODS
Animals
Virgin female Sprague-Dawle rats, 200–225 g in weight and 9–11 weeks of age, were purchased from Hilltop Lab Animals, Inc. (Scottsdale, PA) and were housed in standard polypropylene cages with ad libitum access to food and water in the University of Pittsburgh’s Central Animal Facility. All studies were approved by the University of Pittsburgh’s Institutional Animal Care & Use Committee and were found to meet the standards for humane animal care and use as set by the Animal Welfare Act and the NIH Guide for the Care and Use of Laboratory Animals.
Induction of trinitrobenzenesulfonic acid (TNBS) Colitis
2,4,6-Trinitrobenzenesulfonic acid (TNBS; 5% aqueous solution; Sigma) was instilled intrarectally under 4% isoflurane anesthesia as previously described by Morris [Morris et al., 1989] and modified by Appleyard [Appleyard and Wallace, 1995] to induce colonic irritation. Briefly, TNBS (50 mg/ml) dissolved in 50% ethanol (v/v) was administered via a trans-anal approach (total volume 0.5 ml) using a PE-90 catheter whose tip was placed approximately 6 cm proximal to the anal verge. Control animals received 0.5 ml of normal saline. Surgilube (E. Fougera & Co., Melville, NY) was applied to the perineum to minimize any potential contaminant irritation due to anal leakage. This model of colitis is characterized by local areas of acute inflammation peaking at 4–7 days and is followed by a chronic, mononuclear inflammatory cell infiltrate that persists up to six weeks until it resolves without spontaneous relapse.
Metabolic Cage Measurements
In these experiments, micturition patterns (voided volume and frequency) were measured in metabolic cages over a 24 hr period in awake rats 60–90 days after the induction of TNBS colitis as described above (n=7) or intracolonic saline (n=8). This is considered the “healed” phase of TNBS colitis as stool size and frequency normalize. A period of 60–90 days was utilized to assure that the chronic (active) phase of the colitis had resolved. Because measurement of urine frequency and voided volume in a metabolic cage requires separation of liquid urine and solid stool, the 60–90 day time frame assured clinical resolution of the colitis (as characterized by solid stools) and permitted 24 hr urine measurement (which mandates formed stools).
Animals were placed in the metabolic cages at approximately the same time during each assessment (10 am) to control for diurnal rhythms and variables. At least one control animal littermate was always paired with the treatment animals on any given day (4 total cages) to control for any day-to-day environmental variables. The chambers were housed in a quiet environment, and artificial light was used to replicate the animal’s home cage, day-night cycle (not reversed). The metabolic cages were equipped with a urine collection system that quantitates the urine produced in real time. A computerized data acquisition system was used to collect the data (DATAQ Instruments, Inc., Akron, OH) for later interpretation.
Animals were tested only once or 23+ hrs (<24 hr) in the metabolic cage measurements. Thus, for each animal, 23+ hrs of micturition episodes were recorded. Voided volume was averaged over the course of the 23+ hours. With micturition events numbering more than 14 for most animals in that period, within animal variability was therefore low. Because voiding frequency involves discrete events per unit time, there was no within animal variability without repeating the metabolic cage measurements. Controls were compared with historical controls in and were comparable in all regards.
Mast Cell Histology
Sixty-to-ninety days following intra-rectal TNBS, animals (n=5/group) were euthanized using pentobarbital (100 mg/kg i.p.) (Abbott Laboratories, North Chicago, IL), and urinary bladders were quickly removed and longitudinally transected into 2 equal parts. The part used for histology was fixed overnight in 10% buffered formalin, embedded in paraffin, and sectioned transversely on a sliding microtome at a thickness of 10 μm. Serial sections were mounted and stained with acidified (pH< 2.5) toluidine blue (Sigma, St. Louis, MO). Mast cell numbers and activation (as judged by degranulation and loss of greater than 20% of cellular staining) were determined in a blinded fashion under high power field (hpf) (200x) as previously described [Spanos et al., 1997]. For both control and TNBS treated animals, two random slides each containing 3 sections per slide of the corresponding bladder body were scanned for intact and degranulated mast cells. The two most densely populated mast cell fields from each slide were studied (4 data points per animal), and the results were averaged together for each animal. Within animal variability for the 4 data points ranged from 15–25%. Routine microscopic examination was also conducted in representative tissues stained with hematoxylin/eosin and giemsa stains [Mallory, 1938] to define microscopic morphologic changes if present.
Measurement of Neurotrophic Factors in Pelvic Organs and Associated DRG
Urinary bladders and L6/S1 DRGs (corresponding to lumbosacral afferent input to the urinary bladder and distal colorectum of the rat) were harvested 60–90 days following intra-colonic treatment with TNBS or saline (n=6/group). From each animal both L6 and both S1 DRGs were harvested and pooled according to treatment group. Real-time PCR was used to quantify mRNA levels of stem cell factor (SCF, mast cell growth factor) and nerve growth factor (NGF) in each of these tissues. mRNA expression was analyzed using SYBR Green two-step real-time reverse transcriptase–polymerase chain reaction (PE Applied Biosystems, Foster City, CA). To isolate RNA, frozen tissue samples were placed in 1 ml Trizol reagent (Invitrogen, Carlsbad, CA), homogenized, extracted in chloroform and separated in phase lock gel tubes (Eppendorf, Hamburg, Germany). RNA was precipitated in isopropanol at −20 °C for 1 h then on dry ice for 1 hr and then washed with 75% ethanol and resuspended in water. Extracted RNA was treated with DNase (DNA-free reagent, Ambion, Inc., Austin, TX) to remove genomic DNA, and total RNA concentration was measured using the 260 nm absorbance recorded by a spectrophotometer. Equal aliquots (20 ng) of total RNA from each sample were processed for complementary DNA (cDNA) synthesis using Invitrogen Superscript II reverse transcriptase according to the manufacturer’s instructions. Negative control reactions were run without RNA and without transcriptase to test for contamination
Primers were designed using Primer Express Software (PE Applied Biosystems, Foster City, CA) and purchased from Invitrogen (Carlsbad, CA). GAPDH was used as an endogenous control. SYBR Green PCR amplification was performed using an Applied Biosystems 5700 real-time thermal cycler controlled by a Dell Latitude laptop computer running ABI Prism 7000 SDS software as previously described [Molliver et al., 2005]. Twenty nanograms of cDNA template was added to 50 μl reaction mixtures based on the SYBR Green reagent kit (Applied Biosystems): 1× SYBR Green PCR buffer, 2 mM MgCl2, 200 μM dNTPs, 1.25 U Amplitaq Gold, 0.5 U AmpErase UNG (uracil-N-glycosylase), 250 nM primers. The amplification protocol included 2 min at 50 °C to activate the AmpErase UNG to prevent the re-amplification of any carryover PCR products, 12 min at 95 °C to activate the Amplitaq polymerase, then 40 cycles of 15 s at 95 °C for denaturation and 1 min at 60 °C for annealing and extension. After amplification, a dissociation curve was plotted against melting temperature to ensure amplification of a single product. All samples were run in duplicate, and reactions were run without template and with the reverse-transcriptase negative control reaction products as negative controls. Agarose gel electrophoretic analysis was used to verify the presence of a single product and to ensure that the amplified product corresponded to size predicted for the amplicon.
Primers
Primers for target genes included the following:
| SCF | Sense:
Antisense: |
TGGCGAATCTTCCAAATGACG (5′ TO 3′)
AAACATCCATCCCGGCG |
| NGF | Sense:
Antisense: |
ACTTCAGCATTCCCTTGACACA
ACGGGCAGCTATTGGTTCAG |
| GAPDH | Sense:
Antisense: |
ATGGCACAGTCAAGGCTGAGA
CGCTCCTGGAAGATGGTGAT |
Because, measurement of mRNA required development of methodology including primer development, the refinement and utilization of this technique was later applied. Although metabolic cage studies were not repeated, simultaneous measurement of mast cell histology and bladder mRNA was performed and found to be concordant.
Statistical Analysis
All data are expressed as mean ± SE and were analyzed using GraphPad Prism 3.0 statistical software (San Diego, CA). In the metabolic cage and mast cell quantitation studies, TNBS- and saline-treated animals were compared using unpaired t tests (two-tailed). Where differences in group variances were encountered, the Mann Whitney test was utilized. For the mRNA quantitation studies, mRNA levels were normalized to GADPH, and TNBS-treated animals were compared to the saline controls using a one-way analysis of variance (ANOVA). A p-value < 0.05 was considered significant in all instances.
RESULTS
In general, the TNBS-treated animals showed no signs of a systemic inflammatory response, as they gained weight appropriately and exhibited normal behavioral activities after the resolution of the acute phases of the colitis. There were no fevers, rigors, sepsis or deaths related to cystitis or pyelonephritis. More specifically, at the timepoints evaluated, there were no behavioral abnormalities associated with voiding (i.e. there was no perceivable pain indicators or signs of dysuria or autonomic activation with micturition). Moreover, during retrieval of bladder and colonic tissue, there were no apparent adhesions noted between the 2 organs nor was there any evidence of a locally-spread inflammatory mass (i.e. non-neurogenic inflammation). Gross examination of the urinary bladders did not reveal any macroscopic abnormalities.
As shown in Figure 1, urinary bladder voided volume in awake rats previously treated with intra-colonic TNBS (60–90 days prior) (n=7) was 50% less than saline-treated controls (n=8) (0.62 ± 0.02 mL per void vs. 1.25 ± 0.2 mL per void, respectively) (p<0.005). Within animal variability in voided volume was low and in the range of 15% during the 24 hr recoding period which also includes diurnal contributions. Correspondingly, TNBS treatment increased bladder voiding frequency nearly 50% as compared to controls (0.89 ± 0.08 voids per hour vs. 0.59 ± 0.1 voids per hour, respectively) (p<0.05). Because voiding frequency by definition involves the measurement of discrete events per unit time, there is no within animal variability without repeating the metabolic cage measurements for each animal. Controls, however, were compared with historical controls in the deGroat laboratory (source of the metabolic cages) and were comparable in all regards for animals of the same size and sex.
Figure 1.
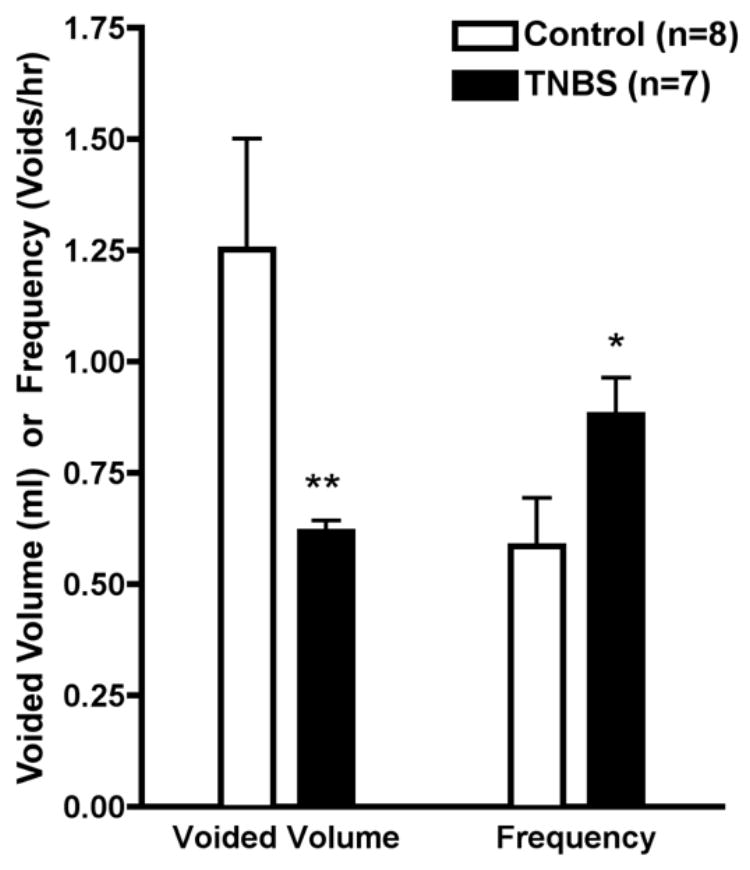
Voiding volume (ml) and frequency (voids/hr) in awake rats previously treated with intra-colonic saline or TNBS 60–90 days prior. (*p< 0.05) (**p<0.005)
Microscopic bladder examination revealed no epithelial damage or polymorphonuclear infiltrate (Figure 2). Mast cells were predominantly located around submucosal and adventitial blood vessels with infiltration of the lamina propria and muscularis propria. A representative photomicrograph for both a control and TNBS-treated rat is shown in Figure 2. While the average number of mast cells were not significantly increased in the TNBS-treated animals (7.1 ± 0.7 per hpf vs 5.4 ± 1.2 per hpf for controls), significant increases in the number and percentage of activated mast cells were observed following TNBS (Figures 3 and 4). The number of activated mast cells increased from 1.05 ± 0.3 per hpf to 2.75 ± 0.5 per hpf (p<0.05), while the percentage of activated mast cells increased from 18.0 ± 7.5% to 37.8 ± 3% (p<0.05).
Figure 2.

Histological localization of urinary bladder mast cells (arrows) in (A) control and (B) TNBS-treated animals (Giemsa, 100x power). U - urothelium; LP - lamina propria; C - capillaries; DM - detrusor muscle.
Figure 3.
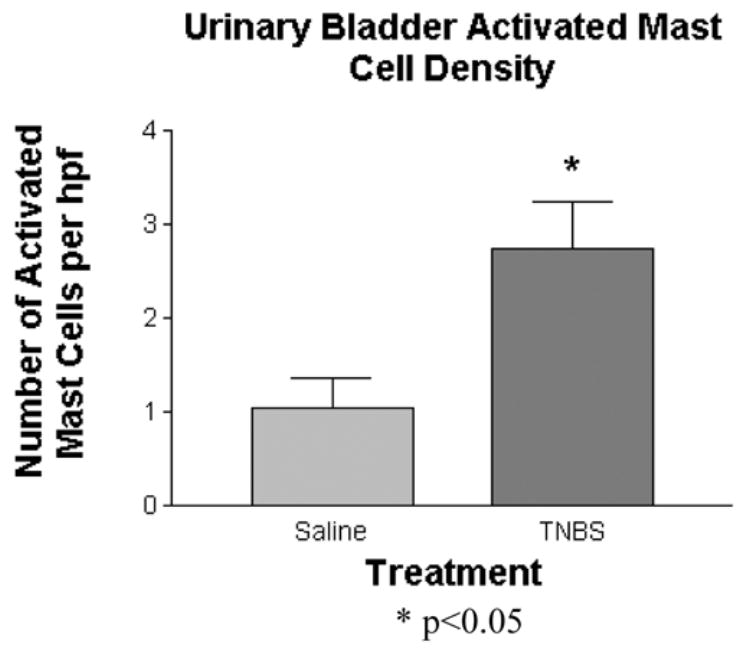
Urinary bladder activated mast cell density following intra-colonic TNBS or saline. The number of activated mast cells per high power field is represented for each group. (*p< 0.05)
Figure 4.

Percentage of activated urinary bladder mast cells following intra-colonic TNBS or saline. (*p< 0.05)
Following the resolution of TNBS colitis, urinary bladder mRNA expression of SCF, the neurotrophic and mast cell stimulating factor, was increased 2-fold (1.0 ± 0.2 vs. 2.0 ± 0.3) (p<0.05) (Figure 5). However, in the L6-S1 DRGs, the common source of bowel and bladder afferent projections, SCF mRNA levels were no different from controls.
Figure 5.
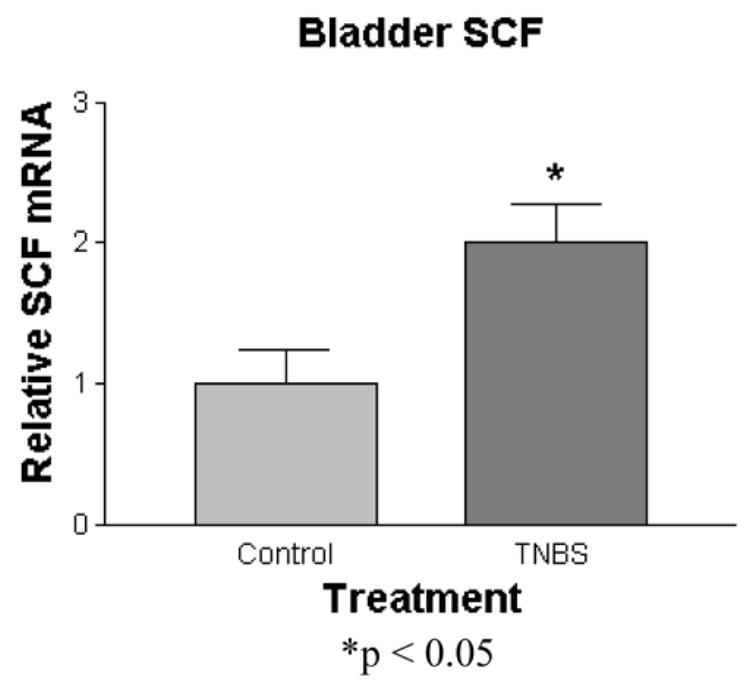
Quantitation of urinary bladder mRNA levels of SCF following intra-colonic TNBS or saline. Treatment groups are normalized to controls. (*p< 0.05)
Similar to SCF, urinary bladder mRNA expression of NGF was significantly elevated (nearly 2-fold) (1.0 ± 0.05 vs. 1.8 ± 0.1) (p<0.01) (Figure 6). Although L6-S1 DRG NGF mRNA levels tended to increase with time, they did so non-significantly (data not shown).
Figure 6.

Quantitation of urinary bladder mRNA levels of NGF following intra-colonic TNBS or saline. Treatment groups are normalized to controls. (*p<0.01)
DISCUSSION
These studies performed in female Sprague-Dawley rats subjected to intracolonic TNBS infusion support the development of chronic, cross-organ sensitization in the pelvis as evidenced by changes in physiology, mast cell histology, and neurotrophin expression in an untreated pelvic organ (urinary bladder). The intimate relationship between mast cell migration, activation, and sensitization, expression of multiple neurotrophic, mast cell stimulatory, and afferent sensitizing factors, and their collective affects upon physiologic pelvic organ function may help further identify some of the putative pathways involved in the induction, maintenance, reactivation, and perhaps overlap of CPP disorders. These findings are both complementary and a natural progression of previous work performed in this laboratory demonstrating that acute cystitis can lower colorectal sensory thresholds to balloon distension and that acute colitis can produce acute irritative micturition patterns and sensitize bladder afferents to mechanical and chemical stimuli [Pezzone et al., 2005; Ustinova et al., 2006]. In these initial studies, the development of pelvic organ cross-sensitization in the acute setting suggested a role for, and subsequent modulation of, pre-existing afferent pathways in the pelvis. With chronic pelvic organ irritation and continued activation of these pelvic cross-organ reflexes (as would occur in the sub-acute and chronic phases of this model of TNBS colitis--up to 6 weeks), one might expect to see chronic changes in pelvic organ physiologic function, pelvic organ afferent processing (both direct and indirect via converging pathways), and pelvic organ morphology, changes reflecting afferent sensitization and perhaps neurogenic inflammation, as shown previously for the bladder by Dupont and colleagues [Dupont et al., 2001] following direct chemical, immune, and mechanical bladder irritation. Although in Dupont’s studies, cross-organ sensitization was not assessed by design [Dupont et al., 2001], it was hypothesized that the neuroplasticity observed in these models of direct bladder irritation could explain the long-term symptoms and pain seen even after resolution of bladder inflammation. Thus, in these and other models of direct pelvic organ irritation, one may hypothesize that the associated neuroplasticity may not necessarily be limited to the irritated organ itself and may involve other pelvic organs as our studies suggest.
In Figure 1, we show that following the induction and clinical resolution of TNBS colitis rats exhibited micturition patterns that were consistent with the development of chronic neurogenic cystitis as manifested by a 50% decrease in voided volume and 50% increase in voiding frequency. Macroscopic and microscopic evaluation of the bladder in this setting revealed the absence of gross or histologic bladder damage as contributing factors. Moreover, there were no apparent adhesions or any evidence of a locally-spread inflammatory mass between the bladder and other pelvic organs such as the colon (i.e. non-neurogenic or transmural irritation). The bladder, a relatively small pelvic organ lying low and anterior in the pelvis and shielded from the colon by the uterus, was at least 2–3 cm away from the foci of the treated colonic segment, which was 6 cm from the anal verge and, by default, very posterior in the abdominal cavity. Supporting a cross-organ, neurogenic etiology, acute studies of pelvic organ cross-sensitization performed in our laboratory demonstrated immediate sensitization of the urinary bladder and external urethral sphincter following acute colonic irritation with TNBS [Pezzone et al., 2005]. Furthermore, follow-up studies in our laboratory employing single unit C-fiber bladder afferent recording revealed that acute colonic irritation (1 hr post TNBS) is capable of sensitizing urinary bladder afferents to mechanical and chemical stimuli, an effect that was prevented by disruption of the neural input to the bladder and suggesting a direct afferent pathway from the colon [Ustinova, et al., 2006]. Although no one has previously shown evidence of cystitis following irritation of other pelvic organs, neurogenic cystitis induced in the central nervous system by pseudorabies virus has been previously reported and was likewise preventable by bladder denervation [Jasmin et al., 1998].
Coincident with the development of chronic cystitis following the induction and resolution of TNBS colitis, the number and percentage of activated urinary bladder mast cells were significantly increased. This finding of enhanced mast cell activation in this setting is not unexpected as a contributing role of mast cells in IC [Theoharides and Sant, 1994] and IBS [Weston et al., 1993; O’Sullivan et al., 2000; Santos et al., 2005] and in other disorders characterized by hyperalgesia and neurogenic inflammation has been previously implicated [Theoharides and Cochrane, 2004]. There is a preponderance of evidence implicating potential pathophysiologic mast cell-nerve interactions in the sensitization of visceral afferent nerves as the mast cell possesses a formidable armamentarium of nociceptive molecules including adenosine phosphates, bradykinin, histamine, leukotrienes, potassium, lymphokines, tumor necrosis factor (TNF), and prostaglandins [Theoharides and Sant, 1991]. Anatomic evidence further supporting this mast cell-mediated modulation of afferent nerve function is apparent in the close apposition of mast cells to nerve fibers in both the human gastrointestinal tract [Newson et al., 1983; Stead et al., 1989] and the urinary bladder [Letourneau et al., 1996]. Interestingly and also reflective of our findings, the CNS-induced neurogenic cystitis following pseudorabies virus infection was also associated with bladder mast cell degranulation in the rat [Jasmin et al., 2000]. The lack of a significant increase in total urinary bladder mast cells in this chronic setting (despite having increased numbers of activated mast cells) might be explained by the fact that completely degranulated mast cells are often difficult to visualize histologically and may have been missed during total mast cell quantitation [Theoharides, 1996].
The etiology and potential physiologic significance of closely juxtaposed mast cells and nerve fibers as observed in the urinary bladder, gastrointestinal tract and other tissues [Keith et al., 1995] has become apparent following the identification of a mast cell growth factor [Huang et al., 1990; Williams et al., 1990; Zsebo et al., 1990] responsible for proliferation of immature and mature mast cells, regulation of their migration, differentiation, and survival, and secretion of their mediators by IgE-dependent and independent mechanisms [Galli et al., 1993]. Mast cell growth factor, also known as stem cell factor (SCF), named for its ability to promote hematopoiesis and mast cell development, binds to a receptor with inherent tyrosine kinase activity encoded by the c-kit proto-oncogene [Chabot et al., 1988; Geissler et al., 1988] and hence, has also been termed kit ligand [Galli et al., 1994]. Signal transduction pathways involving phosphatidylinositiol-3-kinase, Src, JAK/STAT, and Ras-Raf-MAP kinase are activated following the binding of SCF to its c-kit receptor [Linnekin, 1999]. SCF has been identified in the CNS (cortical neurons and microglia), smooth muscle cells of the bladder, cervix, uterus, gastrointestinal tract, striated and cardiac muscle, bone marrow fibroblasts, endothelial cells, and Sertoli cells, while c-kit has likewise been detected in the CNS (hippocampus, cerebellum, dorsal horn of the spinal cord, and microglia) and in mast cells (especially those in the lung, urinary bladder, uterus and gastrointestinal tract), hematopoietic tissue, gonads, and skin [Lammie et al., 1994; Becich et al., 1995]. In our studies, urinary bladder SCF was increased 2-fold following intracolonic TNBS (Figure 6). As SCF has been previously identified in bladder smooth muscle cells, it is likely these cells that are responsible for SCF production in response to intra-colonic TNBS, given the lack of increase in the DRGs. Release of SCF by smooth muscle cells could lead to acute and chronic mast cell migration and sensitization, facilitating degranulation and/or chronic mediator release, all of which could account for the physiologic sequelae observed in our studies and postulated to occur in clinical visceral pain syndromes.
Literature supporting a neuronal role of SCF involving neuron survival and regeneration has been confirmed, as studies have shown that the production of neurotrophic factors such as NGF and cytokines such as IL-1β, IL-6, and TNF-α by microglia are up-regulated by the administration of exogenous SCF, a factor whose secretion may be up-regulated physiologically following neuronal injury [Zhang S-C and Fedoroff, 1996]. Cerebral hypoxia and ischemia are thought to stimulate neurogenesis through trophic factors such as SCF in the CNS [Jin et al., 2002]. SCF has also been shown to be a neurotrophic factor (synergistic with NGF) for DRG neurons, playing a role in neurite outgrowth and regeneration, guiding axons from the DRG [Hirata et al., 1993; Carnahan et al., 1994; Lourenssen et al., 2000]. Moreover, further characterization of these c-kit-positive neurons in the DRG revealed that many contained SP (44%), terminated in spinal cord laminae I and II, and were NGF-responsive, suggesting a nociceptive role as C-fiber afferents [Hirata et al., 1995]. The importance and complexity of these interactions between sensory neurons and SCF is further evident in the finding that SP can induce production of SCF, which, in turn, can up-regulate SP receptors of the NK-1 subtype in bone marrow stroma [Rameshwar and Gascon, 1995]. Hence, SCF may play an important role in afferent sensitization and or neurogenic inflammation.
In conclusion, the development of an animal model capable of assessing the chronic effects of isolated pelvic organ irritation on other pelvic organs with convergent afferent input is important in addressing the etiology of chronic pelvic pain and the overlap of chronic pelvic pain disorders. The combined measurement of in vivo bladder physiologic function (micturition patterns) and the quantitation and determination of urinary bladder mast cell activation along with the measurement of neurotrophic factors, mast cell growth and stimulatory factors, and afferent sensitizing factors in both the urinary bladder and in its associated DRG following chronic irritation of the colon has helped to preliminarily identify some of the mechanisms involved in the development of pelvic visceral cross-sensitization and specifically including the potential roles of mast cell-nerve interactions in the sensitization of visceral nociceptive pathways. Thus, the direct and continued irritation of one pelvic organ (colon) and consequential stimulation of its afferent input may lead to neurogenic stimulation and/or indirect sensitization of another pelvic organ with convergent afferent input (bladder). Upregulation of neurotrophic factors in the non-irritated organ (bladder) may account for end-organ changes and afferent nerve cross-sensitivity in the non-irritated pelvic organ. Importantly, once initiated, cross-organ sensitization might be self-supporting, with both organ systems positively feeding back upon each other, resulting in disease progression in the absence of continued insult. Although afferent sensitization was not specifically assessed in these current studies, future studies aimed at evaluating peripheral and central nociceptive pathways in this and similar models of CPP may help further elucidate the pathophysiolgic mechanisms involved in CPP disorders. In addition, these findings also suggest a possible future role of intentional cross-organ sensitization in model development for the study of potential therapeutics for neurogenic cystitis or colitis in the absence of a direct insult to the mucosa or deeper layers of the organ of interest. Clearly, these findings need to be studied in greater detail as well as in male rats to determine if such responses are sex specific.
Acknowledgments
This work was supported by National Institutes of Health Grants DK02488 and DK066658 (to M.A.P.). Use of metabolic cages for the measurement of urinary voiding patterns was generously provided by Dr. William C. de Groat, Ph.D., University of Pittsburgh, Department of Pharmacology.
References
- Alagiri M, Chottiner S, Ratner V, et al. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology. 1997;49s:52–57. doi: 10.1016/s0090-4295(99)80332-x. [DOI] [PubMed] [Google Scholar]
- Appleyard CB, Wallace JL. Reactivation of hapten-induced colitis and its prevention by anti-inflammatory drugs. Am J Physiol. 1995;269:G119–G125. doi: 10.1152/ajpgi.1995.269.1.G119. [DOI] [PubMed] [Google Scholar]
- Becich MJ, Nomoto M, Inman MG, et al. c-Kit and stem cell factor (mast cell growth factor) expression in interstitial cystitis: Clues to the pathogenesis of detrusor mastocytosis and nerve fiber proliferation. Lab Invest. 1995;72:72A. [Google Scholar]
- Carnahan JF, Patel DR, Miller JA. Stem cell factor is a neurotrophic factor for neural crest-derived chick sensory neurons. J Neurosci. 1994;14:1433–1440. doi: 10.1523/JNEUROSCI.14-03-01433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot B, Stephenson DA, Chapman VM, et al. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- Dmitrieva N, Berkley KJ. Contrasting effects of WIN 55212-2 on Motility of the Rat Bladder and Uterus. J Neurosci. 2002;22:7147–7153. doi: 10.1523/JNEUROSCI.22-16-07147.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont MC, Spitsbergen JM, Kim KB, et al. Histological and neurotrophic changes triggered by varying models of bladder inflammation. J Urol. 2001;166:1111–1118. [PubMed] [Google Scholar]
- Fitzpatrick CC, DeLancey JOL, Elkins TE, et al. Vulvar vestibulitis and interstitial cystitis: A disorder of urogenital-derived epithelium? Obstet Gynecol. 1993;81:860–862. [PubMed] [Google Scholar]
- Galli SJ, Tsai M, Wershil B. The c-kit receptor, stem cell factor, and mast cells. Am J Pathol. 1993;142:965–974. [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Zsebo KM, Geissler EN. The kit ligand, stem cell factor. Adv Immunol. 1994;55:1–96. doi: 10.1016/s0065-2776(08)60508-8. [DOI] [PubMed] [Google Scholar]
- Geissler EN, Ryman MA, Housman DE. The dominant white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Giamberardino MA. Recent and forgotten aspects of visceral pain. European J Pain. 1999;3:77–92. doi: 10.1053/eujp.1999.0117. [DOI] [PubMed] [Google Scholar]
- Gibson TE. Bladder symptoms due to silent sigmoid disease. Am J Surgery. 1969;117:742–744. doi: 10.1016/0002-9610(69)90420-6. [DOI] [PubMed] [Google Scholar]
- Hirata T, Kasugai T, Morii E, et al. Characterization of c-kit-positive neurons in the dorsal root ganglion of the mouse. Dev Brain Res. 1995;85:201–211. doi: 10.1016/0165-3806(94)00205-e. [DOI] [PubMed] [Google Scholar]
- Hirata T, Morii E, Morimoto M, et al. Stem cell factor induces outgrowth of c-kit-positive neurites and supports the survival of c-kit-positive neurons in dorsal root ganglia of mouse embryos. Development. 1993;119:49–56. doi: 10.1242/dev.119.1.49. [DOI] [PubMed] [Google Scholar]
- Hogston P. Irritable bowel syndrome as a cause of chronic pain in women attending a gynaecology clinic. Brit Med J. 1987;294:934–935. doi: 10.1136/bmj.294.6577.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E, Nocka K, Beier DR, et al. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Janni G, Manz HJ, et al. Activation of CNS circuits producing a neurogenic cystitis: Evidence for centrally induced peripheral inflammation. J Neurosci. 1998;18:10016–10029. doi: 10.1523/JNEUROSCI.18-23-10016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin L, Janni G, Ohara PT, et al. CNS induced neurogenic cystitis is associated with bladder mast cell degranulation in the rat. J Urol. 2000;164:852–855. doi: 10.1097/00005392-200009010-00061. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao XO, Sun YJ, et al. Stem cell factor stimulates neurogenesis in vitro and in vivo. J Clin Invest. 2002;110:311–319. doi: 10.1172/JCI15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith IM, Jin J, Saban R. Nerve-mast cell interaction in normal guinea pig urinary bladder. J Comp Neurol. 1995;363:28–36. doi: 10.1002/cne.903630104. [DOI] [PubMed] [Google Scholar]
- Koziol JA. Epidemiology of interstitial cystitis. Urol Clin N Am. 1994;21:7–20. [PubMed] [Google Scholar]
- Lammie A, Drobnjak M, Gerald W, et al. Expression of c-kit and kit ligand proteins in normal human tissues. J Histochem Cytochem. 1994;42:1417–1425. doi: 10.1177/42.11.7523489. [DOI] [PubMed] [Google Scholar]
- Letourneau R, Pang X, Sant GR, et al. Intragranular activation of bladder mast cells and their association with nerve processes in interstitial cystitis. Brit J Urology. 1996;77:41–54. doi: 10.1046/j.1464-410x.1996.08178.x. [DOI] [PubMed] [Google Scholar]
- Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int J Biochem Cell Bio. 1999;31:1053–1074. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- Lourenssen S, Motro B, Bernstein A, et al. Defects in sensory nerve numbers and growth in mutant Kit and Steel mice. Neuroreport. 2000;11:1159–1165. doi: 10.1097/00001756-200004270-00004. [DOI] [PubMed] [Google Scholar]
- Mallory FB. Pathological Technique. Philadelphia: Saunders; 1938. p. 195. [Google Scholar]
- Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: Prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321–337. doi: 10.1016/0029-7844(95)00458-0. [DOI] [PubMed] [Google Scholar]
- Moldwin RM. Similarities between interstitial cystitis and male chronic pelvic pain syndrome. Curr Urol Rep. 2002;3:313–318. doi: 10.1007/s11934-002-0056-x. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Lindsay J, Albers KM, et al. Overexpression of NGF or GDNF alters transcriptional plasticity evoked by inflammation. Pain. 2005;113 :277–284. doi: 10.1016/j.pain.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Morris GP, Beck MS, Herridge MS, et al. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- Newson B, Dahlstrom A, Enerback L, et al. Suggestive evidence for a direct innervation of mucosal mast cells. Neuroscience. 1983;10:565–570. doi: 10.1016/0306-4522(83)90153-7. [DOI] [PubMed] [Google Scholar]
- Novi JM, Jeronis S, Srinivas S, et al. Risk of irritable bowel syndrome and depression in women with interstitial cystitis: A case-control study. J Urology. 2005;174:937–940. doi: 10.1097/01.ju.0000169258.31345.5d. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Clayton N, Breslin NP, et al. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Mot. 2000;12:449–457. doi: 10.1046/j.1365-2982.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: Implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128:1953–1964. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Prior A, Wilson K, Whorwell PJ, et al. Irritable bowel syndrome in the gynecological clinic. Survey of 798 new referrals. Digest Dis Sci. 1989;34:1820–1824. doi: 10.1007/BF01536698. [DOI] [PubMed] [Google Scholar]
- Rameshwar P, Gascon P. Substance P (SP) mediates production of stem cell factor and interleukin-1 in bone marrow stroma: Potential autoregulatory role for these cytokines in SP receptor expression and induction. Blood. 1995;86:482–490. [PubMed] [Google Scholar]
- Ratner V. Interstitial cystitis: A chronic inflammatory bladder condition. World J Urol. 2001;19:157–159. doi: 10.1007/pl00007096. [DOI] [PubMed] [Google Scholar]
- Santos J, Guilarte M, Alonso C, et al. Pathogenesis of irritable bowel syndrome: The mast cell connection. Scand J Gastroenterol. 2005;40:129–140. doi: 10.1080/00365520410009410. [DOI] [PubMed] [Google Scholar]
- Spanos C, Pang X, Ligris K, et al. Stress-induced bladder mast cell activation: Implications for interstitial cystitis. J Urology. 1997;157:669–672. [PubMed] [Google Scholar]
- Stead RH, Dixon MF, Nigel HB, et al. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989;97:575–585. doi: 10.1016/0016-5085(89)90627-6. [DOI] [PubMed] [Google Scholar]
- Theoharides TC. The mast cell: A neuroimmunoendocrine master player. Int J Tissue React. 1996;18:1–21. [PubMed] [Google Scholar]
- Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Sant GR. Bladder mast cell activation in interstitial cystitis. Semin Urol. 1991;9:74–87. [PubMed] [Google Scholar]
- Theoharides TC, Sant GR. The role of the mast cell in interstitial cystitis. Urol Clin North Am. 1994;21:41–53. [PubMed] [Google Scholar]
- Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. In: Drossman DA, Corazziari E, Talley N, Thompson WG, Whitehead WE, McLean VA, editors. The Functional Gastrointestinal Disorders. Degnon Associates; 2000. pp. 351–375. [Google Scholar]
- Ustinova EE, Fraser MO, Pezzone MA. Colonic irritation in the rat sensitizes urinary bladder afferents to mechanical and chemical stimuli: An afferent origin of pelvic organ cross-sensitization. Am J Physiol Renal Physiol. 2006;290:F1478–87. doi: 10.1152/ajprenal.00395.2005. [DOI] [PubMed] [Google Scholar]
- Walker EA, Gelfand AN, Gelfand MD, et al. Chronic pelvic pain and gynecological symptoms in women with irritable bowel syndrome. J Psychosom Obst Gyn. 1996;17:39–46. doi: 10.3109/01674829609025662. [DOI] [PubMed] [Google Scholar]
- Walker EA, Katon WJ, Jemelka R. The prevalence of chronic pelvic pain and irritable bowel in two university clinics. J Psychosom Obst Gyn. 1991;12s:65–70. [Google Scholar]
- Wesselmann U. Neurogenic inflammation and chronic pelvic pain. World J Urol. 2001;19:180–1855. doi: 10.1007/s003450100201. [DOI] [PubMed] [Google Scholar]
- Weston AP, Biddle WL, Bhatia PS, et al. Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig Dis Sci. 1993;38:1590–1595. doi: 10.1007/BF01303164. [DOI] [PubMed] [Google Scholar]
- Whorwell PJ, McCallum M, Creed FH, et al. Non-colonic features of irritable bowel syndrome. Gut. 1986;27:37–40. doi: 10.1136/gut.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DE, Eisenman J, Baird A, et al. Identification of a ligand for the c-kit proto-oncogene. Cell. 1990;63:167–174. doi: 10.1016/0092-8674(90)90297-r. [DOI] [PubMed] [Google Scholar]
- Zhang S-C, Fedoroff S. Neuron-microglia interactions in vitro. Acta Neuropathol. 1996;91:385–95. doi: 10.1007/s004010050440. [DOI] [PubMed] [Google Scholar]
- Zondervan K, Yudkin PL, Vessey MP, et al. The community prevalence of chronic pelvic pain in women and associated illness behavior. Brit J Gen Pract. 2001;51:541–547. [PMC free article] [PubMed] [Google Scholar]
- Zsebo KM, Wypych J, McNiece IK, et al. Identification, purification, and biological characterization of hematopoietic stem cell factor from Buffalo rat liver-conditioned medium. Cell. 1990;63:195–201. doi: 10.1016/0092-8674(90)90300-4. [DOI] [PubMed] [Google Scholar]


