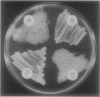
Abstract
In previous work, Rhizobium meliloti SU47 produced its alternative exopolysaccharide (EPSb [also called EPS II]) only in strains that were genetically altered to activate EPSb synthesis. Here we report that EPSb synthesis is not entirely cryptic but occurred under conditions of limiting phosphate. This was shown in several different exo mutants that are blocked in the synthesis of the normal exopolysaccharide, succinoglycan. In addition, EPSb biosynthetic gene expression was markedly increased by limiting phosphate. An apparent regulatory mutant that does not express alkaline phosphatase activity was unable to produce EPSb under these conditions. A mucR mutant that was previously shown to produce EPSb instead of the normal exopolysaccharide, succinoglycan, was not sensitive to phosphate inhibition of EPSb synthesis. No evidence was found to indicate that exoX, which affects succinoglycan synthesis, had any influence on EPSb synthesis. In contrast to limiting phosphate, limiting nitrogen or sulfur did not stimulate EPSb synthesis as it does succinoglycan.
Full text
PDF
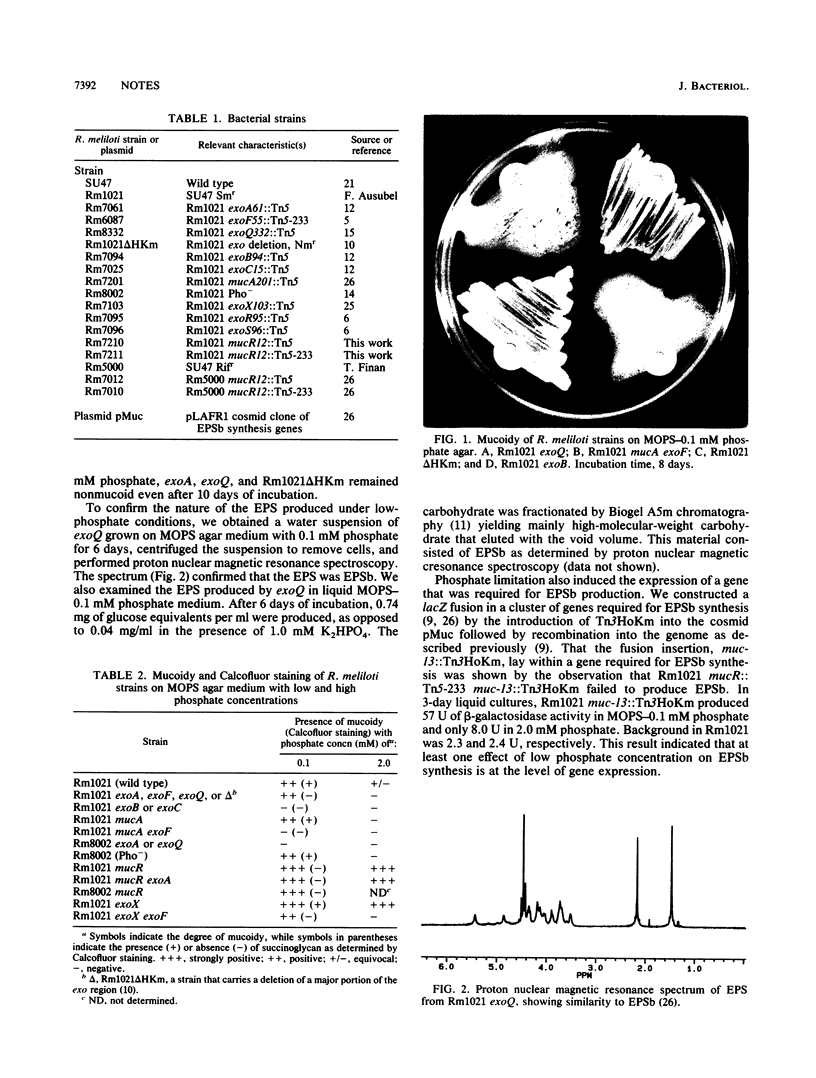
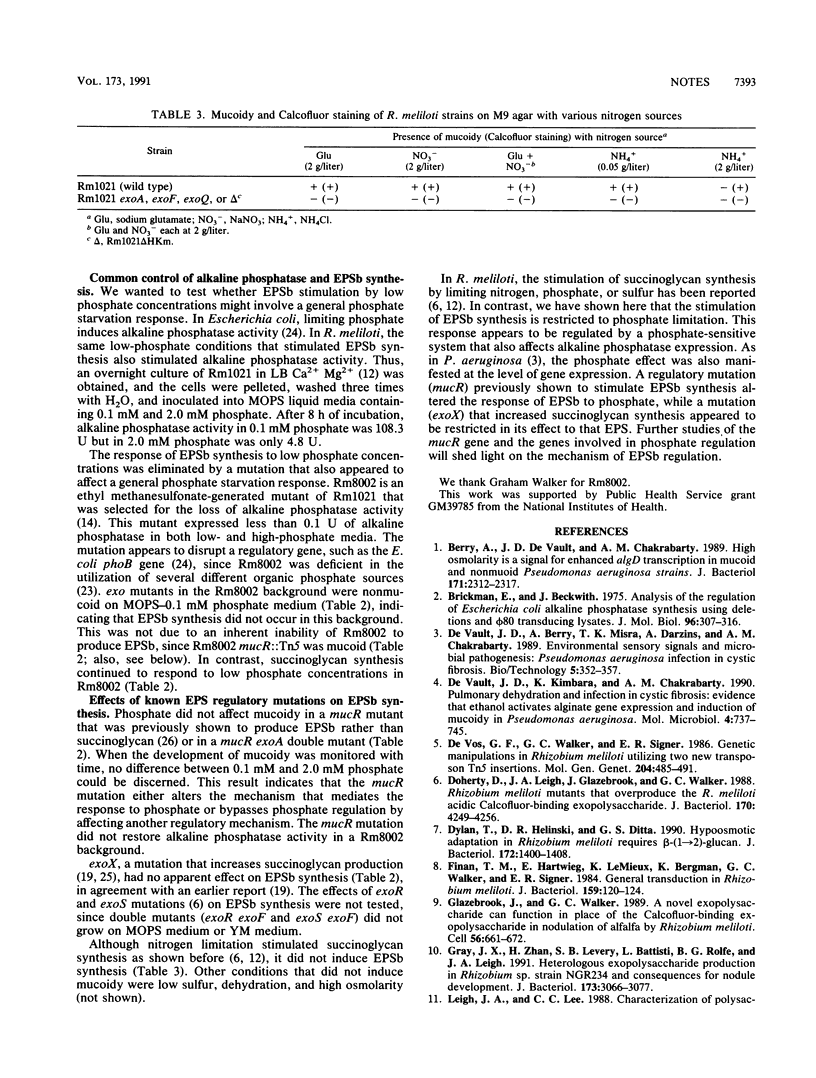

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry A., DeVault J. D., Chakrabarty A. M. High osmolarity is a signal for enhanced algD transcription in mucoid and nonmucoid Pseudomonas aeruginosa strains. J Bacteriol. 1989 May;171(5):2312–2317. doi: 10.1128/jb.171.5.2312-2317.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman E., Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and phi80 transducing phages. J Mol Biol. 1975 Aug 5;96(2):307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- De Vos G. F., Walker G. C., Signer E. R. Genetic manipulations in Rhizobium meliloti utilizing two new transposon Tn5 derivatives. Mol Gen Genet. 1986 Sep;204(3):485–491. doi: 10.1007/BF00331029. [DOI] [PubMed] [Google Scholar]
- DeVault J. D., Kimbara K., Chakrabarty A. M. Pulmonary dehydration and infection in cystic fibrosis: evidence that ethanol activates alginate gene expression and induction of mucoidy in Pseudomonas aeruginosa. Mol Microbiol. 1990 May;4(5):737–745. doi: 10.1111/j.1365-2958.1990.tb00644.x. [DOI] [PubMed] [Google Scholar]
- Doherty D., Leigh J. A., Glazebrook J., Walker G. C. Rhizobium meliloti mutants that overproduce the R. meliloti acidic calcofluor-binding exopolysaccharide. J Bacteriol. 1988 Sep;170(9):4249–4256. doi: 10.1128/jb.170.9.4249-4256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylan T., Helinski D. R., Ditta G. S. Hypoosmotic adaptation in Rhizobium meliloti requires beta-(1----2)-glucan. J Bacteriol. 1990 Mar;172(3):1400–1408. doi: 10.1128/jb.172.3.1400-1408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Hartweig E., LeMieux K., Bergman K., Walker G. C., Signer E. R. General transduction in Rhizobium meliloti. J Bacteriol. 1984 Jul;159(1):120–124. doi: 10.1128/jb.159.1.120-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J., Walker G. C. A novel exopolysaccharide can function in place of the calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell. 1989 Feb 24;56(4):661–672. doi: 10.1016/0092-8674(89)90588-6. [DOI] [PubMed] [Google Scholar]
- Gray J. X., Zhan H. J., Levery S. B., Battisti L., Rolfe B. G., Leigh J. A. Heterologous exopolysaccharide production in Rhizobium sp. strain NGR234 and consequences for nodule development. J Bacteriol. 1991 May;173(10):3066–3077. doi: 10.1128/jb.173.10.3066-3077.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. A., Lee C. C. Characterization of polysaccharides of Rhizobium meliloti exo mutants that form ineffective nodules. J Bacteriol. 1988 Aug;170(8):3327–3332. doi: 10.1128/jb.170.8.3327-3332.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levery S. B., Zhan H., Lee C. C., Leigh J. A., Hakomori S. Structural analysis of a second acidic exopolysaccharide of Rhizobium meliloti that can function in alfalfa root nodule invasion. Carbohydr Res. 1991 Mar 20;210:339–347. doi: 10.1016/0008-6215(91)80135-a. [DOI] [PubMed] [Google Scholar]
- Long S., McCune S., Walker G. C. Symbiotic loci of Rhizobium meliloti identified by random TnphoA mutagenesis. J Bacteriol. 1988 Sep;170(9):4257–4265. doi: 10.1128/jb.170.9.4257-4265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S., Reed J. W., Himawan J., Walker G. C. Genetic analysis of a cluster of genes required for synthesis of the calcofluor-binding exopolysaccharide of Rhizobium meliloti. J Bacteriol. 1988 Sep;170(9):4239–4248. doi: 10.1128/jb.170.9.4239-4248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. W., Capage M., Walker G. C. Rhizobium meliloti exoG and exoJ mutations affect the exoX-exoY system for modulation of exopolysaccharide production. J Bacteriol. 1991 Jun;173(12):3776–3788. doi: 10.1128/jb.173.12.3776-3788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Biosynthesis of microbial exopolysaccharides. Adv Microb Physiol. 1982;23:79–150. doi: 10.1016/s0065-2911(08)60336-7. [DOI] [PubMed] [Google Scholar]
- Zhan H. J., Leigh J. A. Two genes that regulate exopolysaccharide production in Rhizobium meliloti. J Bacteriol. 1990 Sep;172(9):5254–5259. doi: 10.1128/jb.172.9.5254-5259.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan H. J., Levery S. B., Lee C. C., Leigh J. A. A second exopolysaccharide of Rhizobium meliloti strain SU47 that can function in root nodule invasion. Proc Natl Acad Sci U S A. 1989 May;86(9):3055–3059. doi: 10.1073/pnas.86.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]