Abstract
Background
Although adverse neuropsychological and neurological health effects are well known among workers with high manganese (Mn) exposures in mining, ore‐processing and ferroalloy production, the risks among welders with lower exposures are less well understood.
Methods
Confined space welding in construction of a new span of the San Francisco–Oakland Bay Bridge without adequate protection was studied using a multidisciplinary method to identify the dose–effect relationship between adverse health effects and Mn in air or whole blood. Bridge welders (n = 43) with little or no personal protection equipment and exposed to a welding fume containing Mn, were administered neurological, neuropsychological, neurophysiological and pulmonary tests. Outcome variables were analysed in relation to whole blood Mn (MnB) and a Cumulative Exposure Index (CEI) based on Mn‐air, duration and type of welding. Welders performed a mean of 16.5 months of welding on the bridge, were on average 43.8 years of age and had on average 12.6 years of education.
Results
The mean time weighted average of Mn‐air ranged from 0.11–0.46 mg/m3 (55% >0.20 mg/m3). MnB >10 µg/l was found in 43% of the workers, but the concentrations of Mn in urine, lead in blood and copper and iron in plasma were normal. Forced expiratory volume at 1s: forced vital capacity ratios (FEV1/FVC) were found to be abnormal in 33.3% of the welders after about 1.5 years of welding at the bridge. Mean scores of bradykinesia and Unified Parkinson Disease Rating Scale exceeded 4 and 6, respectively. Computer assisted tremor analysis system hand tremor and body sway tests, and University of Pennsylvania Smell Identification Test showed impairment in 38.5/61.5, 51.4 and 88% of the welders, respectively. Significant inverse dose–effect relationships with CEI and/or MnB were found for IQ (p⩽0.05), executive function (p⩽0.03), sustaining concentration and sequencing (p⩽0.04), verbal learning (p⩽0.01), working (p⩽0.04) and immediate memory (p⩽0.02), even when adjusted for demographics and years of welding before Bay Bridge. Symptoms reported by the welders while working were: tremors (41.9%); numbness (60.5%); excessive fatigue (65.1%); sleep disturbance (79.1%); sexual dysfunction (58.1%); toxic hallucinations (18.6%); depression (53.5%); and anxiety (39.5%). Dose–effect associations between CEI and sexual function (p<0.05), fatigue (p<0.05), depression (p<0.01) and headache (p<0.05) were statistically significant.
Conclusions
Confined space welding was shown to be associated with neurological, neuropsychological and pulmonary adverse health effects. A careful enquiry of occupational histories is recommended for all welders presenting with neurological or pulmonary complaints, and a more stringent prevention strategy should be considered for Mn exposure due to inhalation of welding fume.
Manganese (Mn) exposure through welding fume has been reported to cause parkinsonian syndrome, sometimes described as welding fume‐related parkinsonism and often misdiagnosed as Parkinson's disease because of similarities in neurological features including tremor, masked facies and generalised bradykinesia.1 However, important differences in movement disturbance between Mn‐induced parkinsonism (also called manganism) and idiopathic Parkinson's disease (IPD) include an awkward high‐stepping dystonic gait in manganism, which is in stark contrast with the typical shuffling gait in patients with IPD, and the tendency to lose balance by falling backward in IPD and forward in manganism. Additionally, tremor characteristics are different, usually resting tremor in IPD and postural intention tremor in manganism.1 Magnetic resonance imaging (MRI) may be used to differentiate IPD from Mn‐induced parkinsonism if a patient has had excess Mn exposure within the previous 6 months.2 Positive MRIs can show cerebral Mn2+ deposition in both animals and humans, especially in the globus pallidus (possibly in the striatum), by exhibiting a T1‐weighted signal hyperintensity.3 IPD, however, is associated with lesions in the substantia nigra pars compacta, and does not exhibit MRI abnormality in the globus pallidus. Two other key features differentiating between Mn‐induced parkinsonism and IPD are: (1) a younger age of onset for Mn‐exposed workers and (2) little or no response to l‐dopa among Mn‐induced parkinsonism cases.4,5 Case reports of neurological findings in career welders exposed to Mn have shown dystonia bilaterally in the shoulders and four distal limbs, as well as other parkinsonian features—for example, tremor and postural instability.5,6
Welders have been reported to be exposed to “a wide variety of potential respiratory hazards”,7,8 although earlier studies did not show increases in chronic bronchitis.9 However, shipyard welders have been reported to have a higher standardised mortality ratio for lung cancer, a finding which was also supported by the California Occupational Mortality Survey, after adjustment for smoking and asbestos exposure. Sjögren presented evidence of a causal relationship between exposure to stainless steel welding and lung cancer.10
Diagnosis of Mn intoxication in workers actively exposed to excessive Mn in welding fumes can be further supported by biomarkers of exposure indicating increased internal Mn concentrations—for example, in whole blood and to a lesser extent in plasma/serum or urine. Sjögren et al11 reported a mean concentration of 8.4 μg/l for whole blood Mn (MnB) in welders, whereas in other industrial settings the mean MnB ranged from 8.1 to 25.3 µg/l. No clear cutoff value of MnB is currently agreed upon, but HAR postulates that any values >10 µg/l is of concern.
Mn air levels (Mn‐air) as reported in a few welder studies indicate that aerosols in welding operations (external exposure) usually contain <0.5 mg Mn/m3 (total dust), but sometimes they are >1 mg Mn/m3. A paramount feature of welding fume is that most of the airborne particles are in the respirable fraction (particle size <10 µm).12 It is astonishing that reliable levels of respirable Mn particulate have rarely been reported, given the importance of the pulmonary uptake of Mn via the alveoli and its significance for Mn distribution to and effects on the central nervous system.13 It should be pointed out that inhalation exposure to Mn is for most of the jurisdictions regulated on the basis of total (or inhalable) dust: however, in the particular case of welding, a permissible exposure level established on the respirable fraction would enhance health prevention strategies.
Neuropsychological testing methods used over the past two decades have successfully differentiated Mn‐exposed welders from unexposed controls.4,6,11 There have been 13 reports on welders showing deficits for motor, tremor, memory and neurocognitive domains, as well as for sleep, sexual function and vision. Although not all reports dealt with all of these domains, 11 studies indicated slowing of motor speed/efficiency4,6,11,14,15,16,17,18,19,20,21 and tremor3,4,6,14,15,16,17,19,20,21,22; eight found loss of neurocognitive functioning4,6,11,14,16,18,19,21; and six reported diminished memory function.4,6,11,14,18,21 Six studies reported sleep disturbances4,6,11,14,15,17 and three sexual problems.4,6,14 Vision was assessed in only two studies, and both found loss of colour vision associated with Mn exposure.6,14
Welders exposed to Mn‐containing welding fumes and dust have rarely been investigated by clinical neuropsychologists using an epidemiological study design in which actual measurements of internal and external exposure to Mn are studied in relation to outcome variables of a comprehensive test battery (including measures of IQ). Therefore, in the latter part of 2004, we decided to evaluate a welder group with health complaints, identified as employees on the reconstruction project of the San Francisco–Oakland Bay Bridge, which was damaged during the 1989 earthquake. The work on a vulnerable portion of the East span of the bridge began in 2003, requiring confined space welding of 28 anchoring foundations or piers, consisting of steel piles and footing boxes. The welding operations took place below the Bay's surface in unpressurised cofferdams. During the first 1.5 years of welding on the support piers for the new bridge, welders began to express concerns of ill health feared to be the result of exposure to fumes due to confined space welding. During this period the welders were not required to wear personal protective equipment, and ventilation was minimal or ineffective. After receiving health complaints from almost 90% of the welders employed on the project, workers' compensation evaluations were enacted, which supported their report of adverse health effects. A multidisciplinary study group took advantage of this “natural experiment” for undertaking additional clinical evaluations (1) to assess the extent of neurological, neuropsychological, neurophysiological and pulmonary effects, (2) to explore whether these effects were associated with internal and/or external measures of Mn exposure and (3) to define a tentative permissible exposure level for Mn in welding fume.
Methods
Study population
The study group consisted of 49 welders (45 men and 4 women), who were engaged in manual Shielded Metal Arc Welding (SMAW) and automated Flux Cored Arc Welding (FCAW) in confined small spaces during the construction of the new Bay Bridge. The four women, too few to analyse as a group, and two male individuals whose scores on a test of memory malingering23 indicated low effort were evaluated, but excluded from the statistical analysis. All the welders underwent physical examinations before their employment on the bridge and were found to be healthy. The final group of 43 male welders remained for analysis in the current study.
Almost 90% of the welders employed over several months on this project during 2003–4 (based on the employer's confined space entry logs)24 were evaluated for workers' compensation. To determine whether their health complaints could be medically documented, a comprehensive neuropsychological evaluation was conducted. As part of this evaluation, detailed health, lifestyle, work and exposure histories were obtained during individual clinical interviews and during testing. All workers stated that they had previously been in good health and that this was their first experience of confined space welding. None of the welders reported excessive alcohol consumption, which was generally low overall.
Procedures
The participants signed informed consent forms approved by the San Francisco State University's Institutional Review Board, and thereby agreed to use their clinical data from their workers' compensation evaluations, which included Health Insurance Portability and Accountability Act consents.
All tests were double scored using the methods detailed in their respective manuals by specialists of each of the disciplines (neuropsychology, neurology, neurophysiology and pulmonology). Data were then double entered in the SPSS V.13 programme to assure accuracy.
Study design
For the clinical workers' compensation evaluations, RMB, in her role as principal neuropsychologist, conducted clinical history interviews and administered tests of motivation. Five neuropsychologists administered a neuropsychological test battery, which included IQ testing, tests of executive, motor and other cognitive functions; sensory testing including vision and smell; and scales of symptoms, affect and mood.
In January 2005, the welders were also invited for a multidisciplinary investigation, during which they were subjected to neurological examinations and tests for lung function and olfaction. A board certified neurologist (WK) performed neurological examinations and a PhD candidate (MB), trained in neurotoxicology, administered hand tremor and body sway tests. Two certified pulmonary technicians assessed lung function with spirometry. Psychometrician graduate students in psychology administered smell tests, scales of autonomic nervous system function, and a self‐report evaluation of sleep and sexual function. Blood and urine samples were collected by a certified phlebotomist for trace metal analysis. Additional information on exposure histories were recorded by an experienced industrial toxicologist (HAR).
Exposure assessment
Air measurements
Reports of employer‐commissioned air sampling at various locations on the bridge project, for California Division of Occupational Safety and Health (Cal‐OSHA) compliance purposes, were made available by the Cal‐OSHA inspectors. However, the actual sampling in the field was done by independent non‐employer‐affiliated laboratories, and all analyses were carried out autonomously, independent from employer control.
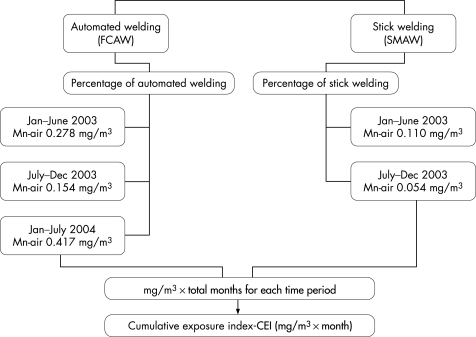
The results of the analyses for Mn and other metals in the confined space air samples taken at the Bay Bridge during January 2003 to June 2004 were averaged over three time periods (January to June 2003, July to December 2003 and January to June 2004) by type of welding: automated welding (FCAW) and manual stick welding (SMAW). For the period July to December 2004, for which no personal air sampling data were available, levels from the preceding 6‐month period were used. A Cumulative Mn Exposure Index (CEI) was calculated for each welder (fig 1) using the self‐reported percentage of time a welder performed FCAW or SMAW during his or her employment in the successive 6‐month periods. Percentage of time was multiplied by estimated average levels of Mn‐air for the two welding types, multiplied by the number of months worked, and then summed over the four time periods.
Figure 1 Construction of the Cumulative Exposure Index (CEI) based on type of welding, air measurements and months worked.
Biomarkers
Samples of 4 and 2 ml blood were collected by venipuncture using a 19‐gauge butterfly catheter and heparinised tubes (BD#367 734). The 2‐ml sample was then centrifuged at 800 g for 10 min at room temperature (about 25°C) to separate the plasma. A spot‐urine sample (50–200 ml) was collected, from which a 1‐ml aliquot was taken for creatinine analysis. Whole blood, plasma and urine were analysed in the trace metal facility at the University of California Santa Cruz (DS). The concentrations of Mn in whole blood, plasma and urine, and the plasma concentrations of copper and iron were determined by high‐resolution inductively coupled plasma/mass spectrometry25; Zeeman graphite furnace atomic absorption spectroscopy was used for lead in whole blood.
Neurological and neuropsychological evaluation
Neurological assessment
For the clinical neurological examination, the neurologist used the motor and the activities of daily living scales of the Unified Parkinson Disease Rating Scale (UPDRS).26 The UPDRS is the most widely used instrument for measuring severity of parkinsonian symptoms qualitatively and quantitatively in clinical research and practice. It has high validity, and high inter‐rater and intra‐rater reliability. Computer assisted tremor analysis system (CATSYS)‐2000 (Danish Product Development) was used to determine postural hand tremor intensity and bodily sway according to standardised procedures.27
Neuropsychological testing
The comprehensive neuropsychological examination included the following tests: for IQ, the Wechsler Adult Intelligence Scale (WAIS‐III) and Wechsler Memory Scale (WMS III)28; for executive function, Rey–Osterrieth Complex Figure,29 Stroop Colour Word30 and Trail Making Tests A and B29; for verbal function, animal naming, Controlled Oral Word Association Test,29 Wide Range Achievement Test 3 reading subtest29 and the Boston Naming Test29; for motor function, Fingertapping, Dynamometer, Grooved Pegboard and Santa Ana Pegboard Test29; for neuropsychiatric symptomatology, the Beck Depression Inventories‐II and Beck Anxiety Inventories,29 the Symptom Checklist 90‐Revised (SCL‐90‐R),31 and Satisfaction with Life Scale and Quality of Life Scale 32; and for malingering, Test of Memory Malingering23 and the Rey 15‐item test.29
Spirometry, olfaction and vision tests
The spirometry test results were evaluated by a board‐certified pulmonologist (RPB). Data analysed are forced expiratory volume at 1 s (FEV1), forced vital capacity (FVC) and the FEV1:FVC ratio. In total, spirometry testing reports were available for three points in time: after working on the bridge for an average of 1.5 months (time 1), after 10.8 months (time 2) and after an average of 20.9 months (time 3) of work on the bridge (study date in January 2005).
For olfactory function testing, we used the University of Pennsylvania Smell Identification Test (UPSIT), which consists of 40 “scratch and sniff” odorants administered according to standardised instructions.33 The scores are summed and interpreted using the UPSIT norms to determine normosmia, microsmia and anosmia.
For the visual function, the Snellen Visual Acuity Test, the Lanthony 15‐hue Test for colour vision and the Vistech Contrast Sensitivity Test were used.34
Missing data
Analyses computing the IQ scores could not be done for eight participants who did not complete all of the subtests of the WAIS or WMS necessary to compute the summary IQs. These participants were given alternate IQ measures in Spanish.
Impairment definition
The neuropsychological tests were scored according to the test publishers' norms, which are adjusted for age and/or education. Scores of 1 standard deviation (SD; for whites) and 1.25 SD (for non‐whites) below the mean of the reported test norms were categorised as impaired.6 The normative data used to compute the impairment level for the tremor are from Després et al.27 Spirometry was evaluated according to the American Thoracic Society Standards and Guidelines35 for mean predicted values to indicate different levels of lung function (mild, moderate and severe).36 A cut‐off of <75 was used to determine abnormality for FEV1:FVC (%). For the UPSIT, clinical olfactory diagnoses were given according to age‐adjusted and sex‐adjusted centile norms from the manual.33 Diagnoses of at least mild microsmia or anosmia were classified as impaired. For the SCL‐90‐R, T scores, mean (SD) 50 (10), based on sex‐adjusted adult norms, were used to derive impairment ratings.
Statistical analyses
Demographic characteristics, biological and exposure results were analysed using descriptive statistics, and for trace metals in whole blood, plasma or urine high normal cut‐off values or normal ranges are shown (table 1). Table 2 shows the neuropsychological mean test scores, SDs and prevalence percentage of impairment using the normative data.
Table 1 Demographics, work and exposure characteristics, and biomarkers for trace elements in Bay Bridge welders.
| Bay Bridge welders (n = 43) | ||||
|---|---|---|---|---|
| n | Mean (%) | SD | Range | |
| Demographics | ||||
| Age (years) | 43 | 43.8 | 10 | 23–66 |
| Years of education | 43 | 12.6 | 2 | 7–17 |
| Ethnicity | ||||
| White | 22 | 51.2 | ||
| Non‐white | 21 | 48.8 | ||
| Smoking habits | ||||
| Current smokers | 8 | 18.6 | ||
| Ex‐smokers | 10 | 23.3 | ||
| Work and exposure | ||||
| Years of welding before Bay Bridge | 43 | 14.2 | 11 | 0–39 |
| Months of welding at Bay Bridge | 43 | 16.5 | 6 | 6–28 |
| Active welders at Bay Bridge* | ||||
| Yes | 22 | 51.2 | ||
| No | 21 | 48.8 | ||
| Type of welding (% of time) | ||||
| FCAW | 40 | 66.5 | 28.5 | 0–100 |
| SMAW | 40 | 28.9 | 25 | 0–100 |
| Respirator worn at Bay Bridge (% of time) | ||||
| Before April 2004 | 42 | 6 | 19.7 | 0–100 |
| After April 2004 | 29 | 82.2 | 28.5 | 0–100 |
| Mn‐air average (mg/m3) | 40 | 0.21 | 0.08 | 0.01–0.38 |
| Mn‐air CEI (mg/m3×month) | 40 | 2.56 | 1.2 | 0.07–4.72 |
| Biomarkers for trace metals | ||||
| Mn‐blood (µg/l)† | 37 | 9.6 | 2.5 | 5.13–15.3 |
| Mn‐urine (µg/g creatinine)†‡ | 36 | 0.28 | 0.460 | 0.00–1.93 |
| Mn‐plasma (µg/l)† | 33 | 0.58 | 0.13 | 0.22–0.85 |
| Pb‐blood (µg/dl)† | 37 | 2.8 | 1.1 | 1.24–6.59 |
| Fe‐plasma (µg/l)§ | 33 | 1127 | 327 | 469–2016 |
| Cu‐plasma (µg/l)§ | 33 | 807 | 120 | 601–1114 |
FCAW, flux cored arc welding; SMAW, shielded metal arc welding.
*Regarded as active welder if the welder left Bay Bridge <1 month before the January 2005 assessment date.
†Cut‐off value upper normal: MnB, 10 µg/l; MnU, 2 µg/g creatinine; Mn‐plasma, unknown; PbB, 25 µg/dl.
‡LOD computed as 0.00003 µg/g creatinine.
§Normal range: Fe, 650–1700 µg/l; Cu, 700–1400 µg/l .37
Table 2 Neuropsychological tests by domain of function.
| Bay Bridge welders (n = 43) | ||||
|---|---|---|---|---|
| n | Mean | SD | Prevalence (%) of impairment | |
| IQ | ||||
| Full IQ | 35 | 95.26 | 12.93 | 20 |
| Performance IQ | 41 | 94.49 | 13.67 | 17.1 |
| Verbal IQ | 35 | 93.69 | 14.17 | 22.9 |
| Cognitive flexibility and executive functioning | ||||
| Stroop colour/word T score | 42 | 39.76 | 13.40 | 45.2 |
| Rey‐Osterreith copy | 43 | 30.60 | 3.73 | 69.8 |
| Design fluency—total correct | 41 | 10.24 | 2.84 | 9.8 |
| Design fluency—percent design accuracy | 41 | 8.73 | 2.69 | 24.4 |
| Design fluency—switching | 41 | 10.61 | 2.64 | 4.9 |
| Sorting—Recognition Description score | 37 | 7.65 | 2.78 | 43.2 |
| Sorting—recognition v free sorting | 37 | 7.30 | 3.15 | 35.1 |
| Information processing speed | ||||
| Stroop colour T score | 42 | 38.98 | 10.59 | 45.2 |
| Stroop word T score | 42 | 39.10 | 11.80 | 40.5 |
| Working memory/attention and concentration/learning | ||||
| WAIS‐III WMI | 40 | 90.6 | 13.24 | 25 |
| Digit span | 41 | 8.44 | 2.37 | 26.8 |
| Digit span—longest digits (forward) | 41 | 6.1 | 1.43 | 17.5 |
| Digit span—longest digits (backward) | 41 | 4.22 | 1.01 | 27.5 |
| Letter–number sequencing | 40 | 8.38 | 3.04 | 32.5 |
| WMS‐III Auditory Immediate Memory Index | 37 | 90.32 | 13.59 | 32.4 |
| Logical memory I | 37 | 7.97 | 3.14 | 43.2 |
| Verbal paired associates I | 37 | 8.65 | 2.19 | 18.9 |
| Word lists 1 recall | 36 | 7.78 | 3.34 | 44.4 |
| Auditory consonant trigrams 3″ | 43 | 10.16 | 3.27 | 51.2 |
| Auditory consonant trigrams 9″ | 43 | 7.86 | 3.25 | 55.8 |
| Auditory consonant trigrams 18″ | 43 | 6.28 | 3.31 | 48.8 |
| Rey–Osterreith immediate recall | 43 | 38.79 | 13 | 46.5 |
| Memory | ||||
| WMS‐III Auditory Delayed Index | 36 | 94.03 | 12.61 | 13.9 |
| Verbal paired associates II | 37 | 8.86 | 2.52 | 18.9 |
| Word lists 2 recall | 35 | 9.57 | 3.38 | 22.9 |
| Rey–Osterreith delayed recall | 43 | 39.02 | 13.18 | 60.5 |
| Visuomotor tracking speed | ||||
| WAIS‐III PSI | 42 | 89.52 | 11.99 | 33.3 |
| Digit symbol coding | 42 | 7.79 | 2.37 | 31 |
| Trail Making Test A T score | 42 | 44.43 | 11.78 | 31 |
| Verbal skills | ||||
| WAIS‐III VCI | 35 | 95.11 | 15.53 | 17.1 |
| WRAT3‐reading | 34 | 93.65 | 13.91 | 23.5 |
| Comprehension | 41 | 8.78 | 2.92 | 24.4 |
| Similarities | 35 | 8.71 | 3.56 | 28.6 |
| Vocabulary | 37 | 8.62 | 3.12 | 35.1 |
| Information | 35 | 9.94 | 2.71 | 17.1 |
| Motor dexterity and strength | ||||
| Grooved pegboard T score (dominant hand) | 43 | 53.93 | 12.27 | 14 |
| Finger tapping Tscore (dominant hand) | 43 | 46.42 | 13.76 | 23.3 |
| Dynamometer T‐score (dominant hand) | 42 | 35.33 | 8.36 | 52.4 |
| Santa Ana pegboard (dominant hand) | 43 | 21.89 | 3.83 | 97.7 |
| Tremor/postural sway | ||||
| Parallel Line Test | 43 | 2.12 | 1.22 | 90.7 |
| CATSYS Tremor: Harmonic Index (right) | 39 | 0.15 | 0.11 | 38.5 |
| CATSYS tremor: Harmonic Index (left) | 39 | 2.32 | 0.56 | 61.5 |
| CATSYS sway 4: sway intensity | 35 | 8.84 | 5.11 | 51.4 |
CATSYS, computer assisted tremor analysis system; VCI, Verbal Comprehension Index; WAIS‐III, Wechsler Adult Intelligence Scale; WRAT, Wide Range Achievement Test 3; WMS III, Wechsler Memory Scale.
Multiple regression analyses were performed to examine dose–effect associations between neuropsychological/neurophysiological test scores or self‐reported adverse health symptoms obtained in the clinical interview and MnB or CEI. For spirometry, the ratio percentage changes from T1 to T3 were modelled using smoking and total years of welding before the Bay Bridge as covariates and MnB or CEI as independent variables.
To avoid bias of confounders in the dose–effect analyses, potential confounding factors (age, years of education, ethnicity and total years of welding before the Bay Bridge) were included as covariates in multiple regressions for the neuropsychological tests when test scores were not previously adjusted for demographic variables in the test publisher norms. Similarly, age, years of education and total years of welding before the Bay Bridge were controlled in multiple logistic regressions examining the association between exposure variables and adverse health symptoms. As smoking among 10 welders ceased at a minimum of ⩾8 years before the study date, the number of cigarettes smoked currently was included as a possible confounder in the dose–effect analyses for spirometry and olfactory tests. Smoking was not adjusted in other dose–effect analyses as it was found to have neither significant association with neuropsychological test performance or self‐reported symptoms, nor significant covariate effects on the relationship between exposure variables and these dependent variables.
Results
Study population characteristics
Table 1 shows the Bay Bridge welder group characteristics of demographics, work and exposure, and biomarkers of trace metals. On average, the welders were 43.8 years old, had 12.6 years of education, had welded already for 14.2 years before the Bay Bridge, and did 16.5 months of confined space welding at the Bay Bridge, where 22 of 43 welders were still active at the end of January 2005. About half of the welder group was composed of whites. As to smoking habits, 25 welders had never smoked and 10 were ex‐smokers who reported cessation of smoking at least 8 years before the actual study date.
Exposure
Before April 2004, the welders reported wearing respirators on average 6% of the time, whereas after this date they wore them 82% of the time. On‐site Cal‐OSHA inspections of the confined spaces in which the welders worked noted deficiencies in orientation and functioning of the ventilation devices and identified overexposure to Mn in welding fumes.
Cal‐OSHA data for May and June 2003 at the Bay Bridge reconstruction site and personal exposure monitoring done by the contracted laboratory during 75–80% of the work shifts showed air levels of Mn ranging from 0.11 to 0.46 mg/m3. Table 1 shows the Mn concentration in air, CEI and trace metal concentrations in whole blood (Mn, Pb), serum/plasma (Mn, Fe, Cu) and urine (Mn).
It is interesting to note that 55% of the average Mn‐air levels exceeded the Cal‐OSHA PEL or ACGIH TLV, which is 0.2 mg/m3 for 8‐h time weighted average (total dust). All biomarkers of trace metals are considered normal, except MnB, the level of which exceeded 10 μg/l in 16 of 37 (43%) welders. Moreover, welders still actively welding at the time of the study (n = 21) had a significantly higher mean (SD) MnB than those who had stopped working at the Bridge 1 month or more ago (n = 16)—that is, 10.3 (2.82) v 8.7 (1.74) μg/l (t35 = 2.00, p = 0.05).
Spirometry
Spirometric findings indicated that the welders' lung function decreased from time 1 to time 3 by 7% for FEV1, by 2% for FVC and by 21.2% for the FEV1:FVC ratio. By the time of the study (time 3), 33.3% of the welders had an abnormal FEV1:FVC ratio. This was supported by the result of a paired t test analysis of FEV1:FVC ratio in non‐smokers for times 1 and 3 (t24 = 2.36, p<0.05). The ratio percentage changes from time 1 to time 3 were regressed with smoking, total years of welding before the Bay Bridge and each exposure variable (MnB or CEI), but no significant dose–effect association was obtained.
Neuropsychological testing
Table 2 shows the neuropsychological test results for selected parameters which are frequently affected in parkinsonism, including subtests of the WAIS‐III (Letter–Number Sequencing, Working Memory), the WMS‐III, the Rey–Osterrieth Complex Figure test, Design Fluency and Free Sorting, Auditory Consonant Trigrams, Stroop, Trailmaking Tests A and B, Grooved Pegboard, Fingertapping, Dynamometer, Santa Ana Pegboard and Parallel Lines Test. Bolded scores indicate the prevalence of welders with impaired performance at least twice as large as expected. DSM‐IV clinical diagnoses in the workers' compensation evaluation resulted in classifying 79% with cognitive disorders.
Neurological and sensory examination
UPDRS results indicate that participants had total mean scores of 6.79 (SD 5.08, range 0–19) on the activities of daily living scale and 6.62 (SD 8.16, range 0–24) for the motor scale. In addition, a bradykinesia score was computed by summing scores of fingertaps, hand movements, rapid alternating movements of hands, leg agility, arising from a chair, gait and body bradykinesia/hypokinesia (mean (SD) 4.15 (6.53), range 0–20). Results from the CATSYS Tests showed impairment of hand tremor intensity (Harmonic Index) for 38.5–61.5% and postural sway intensity for 51.4% in the fourth condition (on foam and blinded; table 2).
Olfactory tests were compared with matched controls (by age, years of education and smoking habits) of a control subject database from the University of Pennsylvania Smell and Taste Center, and showed that 88% of the welders scored below their individually matched controls.33 The mean for welders was significantly lower than that for their matched controls (F1,42 23.06, p<0.001).
Mood
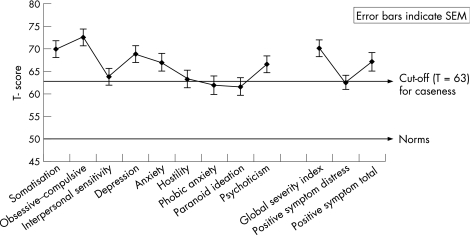
Analyses of affect and mood indicated that the levels of clinical depression and anxiety were >2 SDs above the normative mean, indicating high levels of mood disturbance. Figure 2 shows the mean scores and standard errors of the welders' SCL‐90‐R, which indicate dysphoric mood and affect, signs of withdrawal from life interests, lack of motivation and loss of vital energy in addition to having clinical signs such as nervousness, tension and trembling, with possible panic episodes and feelings of terror. Psychoticism scores also were moderately raised, supporting some of the welders' statements of having hallucinations while welding. The mean (SD) score of the Beck Depression Inventories was 18.9 (10), and 63% of the welders had clinical elevations. The Beck Anxiety Inventories mean score was 22.6 (SD 14.6), with 80.5% clinical elevations. DSM‐IV diagnoses indicate 70% anxiety disorders and 61% depression for the welders.
Figure 2 T scores of the Symptom Checklist 90‐R (SCL‐90‐R), mean (SEM), for the nine clinical and three summary scales.
Based on the Behavioural Risk Factor Surveillance System, the average numbers of days reported for poor physical and mental health were 14 and 15 of 30 days, respectively, indicating significantly more poor health days than those reported by the US Center for Disease Control.
Dose–effect relationships
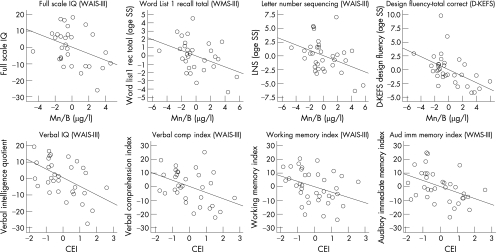
Significant dose–effect relationships between neuropsychological variables and blood Mn or CEI, shown in table 3, resulted from multiple regression analyses with the covariates of years of welding before Bay Bridge and age, years of education and ethnicity when these covariates were not adjusted in their respective norms. For blood Mn, significant associations were obtained for Full Scale IQ; cognitive flexibility and executive function; working memory, attention and concentration/learning; and memory. For CEI, significant associations were obtained for verbal IQ , working memory and concentration/learning, memory and verbal skills. The partialled out effects of the potential covariates show that the welding fume exposure on the Bay Bridge remained significantly associated with impaired scores on tests. All coefficients (β values) had negative signs, indicating a consistent direction of effect—that is, the higher the Mn values, the less favourable the neuropsychological outcomes. A substantial amount of variance was uniquely accounted for by MnB shown in table 3 and CEI as shown in table 4 (expressed by sr2). Partial regression plots (fig 3) illustrate the inverse slopes for the regression lines of the residuals for the age‐adjusted neuropsychological tests with MnB—that is, Full Scale IQ , Word List recall total (WMS‐III), Letter–number sequencing (WAIS‐III) and Design Fluency total correct—and with CEI—that is, Verbal IQ, Verbal Comprehension Index (WAIS‐III) Working Memory Index (WAIS‐III), and Auditory Immediate Memory Index (WMS‐III). Multiple regressions indicate that an MnB increment of 5.0 µg/l results in a decrease in Full Scale IQ of 10 points (B = −2.0, SE = 1), and a CEI increment of 2.4 mg/m3×month in a decrease of 12 points in verbal IQ (B = −5.2, SE = 1.7) and in a loss of 9 points for Working Memory Index (B = −3.8, SE = 1.8).
Table 3 Multiple regression analyses on neuropsychological test scores and using Mn in blood as a predictor.
| Mn in blood (µg/l) | |||||||
|---|---|---|---|---|---|---|---|
| β | sr2 (95% CI of sr2) | p Value | sr2 Control variables | ||||
| Age | Edu | Eth | Yr W | ||||
| IQ | |||||||
| Full IQ | −0.34 | 0.09 (0–0.28) | 0.053 | – | 0.10 | 0.05 | 0.01 |
| Verbal IQ | −0.31 | 0.08 (0–0.25) | 0.061 | – | 0.18 | 0.06 | 0 |
| Cognitive flexibility and executive functioning | |||||||
| Design fluency—total correct | −0.51 | 0.22 (0–0.44) | 0.004 | – | 0.02 | 0.04 | 0.01 |
| Design fluency—percentage design accuracy | −0.43 | 0.15 (0–0.36) | 0.012 | – | 0.05 | 0.01 | 0.04 |
| Design fluency—switching | −0.39 | 0.13 (0–0.32) | 0.027 | – | 0.01 | 0.09 | 0.01 |
| Working memory/attention and concentration/learning | |||||||
| WAIS‐III WMI | −0.29 | 0.07 (0–0.23) | 0.095 | – | 0.11 | 0.14 | 0 |
| Letter–number sequencing | −0.38 | 0.12 (0–0.32) | 0.035 | – | 0.09 | 0.00 | 0.01 |
| Word lists 1 recall | −0.29 | 0.07 (0–0.23) | 0.042 | – | 0.41 | 0.01 | 0.01 |
| Memory | |||||||
| WMS‐III WMI | −0.36 | 0.11 (0–0.3) | 0.061 | – | 0.02 | 0 | 0.02 |
| WMS‐III Auditory Delayed Index | −0.3 | 0.08 (0–0.25) | 0.093 | – | 0.07 | 0.04 | 0.09 |
| Verbal Paired Aassociates II | −0.40 | 0.14 (0–0.35) | 0.041 | – | 0.01 | 0.01 | 0.01 |
| Verbal skills | |||||||
| WAIS‐III VCI | −0.27 | 0.06 (0–0.21) | 0.094 | – | 0.18 | 0.08 | 0 |
Edu, education; Eth, ethnicity; sr2, squared semi‐partial correlation; VCI, Verbal Comprehension Index WAIS‐III, Wechsler Adult Intelligence Scale; WMS III, Wechsler Memory Scale; Yr W, years of prior welding.
–demographic variable (age) was not entered in multiple regressions.
Table 4 Multiple regression analyses on neuropsychological test scores using Mn in Cumulative Exposure Index (CEI) as a predictor.
| CEI (mg Mn/m3 × month) | |||||||
|---|---|---|---|---|---|---|---|
| β | sr2 (95% CI of sr2) | p Value | sr2 control variables | ||||
| Age | Edu | Eth | Yr W | ||||
| IQ | |||||||
| Verbal IQ | −0.44 | 0.16 (0–0.37) | 0.004 | – | 0.16 | 0.03 | 0 |
| Cognitive flexibility and executive functioning | |||||||
| Stroop colour‐word T score | −0.30 | 0.07 (0–0.22) | 0.097 | – | 0.04 | 0.01 | 0.01 |
| Working memory/attention and concentration/learning | |||||||
| WAIS‐III WMI | −0.35 | 0.1 (0–0.26) | 0.041 | – | 0.08 | 0.02 | 0.01 |
| Digit span (forward) | −0.30 | 0.07 (0–0.21) | 0.081 | – | 0.06 | 0.06 | 0.02 |
| Letter–number sequencing | −0.39 | 0.12 (0–0.3) | 0.036 | – | 0.05 | 0 | 0.02 |
| WMS‐III Auditory Immediate Memory Index | −0.42 | 0.14 (0–0.34) | 0.017 | – | 0.03 | 0.05 | 0.06 |
| Verbal Paired Associates I | −0.5 | 0.2 (0–0.42) | 0.007 | – | 0.01 | 0.01 | 0.08 |
| Auditory consonant trigrams total score | −0.34 | 0.09 (0–0.24) | 0.071 | 0.05 | 0.02 | 0 | 0.05 |
| Memory | |||||||
| WMS‐III WMI | −0.38 | 0.11 (0–0.29) | 0.052 | – | 0 | 0 | 0.01 |
| Logical memory I | −0.31 | 0.08 (0–0.24) | 0.09 | – | 0.03 | 0.08 | 0.03 |
| Verbal skills | |||||||
| WAIS‐III VCI | −0.35 | 0.1 (0–0.28) | 0.026 | – | 0.19 | 0.05 | 0 |
| Comprehension | −0.51 | 0.2 (0–0.41) | 0.003 | – | 0.06 | 0 | 0 |
| Similarities | −0.34 | 0.09 (0–0.27) | 0.053 | – | 0.09 | 0.06 | 0 |
| Vocabulary | −0.43 | 0.15 (0–0.35) | 0.006 | – | 0.13 | 0.03 | 0 |
CEI, Cumulative Exposure Index; sr2, squared semi‐partial correlation; WAIS‐III, Wechsler Adult Intelligence Scale; WMS III, Wechsler Memory Scale; VCI, Verbal Comprehension Index.
–demographic variable (age) was not entered in multiple regressions.
Figure 3 Graphic representation of partial regression graphs for selected tests and manganese in blood (MnB) and Cumulative Exposure Index (CEI).
Logistic regression analyses for symptoms while working were performed to examine their relationship with MnB or CEI, by controlling possible confounders such as age, years of education and years of welding before the Bay Bridge. A unit increase in MnB showed a significant association with depression (OR = 1.5, p<0.05, 95% CI 1.0to 2.2). There were also significant associations between the CEI and sexual dysfunction (OR = 2.6, p<0.05, 95% CI 1.2 to 5.5), headache (OR = 2.0, p<0.05, 95% CI 1 to 4), depression (OR = 2.8, p<0.01, 95% CI 1.3 to 6) and fatigue (OR = 2.7, p<0.05, 95% CI 1.3 to 5.9). No significant association was obtained for hallucination, sleep disturbance, tremors, anxiety and numbness. Bonferroni adjustments were not made as each symptom is considered its own domain.
Discussion
Although there is a large body of literature on the association between occupational Mn exposure and ill health, a controversy remains whether Mn exposure in welders produces welding‐related symptoms and associated specific deficits in neuropsychological and neurological function. Many of the previous welder studies did not report/measure air Mn, did not have the type of extensive exposure (air Mn) and biomarker measurements (blood, plasma and urine), or lacked the multidisciplinary methods (neurological, neuropsychological and respiratory assessment) used in the present study. There are as yet no reports of groups of confined space welders working in poor ventilation conditions without adequate personal protection equipment who underwent full clinical neuropsychological and neurological examinations. The Bay Bridge welders afforded a unique opportunity to study health effects in the context of a “natural experiment” for this type of welding fume exposures. Although respiratory protection should have been required according to Cal‐OSHA regulations and NIOSH guidelines, this was not done.24 This group of middle age welders, who were pre‐screened during a competitive hiring process to assure good health and fitness, were highly skilled, competent and physically fit at the beginning of their employment on the bridge.
A major asset of the study is that the neurological effects identified in the Bay Bridge welders were detected by a neurologist using UPDRS examination, a reliable clinical tool widely used for assessing parkinsonian syndromes. Abnormal mean scores for UPDRS and bradykinesia were found in the welder group. Another important result is the detection of increased hand tremor and body sway with the CATSYS instrument, which allows objective measurements based on physics. Tremor, a hallmark of parkinsonism, was found to be present 39–90% of the time on three different methods of tremor testing. Motor dexterity and speed also was impaired 52–95% of the time on various neuropsychological measures. Welders, by the nature of their job, are required to have good speed and efficient motor performance, and are expected to have scored above average before their confined space welding period. The present results may underestimate the true effects size—this could be answered in a longitudinal study beginning with apprentice welders.
The study also showed strong suggestive evidence that as the welders continued to be exposed to Mn in welding fumes on the bridge, their intelligence level may have declined by as much as 10–12 IQ points, although some uncertainty cannot be ruled out because of the small sample size, lack of baseline and longitudinal data. Although lead has been acknowledged as a neurotoxicant lowering IQ in children, this is the first report of adults having IQ decrements associated with occupational Mn exposure. Recently, Wright et al38 reported findings of an association between Mn in hair and lowered IQ in 32 children from Ottawa County in northeastern Oklahoma who lived at the Tar Creek Superfund clean‐up site, slated for clean‐up by the US Environmental Protection Agency. Wasserman et al39 reported that exposures to Mn in tube‐well water in Araihazar, Bangladesh, produced similar decrements as those reported here in welders—that is, Mn exposure correlated with Full Scale IQ, performance and verbal IQ raw scores. This scientific convergence of findings suggests a detrimental effect of Mn on cognitive function resembling the negative effects of lead in children (blood lead levels were negligible in the welders)—regardless of the route of exposure (inhalation or oral). The finding of a dose–effect association between increased internal Mn exposure (moderate elevations of MnB) and lower IQ in a group of welders is of concern and should be followed up with larger observational studies of welding in confined spaces and other occupational settings with Mn exposure. Although the level of impairment for the neuropsychological test results is relatively small compared with more extreme findings of long‐term welders reported previously,6,14 the inverse dose–effect relationships between Mn exposure and IQ or other cognitive functions obtained in the present study suggest an increased population‐attributable risk and represent a change, albeit small, in the distribution of IQ scores above some threshold of Mn exposure.
Overall, the skilled welder group, when interviewed by the clinical neuropsychologists, reported good mental health before welding on the bridge. After employment at the bridge, 63% and 81% scored clinically depressed and anxious, respectively. Mood effects have been described in many studies of Mn exposure.4,6,14,40 Sleep and sexual disturbance, such as loss of libido and decreased potency, were reported by all but one welder. About 70% of welders who had workers' compensation evaluation were referred for clinical psychotherapeutic interventions.
Good or adequate olfactory ability contributes significantly to the enjoyment of life, and olfactory changes serve as an early warning system for the detection of overexposure to chemicals, fire, gas, spoiled foods or dangerous levels of air contamination.33 Olfactory loss has been associated with Parkinson's disease and head trauma,41 but inhalation exposure to manganese fume/dust may also entail olfactory changes as reported in a study of Mn‐exposed ferromanganese‐alloy workers.42 Recent studies in rats strongly suggest olfactory nerve transport as a significant route for central nervous system uptake of manganese from inhaled fine43 (mass median aerodynamic diameter 1.3 µm) or ultrafine44 (count median diameter 0.031 µm) Mn‐oxide aerosols. Inhalation exposure of fine (6 h/day, 15 days; 3 mg/m3) and ultrafine (6 h/day, 12 days; 0.5 mg/m3) particles resulted in, respectively, 1.7 and 3.5 higher Mn concentrations in olfactory bulbs of exposed rats compared with controls. For ultrafine Mn‐oxide aerosol exposure, an increased gene expression was found for tumour necrosis factor α in the olfactory bulb, frontal cortex, midbrain and striatum. For the olfactory bulb, at least a twofold increase over controls was shown for expression of other genes involved in stress response and inflammation.44 Elder et al44 stated that, as the olfactory neuronal translocation pathway was also shown in non‐human primates, it is likely to be operative in humans as well. These experimental findings offer a strong basis for a causal association between the Bay Bridge welders' changes in smell identification and inhalation exposure to Mn from confined space welding generating fine/ultrafine particle aerosols. With more than three quarters of the welders showing impaired sense of smell, this loss leaves them deprived of olfaction as a sensory warning sign making them potentially more vulnerable to future injuries.
Most of the welders reported having developed respiratory symptoms (93%). The evolution of pulmonary abnormalities suggests that the longer they welded in the confined spaces on the Bay Bridge, the more the air flow obstruction developed. One third of the welder group presented with a mild obstructive syndrome at an average of 20.9 months after they began working on the bridge. They were referred for further pulmonary follow‐up. The spirometric results suggest that further testing is indicated, such as inhaled bronchial challenge or clinical exercise testing. Those welders who continue to exhibit welding‐related respiratory symptoms should also be considered candidates for additional pulmonary testing.36
Both the raised MnB levels and the increase in Mn levels in the air of the Bay Bridge welders' working environment suggest that the measurable exposure to Mn is the likely cause of their ill physical and mental health. However, MnB analyses are useful only if they are done within <1 month of cessation of welding. A follow‐up is needed to determine if the welders' condition has improved since leaving this work site. The confined space welding and simultaneous unprotected Mn exposure on the bridge were relatively brief (on average 16.5 months), which may make reversibility of their symptoms and deficits possible.
Both neurological and neuropsychological methods were found to be complementary in the diagnosis of welding‐associated parkinsonism and, moreover, neuropsychological testing is shown to contribute significantly to the clinical neurological and pulmonary definition of a comprehensive portrait for health effects in Mn‐exposed welders. A more complete portrait includes also tremor, IQ and other cognitive deficits, mood disturbance, sleep and sexual perturbations, changes in smell, and surprisingly early lung function effects as well, most probably linked to the size of welding fume particulate being predominantly in the respirable range.
Policy implications
Welders should undergo medical/occupational screening for symptoms related to manganese (Mn) exposure, at minimum once per year.
Appropriate regulatory agencies (OSHA) should monitor welders for safe working conditions, wearing personal protective equipment should be supervised, and air measurements of Mn and other toxic metals should be performed and reviewed on a regular basis (at a minimum of once per month).
The adverse health effects from Mn exposure in the welders described here occurred at average exposure levels at or slightly above 0.2 mg/m3. Therefore, the current permissible exposure level of California Division of Occupational Safety and Health for Mn exposure (total dust) should be reconsidered for welders, because welding fume aerosol falls nearly completely within the respirable fraction allowing very fine particulate a greater absorption rate of manganese via deposition in the alveolar region of the lung.
A limitation of this study is the lack of a matched control group for comparison. However, as can be seen in the welders' demographic characteristics, they are similar to the standardisation samples of the neuropsychological tests used. This similarity suggests that comparisons with the standardised age norms of the tests used are likely appropriate. Although the welders were not drawn at random, a selection bias is not likely because, according to several foremen, most (90%) of the welders worrying about health effects from welding fume exposure on the Bay Bridge have been included in our sample and are thus likely representative of the overall group. Significant dose–effect associations in this relatively small group support and strengthen the finding of multiple adverse health effects in these welders, despite the conservative approach used by taking into account up to four possible covariates in the multiple regression models. Although this may have had some influence in obtaining statistical significance, the remaining “explained” variance after adjustment for the covariates still supports inverse associations between MnB or CEI and several test performances. As the sample size was not under the investigator's control, replication using a larger sample of welders would be desirable to confirm these findings.
It is highly recommended for occupational physicians and other health professionals, who may evaluate or treat a welder, to consider exposure to Mn fumes as a possible cause of their ill health. The evaluating physician should refer such welders to neuropsychologists and neurologists for further assessments, which will assist in diagnostic considerations. Clearly, the loss of almost 1 SD of IQ, if this loss persists, will affect the welders' lives permanently, lowering their potential to earn a good living and their learning of new information which is required for work and for independent living skills. It is a high cost to society, as aptly stated by Weiss 45: “That cost is the decline in functional capacity. If the disease is a gradually progressive one, the ability of its victims to function effectively and efficiently will be impaired at stages of the disease far earlier than its eventual detection.” Given these findings, enforcing protection by agencies such as NIOSH and Cal‐OSHA is essential, and ongoing inspections to ascertain compliance can yield significant benefit to welders and to the society.
Acknowledgements
We thank Dr Sabine Gysens, Dr Stephen Rauch and Dr Patricia Perez‐Arce for their review of this manuscript and their helpful comments. We also appreciate Dr Edward Baker for his encouragement and support during the initial scientific planning phase of this research. We thank Dr Patricia Perez‐Arce and Dr Tatjana Novakovic for assisting in the neuropsychological assessments of the welders, and Dr Ralf Schwarzer for his advice on the advanced statistical analyses and their interpretation. Additionally, We appreciate Travis Riddle, Ana Anayansi and Sergio Rizzo (SFSU interns) for their editorial help and thank Dustin Cantrera for the exposure assessment computerisation. Clinical psychology graduate students (Linda Mora, Katie Pearson, Lisa Anderson, Aaron Estrada and Markus Doebler) assisted in the testing on the study days. We thank Dr Robert Harrison and Dr Adam Duhan for providing additional medical consultations. Above all, we thank the welders who came to the clinical and study appointments numerous times and were willing to share much of their personal background, symptoms and worries, and work experiences, as well as to undergo such extensive testing to contribute to this research.
Abbreviations
Cal‐OSHA - California Division of Occupational Safety and Health
CATSYS - computer assisted tremor analysis system
CEI - Cumulative Exposure Index
FCAW - Flux Cored Arc Welding
FVC - forced vital capacity
FEV1 - forced expiratory volume at 1 s
IPD - idiopathic Parkinson's disease
Mn‐air - manganese air levels
MnB - whole blood manganese
MRI - magnetic resonance imaging
SCL‐90‐R - Symptom Checklist 90‐Revised
SMAW - Shielded Metal Arc Welding
UPDRS - Unified Parkinson Disease Rating Scale
UPSIT - University of Pennsylvania Smell Identification Test
WAIS‐III - Wechsler Adult Intelligence Scale
WMS III - Wechsler Memory Scale
Footnotes
Funding: RMB was paid by the participants' employer to conduct neuropsychological evaluations of the employees as part of State and Federal Workers' Compensation administrative law proceedings. The welders' Workers Compensation attorneys made a contribution to the medical assistants and their incidental supplies during the two days of the study in January 2005. The welder participants, in turn, agreed to participate for free in the study.
Competing interests: None.
Parts of this research have been presented on invitation at the NIOSH meeting in Morgantown, WV (June 2005), at the 9th International Symposium on Neurobehavioral Methods and Effects in Occupational and Environmental Health in Gyeongju, Korea (September 2005), at the ICOH meeting on Health Effects of Metals in Brescia, Italy (June 2006), and at the California State Department of Health Services in Oakland, California (September 2006).
References
- 1.Feldman G. Manganese. In: Feldman G, ed. Occupational and environmental neurotoxicology. New York, NY: Lippincott‐Raven Press, 1999168–188.
- 2.Lucchini R, Albini E, Placidi D.et al Brain magnetic resonance imaging and manganese exposure. Neurotoxicology 20005769–775. [PubMed] [Google Scholar]
- 3.Kim Y. Neuroimaging in manganism. Neurotoxicology 200627369–372. [DOI] [PubMed] [Google Scholar]
- 4.Bowler R M, Koller W, Schulz P. Parkinsonism due to manganism in a welder: neurological and neuropsychological sequelae. Neurotoxicology 200627327–332. [DOI] [PubMed] [Google Scholar]
- 5.Koller W C, Lyons K E, Truly W. Effect of levodopa treatment for parkinsonism in welders: a double blind study. Neurology 200462730–733. [DOI] [PubMed] [Google Scholar]
- 6.Bowler R M, Gysens S, Diamond E.et al Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology 200627315–326. [DOI] [PubMed] [Google Scholar]
- 7.Martin C J, Guidotti T L, Langard S. Respiratory hazards of welding. Clin Pulm Med 19974194–204. [Google Scholar]
- 8.Antonini J. Health effects of welding. Crit Rev Toxicol 20033361–103. [DOI] [PubMed] [Google Scholar]
- 9.McMillan G H G, Plethybridge R J. A clinical radiological and pulmonary case‐control study of 135 dockyard welders aged 45 years and over. J Soc Occup Med 1984343–23. [DOI] [PubMed] [Google Scholar]
- 10.Sjögren B. Effects of gases and particles in welding and soldering. In: Zenz C, Dickerson OB, Horvath EP Jr, eds. Occupational medicine. 3rd ed. St Louis: Mosby Press, 1994, 917–23, ISBN 6801666767
- 11.Sjögren B, Iregren A, Frech W.et al Effects on the nervous system among welders exposed to aluminum and manganese. Occup Environ Med 19965332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris M.Welding health and safety: a field guide for OEHS professionals. Fairfax, VA: American Industrial Hygiene Association, 2002
- 13.Roels H A, Ghyselen P, Buchet J P.et al Assessment of the permissible exposure level to manganese in workers exposed to manganese dioxide dust. Br J Ind Med 19924925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowler R M, Gysens S, Diamond E.et al Neuropsychological sequelae of exposure to welding fumes in a group of occupationally exposed men. Int J Hyg Environ Health 20032061–13. [DOI] [PubMed] [Google Scholar]
- 15.Wang J D, Huang C C, Hwang Y H.et al Manganese induced parkinsonism: an outbreak due to an unrepaired ventilation control system in a ferromanganese smelter. Br J Ind Med 198946856–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Racette B A, McGee‐Minnich L, Moerlein S M.et al Welding‐related parkinsonism: clinical features, treatment, and pathophysiology. Neurology 2001568–13. [DOI] [PubMed] [Google Scholar]
- 17.Chandra S V, Seth P K, Mankeshwar J K. Manganese poisoning: clinical and biochemical observations. Environ Res 19747374–380. [Google Scholar]
- 18.Nelson K, Golnick J, Korn T.et al Manganese encephalopathy: utility of early magnetic resonance imaging. Br J Ind Med 199350510–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y, Kim J W, Ito K.et al Idiopathic parkinsonism with superimposed manganese exposure: utility of positron emission tomography. Neurotoxicology 199920249–252. [PubMed] [Google Scholar]
- 20.Discalzi G, Pira E, Hernandez E H.et al Occupational Mn parkinsonism: magnetic resonance imaging and clinical patterns following CaNa2EDTA chelation. Neurotoxicology 200021863–866. [PubMed] [Google Scholar]
- 21.Sadek A H, Rauch R, Schulz P E. Parkisonism due to manganism in a welder. Int J Toxicol 200322393–401. [DOI] [PubMed] [Google Scholar]
- 22.Beuter A, Lambert G, MacGibbon B. Quantifying postural tremor in workers exposed to low levels of manganese. J Neurosci Methods 2004139247–255. [DOI] [PubMed] [Google Scholar]
- 23.Tombaugh T.Test of memory malingering (TOMM). New York: Multi‐Health System (MHS), 1996
- 24.Park R M, Bowler R M, Eggerth D E.et al Issues in neurological risk assessment for occupational exposures: the Bay Bridge welders. Neurotoxicology 200627373–384. [DOI] [PubMed] [Google Scholar]
- 25.Gwiazda R G, Lee D, Sheridan J.et al Low cumulative manganese exposure affects striatal GABA but not dopamine. Neurotoxicology 2002951–8. [DOI] [PubMed] [Google Scholar]
- 26.Goetz G C, LeWitt P A, Weidenman M. Standardized training tools for the UPDRS activities of daily living scale: newly available teaching program. Mov Disord 2003181455–1458. [DOI] [PubMed] [Google Scholar]
- 27.Després C, Lamoureux D, Beuter A. Standardization of a neuromotor test battery: the CATSYS system. A. Sensitivity and specificity of a portable system measuring postural tremor. Neurotoxicology 200021725–735. [PubMed] [Google Scholar]
- 28.Wechsler D.WAIS‐III & WMS‐III technical manual. San Antonio; The Psychological Corporation 1997
- 29.Lezak M D, Howieson D B, Loring D W.Neuropsychological assessment. New York: Oxford University Press, 2004
- 30.Golden J G.Stroop Color Word Test: a manual for clinical and experimental uses. Chicago, IL: Stoelting Company, 19783–20.
- 31.Derogatis L R.SCL‐90‐R administration, scoring and procedures manual. Townson, MD: Clinical Psychometric Research, 1992
- 32.Hennessy C H, Moriarty D G, Zack M M.et al Measuring health‐related quality of life for public health surveillance. Public Health Rep 1994109665–672. [PMC free article] [PubMed] [Google Scholar]
- 33.Doty R L.The smell identification test administration manual. 3rd edn. White Horse Pike, Haddon Heights, NJ: Sensonics, 1995
- 34.Lanthony P. The desaturated panel D‐15. Doc Ophtalmol 197846185–189. [DOI] [PubMed] [Google Scholar]
- 35.American Thoracic Society Evaluation of impairment/disability secondary to respiratory disorders. Am Rev Respir Dis 19861331205–1209. [DOI] [PubMed] [Google Scholar]
- 36.Bowler R P. Pulmonary function tests. In: Bowler RB, Cone JE, eds. Occupational medicine secrets. Philadelphia, PA: Hanley & Belfus, 1999
- 37.Tietz N W, Andersen B D.Textbook of clinical chemistry. Philadelphia, PA: WB Saunders, 1986
- 38.Wright R O, Amarasiriwardena C, Woolf A D.et al Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school‐age children residing near a hazardous waste site. Neurotoxicology 200627210–216. [DOI] [PubMed] [Google Scholar]
- 39.Wasserman G A, Liu X, Parvez F.et al Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environ Health Perspect 2006114124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowler R M, Mergler D, Sassine M.et al Neuropsychiatric effects of manganese on mood. Neurotoxicology 199920367–378. [PubMed] [Google Scholar]
- 41.Doty R L, Yousem D M, Pham L T.et al Olfactory dysfunction in patients with head trauma. Arch Neurol 1997541131–1140. [DOI] [PubMed] [Google Scholar]
- 42.Mergler D, Huel G, Bowler R.et al Nervous system dysfunction among workers with long‐term exposure to manganese. Environ Res 199464151–180. [DOI] [PubMed] [Google Scholar]
- 43.Fechter L D, Johnson D L, Lynch R A. The relationship of particle size to olfactory nerve uptake of a non‐soluble form of manganese into brain. Neurotoxicology 200223177–183. [DOI] [PubMed] [Google Scholar]
- 44.Elder A, Gelein R, Silva V.et al Translocation of inhaled ultrafine manganese oxide particles to the central nervous system.Environ Health Perspect 20061141172–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss B. Economic implications of manganese neurotoxicity. Neurotoxicology 200627362–368. [DOI] [PubMed] [Google Scholar]