The use of saliva as a diagnostic fluid is a relatively recent trend. This is not surprising when one considers its many advantages and the fact that saliva contains a wide array of constituents.1 Saliva collection is non‐invasive compared with phlebotomy, and, as a result, more acceptable to patients. As obtaining saliva is easy, self‐collection after instruction is possible and there is no need for trained staff. Moreover, it does not carry the risk of needle‐stick injuries. Saliva collection is also less likely to cause stress compared with other invasive procedures such as phlebotomy, an important consideration when researching biomarkers of stress. Lastly, saliva samples can reflect real‐time levels of biomarkers, unlike other biological fluids, such as urine, which is stored in the bladder for a few hours before sampling.
Biomarkers in saliva
A wide range of biomarkers is measurable in saliva, including heavy metals (eg, lead), hormones (eg, cortisol, dehydroxyepiandrosterone (DHEA)), toxins and their metabolites (eg, cotinine), enzymes (eg, lysozyme, α‐amylase), immunoglobulins (eg, IgA), other proteins (eg, eosinophil cationic protein) and DNA. Researchers are also studying the proteomic components of saliva in the hope of identifying novel biomarkers of disease.2,3
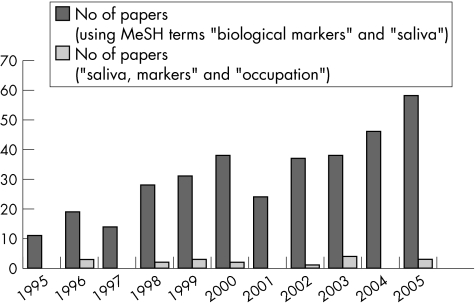
A search of PubMed using the medical subject heading terms “saliva” and “biological markers” showed an increase in the number of studies using salivary biomarkers in the past decade (fig 1). However, when a search using the terms “saliva”, “markers” and “occupation” was performed, the numbers of occupational health papers using salivary markers lagged far behind. Of the 20 papers obtained from the second search, a review of abstracts showed that only 11 had actually used saliva biomarkers in occupational health research. These 11 papers were classified according to areas of occupational health research and the biomarker studied (table 1). The subsequent part of this paper will discuss these four broad groups of salivary biomarkers in two main parts: general research findings of the salivary biomarker and its use in occupational health research.
Figure 1 Number of articles per year in PubMed using respective search terms. MeSH, medical subject headings.
Table 1 Papers obtained from PubMed search using search terms “saliva”, “markers” and “occupation” classified according to the field of application in occupational and environmental medicine and type of biomarker.
Stress
Work stress, a major cause of anxiety, depression, burnout and staff turnover, has been shown to correlate with several salivary biomarkers. The choice of salivary biomarker to examine the body's response to occupational stress depends on the type of stress studied. Chronic stress is associated with the activation of the hypothalamic–pituitary–adrenal (HPA) axis (measured by salivary cortisol), as well as with the depression of immune function (measured by salivary IgA and lysozyme). Acute stress is associated with activation of the sympatho–adreno–medullary system, which is reflected by salivary α‐amylase and chromogranin A. The half‐life of these stress biomarkers should also be considered: t½ of salivary cortisol is about an hour and that of salivary chromogranin A is 15–20 min.
Salivary cortisol
Cortisol is thought to enter saliva by passive diffusion or by other means independent of an active transport mechanism.15 Its level in saliva is lower than that in blood (table 2). Salivary cortisol correlates closer with free physiologically active serum cortisol fraction than with total serum cortisol, which contains the physiologically inactive protein‐bound cortisol fraction.16 Salivary free cortisol correlates better with serum adrenocorticotrophin (given a 15‐min time delay) than serum cortisol, and adrenocorticotrophin is believed to more accurately reflect secretory activity in the HPA axis. An obvious advantage of salivary over serum cortisol measurement is the minimisation of stress from fear of needles during collection, which may bias the results.17 Salivary cortisol secretion, like serum cortisol, displays marked circadian rhythm, characterised by low levels during slow‐wave nocturnal sleep, steady increase during late sleep and peaking just after awakening. Levels then decline rapidly, followed by a sustained gradual decrease for the rest of the day.18 To compensate for diurnal variation, single‐sample determination is usually taken at the same time of the day.
Table 2 Comparative blood and saliva levels of selected biomarkers.
| Biomarker | Normal range | |
|---|---|---|
| Blood | Saliva | |
| Cortisol | 2–25 mg/dl (serum) | 3.5–27.0 mg/dl |
| Morning range | 7–25 mg/dl | 2.0–4.5 mg/dl |
| Evening range | 2–25 mg/dl | 1.0–3.0 mg/dl |
| Chromogranin A | 20–30 ng/ml (serum) | 0.30–0.45 pmol/mg protein |
| α‐Amylase | 0.05–0.125 U/ml (serum) | 19–308 U/ml |
| Secretory IgA | – | Concentration: 100–900 µg/ml |
| Secretion rate: 5–150 µg/min | ||
| Lysozyme | 1–15 µg/ml (serum) | Concentration: 10–300 µg/ml |
| Secretion rate: 3–120 µg/min | ||
| Lead | 5–15 µg/dl (whole blood) | 0.7–7.5 µg/dl (15–50% of whole blood lead) |
| Cotinine (non‐smokers) | <10 ng/ml (serum) | <10 ng/ml (10–40% higher than blood) |
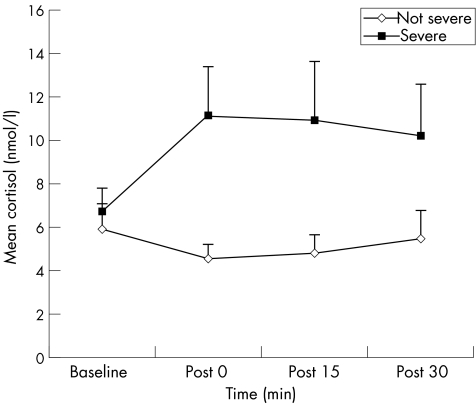
Salivary cortisol has been measured in occupational stress studies on medical personnel, emergency personnel (eg, firefighters, rescue workers) and students. In one study, 20 experienced Dutch male ambulance paramedics were studied during emergency situations.4 Salivary cortisol was measured at baseline (time of incoming emergency call), immediately after delivery of the patient to hospital, and 15 and 30 min thereafter. Greater salivary cortisol reactions were observed during and after handling patients in direct life‐threatening situations than during non‐life‐threatening situations (p = 0 immediately after delivery, p = 0.02 at 15 min and p = 0.07 at 30 min thereafter; fig 2). Weibel et al19 sampled saliva cortisol in emergency ambulance dispatchers during work time every 2 h between 09:00 to 19:00 h during a usual work day, with matched controls during their leisure time. They found significantly increased levels of cortisol in emergency ambulance dispatchers during work time than in controls. Subjective perception of emotional stress positively correlated with total cortisol concentrations per day. It was also found that salivary cortisol positively correlated with anxiety and depressive symptoms using the General Health Questionnaire (GHQ‐28), and with post‐traumatic stress symptoms using the Impact of Events Scale and Post‐Traumatic Symptom Scale, in 65 rescue service workers.5 Among university students, salivary cortisol levels had increased before class assessments, and were positively correlated with pre‐examination stress scores.6,20 These studies show that salivary cortisol levels increase with acute stress.
Figure 2 Cortisol reactivity during handling of patients in emergency situations (n = 33) for severe versus non‐severe patients.4
There is some evidence that chronic stress can paradoxically reduce or blunt cortisol response to stress. In a cross‐sectional study, self‐perceived work‐related stress and saliva cortisol at the start and end of the morning shift were compared between 23 nurses from the emergency department and 50 nurses from general wards.21 Nurses from the emergency department who reported higher levels of work stress on the modified mental health Professional Stress Scale had lower levels of saliva cortisol at the start of shift (geometric mean (GM), 9.10; 95% confidence interval (CI) 6.62 to 12.42 (nmol/l)) than nurses from general wards (GM, 15.45; 95% CI 11.96 to 20.14 (nmol/l)). The logarithm of salivary cortisol concentration was negatively correlated with Professional Stress Scale score. Low levels of cortisol have also been found in the plasma of adolescents with post‐traumatic stress disorders22 and in the saliva of earthquake‐related trauma victims23 and teachers with stress exhaustion (“burn‐out”).24 It is believed that when the HPA axis is subjected to prolonged increased levels of cortisol caused by chronic stress, glucocorticoid receptors in the hypothalamus and pituitary gradually become down regulated.25 The altered HPA axis results in a flattened diurnal cortisol curve and smaller diurnal variation.26,27
Chromogranin A and α‐amylase
Saliva chromogranin (Cg)A and α‐amylase have been shown to be biomarkers of acute stress. CgA is a 48‐kDa acidic glycoprotein stored and secreted by exocytosis from vesicles in the adrenal medulla and sympathetic nerves along with catecholamines. Its secretion correlates with sympathoadrenal release of catecholamines.28 CgA has been localised in human submandibular glands using immunohistochemistry and in‐situ hybridisation.29 Reactivity was stronger in serous cells than in ductal cells. CgA has also been detected in secretory granules of serous and ductal cells by immunoelectron microscopy. Giampaolo et al30 reported a circadian rhythm for CgA in normal subjects, with peak values during the night (at 23:00 h, mean (standard deviation) 65.4 (9.0) µg/l) and a nadir in the morning (at 08:00 h, mean (SD) 43.1 (6.6) µg/l). CgA has a half life of about 18.4 min in blood,28 but the correlation between saliva and serum CgA has not yet been reported.
Salivary α‐amylase is a major enzyme in humans and is secreted from the salivary glands in response to adrenergic stimulation. α‐Amylase degrades starch, first to oligosaccharides and then to maltose and glucose, by hydrolysing α‐1,4‐glucan bonds. Amylase is also found in the pancreas, fallopian tubes, lung, prostate and ovarian tissues. The half life of amylase in plasma is about 12–24 h31 but the half life in saliva has not been reported. A diurnal rhythm for saliva α‐amylase has been described, with levels lowest in the morning and highest in the late afternoon, contrary to cortisol and salivary IgA.32,33
Both salivary CgA and α‐amylase are considered biomarkers of the stress response by the sympatho–adreno–medullary system, unlike cortisol, which is considered a biomarker of stress response by the HPA system. Nakane et al found that salivary CgA‐like immunoreactivity acutely increased in nine adult male volunteers just before they made a public oral presentation compared with a control day,34 and also increased in 12 female students carrying out a word‐processing task for 40 min.35 However, other studies found that pre‐academic assessment stress scores in 31 dental undergraduates correlated with higher cortisol levels but not with CgA,6 and that the State Anxiety Inventory score in subjects exposed to arithmetic stress also did not correlate with CgA.36 The incongruent findings on the stress–CgA relationship could perhaps be due to different levels of stressors in these studies. Further research is needed to clarify this association.
The evidence for α‐amylase being a marker for acute stress seems to be better. Noto et al found that anxiety from arithmetic stress was significantly associated with high salivary α‐amylase levels. Nater et al37 recently found that salivary α‐amylase was significantly increased in 30 healthy young men during the Trier Social Stress Test (mental arithmetic task and free speech in front of an audience) compared with resting conditions. Other authors have found correlations between saliva α‐amylase and adrenergic blockade with β‐blockers, physical exercise, examination stress and extreme temperature stress.38,39,40
Stress and immunity
The relationship between mental stress, the HPA endocrine system and immunity is complex. Psychological stressors increase glucocorticoid levels through increased adrenal activity. Increased glucocorticoid levels inhibit the functions of lymphocytes, macrophages and monocytes, and may increase the susceptibility of individuals to infection.
In a cross‐sectional study of 132 nurses, nurses from the emergency department who reported higher self‐perceived stress exhibited significantly lower levels of salivary secretory IgA and lysozyme. The nurses from the emergency department also reported more frequent episodes of upper respiratory tract infections and sickness absence days because of upper respiratory tract infections than did nurses from general wards.41 Although the study was cross‐sectional and retrospective, it lends supporting evidence for the link between stress, depressed immunity, increased infection rate and sickness absence.
Immunoglobulin A
Secretory IgA is the dominant antibody in saliva and exists as a dimer of IgA molecules linked by a polypeptide (J‐chain), and it is the polypeptide secretory component that makes secretory IgA resistant to proteolysis in an enzyme‐rich environment. Secretory IgA has a half life of 3–6 days and a synthesis rate of 66 mg/kg/day.42
Salivary secretory IgA has been used in several studies as a marker of stress. The protective effect of secretory IgA in the upper gastrointestinal tract is dependent on both the absolute secretory IgA concentration and the salivary flow, so salivary secretory IgA studies usually report secretory IgA secretion rates (µg/min) (defined as concentration of secretory IgA in saliva (µg/ml) multiplied by saliva flow rate (ml/min)) instead of concentration alone.43
In a study of 48 healthy adults whose morning and afternoon saliva were collected for seven consecutive days, salivary secretory IgA secretion rate in the elderly was found to be significantly lower than in the young, mainly because of lower salivary flow rates in older subjects.44 Intra‐individual salivary flow rates and secretory IgA secretion rates among young subjects were found to be significantly higher in the morning than in evening samples, although this difference was not found in the elderly. No correlation was found between salivary secretory IgA secretion rate and concentrations of total proteins in saliva for young or old subjects, suggesting that secretory IgA secretion in saliva is independent of total saliva protein secretion. The study also found that self‐perceived stress during evening saliva sampling negatively correlated with saliva secretory IgA secretion rate.
Hucklebridge et al systematically studied the awakening response of salivary secretory IgA concentrations in 30 healthy young adults (0, 10, 20 and 30 mins after awakening), and the diurnal rhythm of salivary secretory IgA concentrations in six healthy day‐active young adults (hourly intervals for the first 4‐h of the day and three more samples at 2‐h intervals thereafter). They found that salivary secretory IgA reached a peak in the first 30 min after awakening and declined over the next 4 h, reaching a plateau for the rest of the day.45 The study population comprised of adults between 20–45 years of age, so it is still uncertain if this diurnal pattern is the same in other age groups. Hucklebridge et al also found that the diurnal pattern of salivary secretory IgA concentration correlated closely with the diurnal pattern of saliva cortisol. However, it is still unclear whether the diurnal variation of salivary secretory IgA occurred in response to cortisol secretion or another diurnal‐related control mechanism.
Salivary secretory IgA has been found to be negatively correlated with self‐perceived occupational stress among nurses and students. A cross‐sectional study on 106 nurses from the emergency department and 56 nurses from general wards, by administering a modified mental health Perceived Stress Scale and measuring salivary secretory IgA and lysozyme concentrations, showed that nurses from emergency department reported higher levels of stress than nurses from general wards (mean 1.51 v 1.30), and had significantly lower salivary secretory IgA secretion rates (GM 49.1 v 68.2 µg/min).10 Another cross‐sectional study of saliva and Stress Assessment Score among 124 female nurses from surgical wards, operating theatres, medical wards and outpatient clinics showed that salivary secretory IgA secretion rate negatively correlated with self‐reported stress among the nurses (Spearman's r = −0.22, p = 0.01).46,47
In a more recent cross‐sectional study, saliva was collected and a 38‐item dental environmental stress questionnaire was given to 130 dental undergraduate and postgraduate students. Logarithms of salivary secretory IgA secretion rates were found to be inversely correlated to Cohen's Perceived Stress Scale in this population (Spearman's r = −0.20, p<0.05).48
Lysozyme
Lysozyme is a low molecular weight cationic protein synthesised and continuously released from monocytes and macrophages, and is widely distributed in human tissues and secretions. It is considered to be part of the innate immune system. It has enzymatic activity, cleaving β‐1,4 glycosidic bonds between muramic acid and N‐acetylglucosamine residues in the bacterial peptidoglycan cell wall,49 and other antimicrobial properties such as inhibition of bacterial growth and metabolism.
A study by Perera et al reported a negative correlation between perceived stress levels and salivary lysozyme in 39 students, and increased levels after an examination.50 Ng et al initially reported that saliva lysozyme did not correlate with self‐reported levels of stress,11 but the same group of investigators later found that logarithms of salivary lysozyme levels correlated with the mental health Professional Stress Scale.10 A possible reason for this later finding was that the method used to measure lysozyme initially (lysoplate method) was inferior to the more sensitive ELISA method used in the later study.
Heavy metals
Heavy metals such as lead and cadmium are important occupational toxins and can also be found in saliva.
Salivary lead
Plasma lead has two components: protein‐bound lead, which is non‐diffusible, and unbound diffusible lead, which is directly excreted into the saliva. The level of salivary lead is about 15–50% of that in whole blood (table 2), and it exists in the unbound form in saliva.
The notion of using salivary lead for biological monitoring is attractive. A study by P'an reported a strong correlation between saliva and blood lead (Pearson's correlation coefficient, r = 0.72) among adult males over a wide range of blood lead measurements.51 However, when the scattergram presented in P'an's study is examined, it becomes clear that at blood lead levels >500 µg/l, salivary lead levels increase almost exponentially. In addition, the correlation of salivary with blood lead at levels of blood lead >500 µg/l seems to be better than the correlation of data points at blood lead levels <500 µg/l.
In a later study, it was found that salivary lead in occupationally exposed adults correlated poorly with blood lead levels between 100 and 500 µg/l.14 Similar findings have been reported in other non‐occupational populations52,53,54,55 and in more recent epidemiological community studies in Thailand and Mexico.56,57
Moreover, blood contamination of saliva during sampling can spuriously raise salivary lead levels because the lead level in whole blood is 2–6 times higher than that in saliva. Thus, in practice, the use of saliva lead in biomonitoring is probably limited to situations of higher levels of lead exposure, and blood contamination of saliva is a potential problem. Measurement of lower levels of lead in the saliva can also pose technical challenges.
Salivary cadmium
Cadmium is another heavy metal that has been detected in the saliva. The secretion of cadmium into saliva seems to occur by passive diffusion, as shown by in vitro studies on the submaxillary gland of rats,58 but this finding has not been confirmed with human studies. White et al has pointed out that considerable care should be taken in collecting saliva samples for cadmium analysis, because of the risk of contamination during sampling.59
Gervais et al found that 16 workers who were exposed to cadmium dusts and fumes had higher levels of saliva cadmium than non‐exposed workers (59.7 (19.2) v 10.9 (0.8) µg/l, respectively), and that the concentration of cadmium was higher in saliva than in blood (9.8 (1.3) v 6.6 (0.8) µg/l, respectively).60 Cadmium in the saliva of 100 dental students living in Mexico was found to be inversely associated with age (⩽26 years, 2.3 (2.0) µg/l v >26 years, 2.0 (2.5) µg/l).61 However, it is uncertain whether this was due to age in itself or due to other factors such as smoking, environmental exposure, or different absorption or excretion patterns in younger individuals.
Box 1: Methodological issues during sampling of salivary biomarkers
Appropriateness of collection methods for biomarker studied
When using cotton‐based saliva sampling method:
Testosterone, progesterone, oestradiol and dehydroxyepiandrosterone (DHEA) levels are artificially high.
Secretory IgA levels are artificially low.
Cortisol, DHEA sulphate, and cotinine levels are not affected.
Recording of time and duration of collection
Depends on whether biomarkers concentrations are affected by different saliva flow rates.If affected, then biomarker secretion rates should be measured.
Use of screening questions
Use of screening questions such as recent infections, smoking and medication drug history are important, as these can affect levels of certain salivary biomarkers.
Use of single measurements versus several measurements in a subject
To perform valid comparisons of salivary biomarkers between subjects using single measurements, all samples should be collected at the same time and when their levels are representative of the diurnal mean.
Several measurements of salivary biomarkers in a day is also appropriate if the total mean diurnal levels are desired; area under the concentration versus time curve with respect to zero (AUC) should then be measured.
Comparability and sensitivity to existing methods
Saliva sampling needs to be compared against current biomonitoring gold standards.
More sensitive assay techniques are preferred.
Contamination by blood or workplace exposure
Saliva can be contaminated with blood by periodontal disease, dental caries, vigorous teeth‐brushing or oral mucosal injury.
If the levels in blood are significantly higher than those in saliva, the quantitative measurement of salivary concentrations may be spuriously high with blood contamination.
Environmental exposure to the biomarker measured can be minimised by mouth rinsing and collection away from worksite.
Storage and stability issues
For example, secretory IgA and lysozyme concentrations in saliva stored at −30°C are stable for 3 months but significantly decline after 8 months.
Metabolites in saliva
Cotinine
The major pathway of nicotine metabolism is oxidation to cotinine by the cytochrome 2A6 enzyme system in the liver.62 It has been estimated that an average of 70–80% of the nicotine absorbed by a smoker is metabolised to cotinine. The half lives of salivary and plasma cotinine are similar, and the clearance and distribution values in saliva are directly proportional to the corresponding values in plasma. Clearance and half life of cotinine have been shown to be ethnically dependent. For example, the average clearance of cotinine is about 20% slower in African Americans than in Caucasian Americans. In Chinese Americans, the average renal clearance of cotinine was 40% slower than in Caucasian Americans, but the average non‐renal clearance was 30% faster. In addition, interindividual variation was found between nicotine intake and cotinine levels because of genetic polymorphisms of the cytochrome 2A6 enzymes and intraindividual variation from concurrent intake of drugs that affect the activity of the cytochrome 2A6 enzymes.
Nevertheless, cotinine is highly specific and sensitive to tobacco use in the absence of nicotine replacement therapy, and has been shown to correlate well with recent nicotine exposure.63 Cotinine in saliva is stable at room temperature (approximately 22°C) for up to 2 weeks, and, in a paper by Feyerabend et al, it was found to correlate well with plasma (r = 0.82) and urine (r = 0.91) levels in 85 non‐smokers.64 Its salivary concentrations are about 10–40% higher than in plasma, but can vary depending on stimulation of saliva production.
In terms of exposure characterisation of individuals, Etzel suggested that persons with salivary cotinine levels <0.1 ng/ml are non‐smokers with no passive exposure; those with cotinine concentrations between 0.1 and 30 ng/ml are non‐smokers who have probable passive tobacco exposure; those with cotinine concentrations between 30 and 100 ng/ml are occasional smokers who may have passive exposure; and those levels with >100 ng/ml are active smokers.65 Salivary cotinine has been used in environmental research to document decrease in environmental tobacco smoke exposure among smokers and non‐smokers in the workplace after work‐site anti‐smoking policies were implemented.66,67,68
Other salivary biomarkers
There are many other biomarkers that have been measured and studied in saliva. However, the rest are currently inadequately characterised, exist in very low concentrations or are not clinically useful.69 For example, there are conflicting results regarding correlation of L‐thyroxine levels between serum and saliva. Salivary growth hormone has been shown to correlate with serum levels but because its concentration in saliva is a thousand‐fold lower than in serum, its measurement in saliva is difficult. Melatonin can be measured in saliva and has been used in studies on shift work.70 Its salivary levels exhibit circadian rhythm and may be undetectable during daylight exposure. Moreover, because the clinical significance of melatonin is still uncertain, its current usefulness in research may be limited. On the other hand, the use of saliva biomarkers of alcohol and drug abuse is well‐established, and there are good reviews on this subject.71,72 However, this will not be discussed in this paper.
DNA in saliva
As the cost of large‐scale DNA sequencing and genotyping has decreased in the past few years, there have been increasing numbers of studies determining the genetic determinants of common complex diseases. Such large‐scale genetic epidemiological studies need convenient and efficient methods of obtaining genomic DNA from subjects. Commercial kits for DNA extraction from saliva are already available, and this method of DNA sampling is more acceptable than the traditional method of using whole blood. Ng et al have shown that saliva is a viable alternative source of human genomic DNA, yielding an average of 35 µg of DNA of reasonable quality from 2 ml of saliva.73 They also estimated that the amount of DNA obtained from 2 ml of saliva is sufficient for an average of 1750 genetic markers, assuming that each polymerase chain reaction requires 2 ng of DNA. The amount of DNA that can be extracted from saliva can be increased by brushing the inside of each cheek 30 times with a sterile swab, which results in a higher fraction of human cells, as reported by Quinque et al.74
Methodological issues yet to be resolved
Before saliva biomarkers become widely used in health settings, many methodological issues need to be resolved. Box 1 gives a summary of these methodological issues, which are discussed below.
Appropriateness of collection methods for biomarker studied
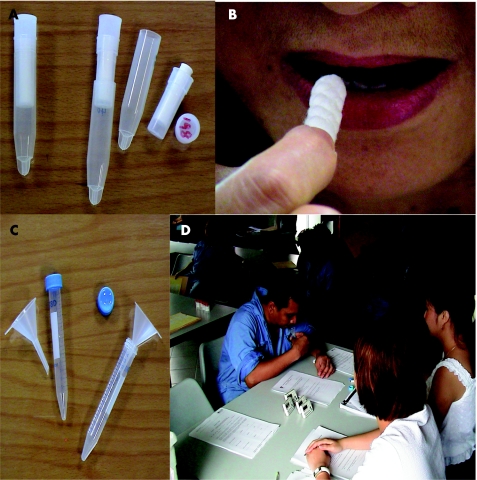
There are several methods to collect saliva, which can be classified into two main techniques: cotton‐based and non‐cotton‐based. Cotton‐based techniques include using a simple cotton dental roll to specialised devices such as the Salivette (fig 3) or Sorbette. Salivette contains a cotton roll that is sucked or chewed for a minute, whereas Sorbette is a cellulose‐cotton tip “eyespear” applicator placed under the tongue to absorb saliva into a capillary tube in its stem. Sorbette offers methodological advantages because it is smaller and can be held during sampling, features that make it particularly suitable for children and the frail elderly. Both cotton‐based methods require centrifugation to extract the saliva. Non‐cotton‐based methods of saliva collection include passive drool (fig 3), spit samples and expectoration through a plastic straw into a collection vial.
Figure 3 Saliva collection tools: Salivette (A), where the subject chews or sucks on the cotton roll (B), and the passive drool method (D) with a funnel and test tube (C).
When cotton‐based saliva sampling methods are used, salivary levels of steroid hormones such as testosterone, progesterone, oestradiol and DHEA may be artificially high, whereas for salivary secretory IgA, the levels may be artificially low. In contrast, salivary cortisol, DHEA sulphate and cotinine may not be affected by the cotton‐based method. The reason for these differences is still uncertain, but Shirtcliff et al postulated that an interfering substance may be filtered out by cotton, resulting in increased availability of steroid hormones for analysis or that plant hormones may be cross‐reacting with secretory IgA.75 Strazdins et al found that the cotton‐based method reduced the concentration of both salivary cortisol and secretory IgA compared with passive saliva collection.76 However, there seem to be differences even when different types of cotton‐based methods (eg, Sorbette “eyespear” and Salivettes) are used. Awareness of such sampling issues is important in ensuring measurement validity in any salivary biomarker assay.
Key points
Saliva collection is non‐invasive, relatively simple, less likely to cause stress and can reflect real‐time levels of biomarkers.
Biomarkers used in occupational health studies include heavy metals (eg, lead), hormones (eg, cortisol), toxins and their metabolites (eg, cotinine), enzymes (eg, lysozyme and α‐amylase), immunoglobulins (eg, secretory IgA) and genetic material.
Stress markers found in saliva include cortisol, chromogranin A and α‐amylase.
Biomarkers of immunity found in saliva include secretory immunoglobulin A and lysozyme.
Salivary lead may be used in toxicity studies if whole blood lead levels are >50 µg/dl.
Salivary cotinine, a metabolite of nicotine, may be used to characterise smokers and measure environmental tobacco smoke exposure
Methodological issues to consider when using salivary biomarkers include sampling method, measurement of saliva flow rates, screening questions, circadian variation (if any), blood and environmental contamination, and stability during storage.
Recording of time and duration of collection
If the secretion rate is desired, then saliva collection should be timed. Salivary biomarker secretion rate is dependent on saliva flow rate, and stimulation of saliva flow rate can be achieved by various means (eg, oral instillation with citric acid). Whether the saliva collection method is a stimulated or unstimulated, the procedure should be specified and the method of salivary stimulation should be reported.
The decision whether to use salivary biomarker concentration or secretion rate as measurement units depends on whether biomarker concentrations vary with different saliva flow rates, in which case salivary secretion rates would be preferred.
Use of screening questionnaires
Several conditions may affect the levels of various salivary biomarkers. For example, Hibel et al recently reported that saliva cortisol reactivity to challenge tasks was less pronounced for infants taking paracetamol (acetaminophen) and mothers taking pure agonist opioids, and greater for mothers taking oral or transdermal contraceptives and acetylsalicylic acid.77 These drug differences remained significant after controlling for a wide range of possible confounding variables such as sampling frame, fever, maternal anxiety and depression, and socioeconomic factors. It would be impractical for any study to exclude participants using any of these common over‐the‐counter drugs, so it is recommended that drug history be obtained during saliva collection and statistically controlled to ensure measurement validity of salivary biomarkers.
Recent upper respiratory infections may affect salivary IgA and lysozyme levels, smoking may affect cortisol and α‐amylase, and other drugs (eg, corticosteroids) may affect IgA and lysozyme. These factors should be considered and appropriate screening questions included in study protocols.
Use of single measurements versus several measurements in a subject
To perform valid comparisons of salivary biomarkers between subjects using single measurements, all samples should be collected at the same time and when their levels are representative of the diurnal mean. For example, saliva cortisol, secretory IgA and α‐amylase could be sampled in the afternoon because their levels rise sharply just after awakening and become stable by the afternoon.
Several measurements of salivary biomarkers in a day are appropriate if the total mean diurnal level is desired; measurement of area under the concentration versus time curve with respect to zero would then be preferred.18
Comparability and sensitivity of saliva sampling with existing biomonitoring methods
Saliva sampling, being a new way of measuring biomarkers, needs to be compared against the current gold standards of biological monitoring before being widely accepted. For example, the correlation between serum and salivary cotinine is strong, but this relationship is not seen with blood and salivary lead in instances of low levels of lead exposure. The sensitivity of the assay technique should also be considered as illustrated by lower sensitivity of the lysoplate method compared with the ELISA method for measuring salivary lysozyme levels.
Contamination by blood or workplace exposure
Contamination of saliva with blood is another issue in sampling, as shown by saliva lead measurements. Considering that the concentration of salivary biomarkers can be 10–100‐fold higher in blood than in saliva (table 2), blood contamination (by periodontal disease, dental caries, injury or vigorous teeth brushing) can significantly affect the quantitative estimate of their salivary concentrations.78 A possible solution to minimise contaminated samples could be to check for blood contamination using occult blood testing techniques during collection. Such samples should be discarded and saliva collection repeated. Contamination of samples from environmental sources of the biomarker during collection may also occur, and this may lead to large measurement errors. This can be minimised by collecting samples away from the worksite and ensuring that the subjects rinse their mouths thoroughly before collection. A period of time should also elapse between mouth rinsing and sampling for attainment of steady‐state levels of biomarkers for more accurate measurements. Lastly, personal habits such as the chewing of betel nut may give rise to contamination of saliva by other extraneous substances, and hence should be excluded before sampling.
Storage and stability issues
The stability of biomarkers during storage is also another methodological issue that needs to be considered. A study found that the concentrations of secretory IgA and lysozyme in saliva stored at −30°C remained stable for up to 3 months but significantly declined after 8 months.79 DNA yield and genotyping fidelity are not significantly diminished if samples are stored at room temperature for 6 months,80 a finding that was also supported by a later report.74 In another study,73 it was also found that DNA can still be extracted successfully from saliva for at least a 1 month when stored at −70°C, an important finding with regard to measurement validity in the field of research into salivary biomarkers.
Conclusion
Salivary biomarkers offer a novel approach in occupational health practice with their ease of collection and potentially wide scope for application. Currently, they are beginning to be established in the assessment of occupational stress (using cortisol and α‐amylase) and oral mucosal immunity (using secretory IgA). However, there is a need for awareness and understanding of their biological significance and the methodological issues involved when using them. Further research and validation studies are needed for less characterised biomarkers before they can be used in occupational and environmental health. To date, much of the current research in salivary biomarkers has been focused on discovering correlations on a group basis. Further research on the individual level is needed before salivary biomarkers can be used in daily practice.
Acknowledgements
This work was supported by grant number R‐186‐000‐077‐101 from the Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
QUESTION (SEE ANSWER ON P 149)
What is the future of salivary biomarkers in occupational health?
Footnotes
Competing interests: GC‐HK and DS‐QK have no affiliations with any pharmaceutical or laboratory diagnostics company.
References
- 1.Malamud D, Tabak L. Saliva as a diagnostic fluid. Ann N Y Acad Sci 19936941–348. [PubMed] [Google Scholar]
- 2.Vitorino R, Lobo M J, Ferrer‐Correira A J.et al Identification of human whole saliva protein components using proteonomics. Proteomics 200441109–1115. [DOI] [PubMed] [Google Scholar]
- 3.Xie H, Rhodus N L, Griffin R J.et al A catalogue of human saliva proteins identified by free flow electrophoresis‐based peptide separation and tandem mass spectrometry. Mol Cell Proteomics 200541826–1830. [DOI] [PubMed] [Google Scholar]
- 4.Sluiter J K, van der Beek A J, Frings‐Dresen M H. Medical staff in emergency situations: severity of patient status predicts stress hormone reactivity and recovery. J Occup Environ Med 200360373–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aardal‐Ericksson E, Eriksson T E, Holm A C.et al Salivary cortisol and serum prolactin in relation to stress rating scales in a group of rescue workers. Biol Psychiatry 199946850–855. [DOI] [PubMed] [Google Scholar]
- 6.Ng V, Koh D, Mok B Y Y.et al Salivary biomarkers associated with academic assessment stress among dental undergraduates. J Dent Educ 2003671091–1094. [PubMed] [Google Scholar]
- 7.Heinrichs M, Wagner D, Schoch W.et al Predicting posttraumatic stress symptoms from pretraumatic risk factors: a 2‐year prospective follow‐up study in firefighters. Am J Psychiatry 20051622276–2286. [DOI] [PubMed] [Google Scholar]
- 8.Fischer J E, Calame A, Dettling A C.et al Objectifying psychomental stress in the workplace–an example. Int Arch Occup Environ Health 200073S46–S52. [DOI] [PubMed] [Google Scholar]
- 9.Sluiter J K, Frings‐Dresen M H, Meijman T F.et al Reactivity and recovery from different types of work measured by catecholamines and cortisol: a systematic literature overview. J Occup Environ Med 200057298–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Koh D, Ng V.et al Self‐perceived work‐related stress and the relationship with salivary IgA and lysozyme among emergency department nurses. J Occup Environ Med 200259836–841.This paper with the paper by Ng et al (1999) show the importance of using a sensitive assay to detect salivary biomarkers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng V, Koh D, Chan G.et al Are salivary IgA and lysozyme biomarkers of stress among nurses? J Occup Environ Med 199941920–927. [DOI] [PubMed] [Google Scholar]
- 12.Mulcahy M, Evans D S, Hammond S K.et al Secondhand smoke exposure and risk following the Irish smoking ban: an assessment of salivary cotinine concentrations in hotel workers and air nicotine levels in bars. Tob Control 200514384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaKind J S, Jenkins R A, Naiman D Q.et al Use of environmental tobacco smoke constituents as markers for exposure. Risk Anal 199919359–373. [DOI] [PubMed] [Google Scholar]
- 14.Koh D, Ng V, Chua L H.et al Can salivary lead be used for biological monitoring of lead exposed individuals? J Occup Environ Med 200360696–698.This paper found that salivary lead levels correlated poorly with blood lead levels <50 µg/dl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirschbaum C, Hellhammer D H. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology 199419313–333.This paper provides a good review on the methodological issues and applications of salivary cortisol. [DOI] [PubMed] [Google Scholar]
- 16.Hiramatsu R. Direct assay of cortisol in human saliva by solid phase radioimmunoassay and its clinical applications. Clin Chim Act 1982117239–249. [DOI] [PubMed] [Google Scholar]
- 17.Meeran K, Hattersley A, Mould G.et al Venepuncture causes rapid rise in ACTH. Br J Clin Pract 199347246–247. [PubMed] [Google Scholar]
- 18.Edwards S, Clow A, Evans P.et al Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci 2001682093–2203.This landmark paper reported that salivary free cortisol response was related to awakening and not just time. It also found that area under the concentration curve was representative of the diurnal activity but area under the response curve was not. [DOI] [PubMed] [Google Scholar]
- 19.Weibel L, Gabrion I, Aussedat M.et al Work‐related stress in an emergency medical dispatch center. Ann Emerg Med 200341500–506. [DOI] [PubMed] [Google Scholar]
- 20.Ng V, Koh D, Chia S E. Examination stress, salivary cortisol, and academic performance. Psychol Rep 2003931133–1134. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Koh D, Ng V.et al Salivary cortisol levels and work‐related stress among emergency department nurses. J Occup Environ Med 2001431011–1018. [DOI] [PubMed] [Google Scholar]
- 22.Boscarino J A. Posttraumatic stress disorder, exposure to combat and lower plasma cortisol among Vietnam veterans: findings and clinical implications. J Consult Clin Psychol 199664191–201. [DOI] [PubMed] [Google Scholar]
- 23.Goenjian A K, Yehua R, Pynoos R S.et al Basal cortisol, dexamethasone suppression of cortisol, and MHPG in adolescents after the 1988 earthquake in Armenia. Am J Psychiatr 1996153929–934. [DOI] [PubMed] [Google Scholar]
- 24.Pruessner J C, Hellhammer D H, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med 199961197–204. [DOI] [PubMed] [Google Scholar]
- 25.Uno H, Eisele S, Sakai A.et al Neurotoxicity of glucocorticoids in the primate brain. Horm Behav 199428336–348. [DOI] [PubMed] [Google Scholar]
- 26.Dallman M F. Stress update. Adaptation of the hypothalamic‐pituitary‐adrenal axis to chronic stress. Trends Endocrinol Metab 1993462–69. [DOI] [PubMed] [Google Scholar]
- 27.McEwan B S. Protective and damaging effects of stress mediators. N Engl J Med 1998338171–179. [DOI] [PubMed] [Google Scholar]
- 28.O'Conner D T, Bernstein K N. Radioimmunoassay of chromogranin A in plasma as a measure of exocytic sympathoadrenal activity in normal subjects and patients with phaeochromocytoma. N Engl J Med 1984311764–770. [DOI] [PubMed] [Google Scholar]
- 29.Saruta J, Tsukinoki K, Sasaguri K.et al Expression and localization of chromogranin A gene and protein in human submandibular gland. Cells Tissues Organs 2005180237–244. [DOI] [PubMed] [Google Scholar]
- 30.Giampaolo B, Angelica M, Antonio S. Chromogranin ‘A' in normal subjects, essential hypertensives and adrenalectomized patients. Clin Endocrinol (Oxf) 20025741–50. [DOI] [PubMed] [Google Scholar]
- 31.Koay E S C, Walmsley R N.Handbook of chemical pathology. Chapter 13, 1st edn. Singapore: PG Medical Books, 1989229–230.
- 32.Li T L, Gleeson M. The effect of single and repeated bouts of prolonged cycling and circadian variation on saliva flow rate, immunoglobulin A and alpha‐amylase responses. J Sports Sci 2004221015–1024. [DOI] [PubMed] [Google Scholar]
- 33.Rohleder N, Nater U M, Wolf J M.et al Psychosocial stress‐induced activation of salivary alpha‐amylase: an indicator of sympathetic activity? Ann N Y Acad Sci 20041032258–263. [DOI] [PubMed] [Google Scholar]
- 34.Nakane H, Asami O, Yamada Y.et al Salivary chromogranin A as an index of psychosomatic stress response. Biomed Res 199819401–406. [Google Scholar]
- 35.Nakane H, Asami O, Yamada Y.et al Effect of negative air ions on computer operation, anxiety and salivary chromogranin A‐like immunoreactivity. Int J Psychophysiol 20024685–89. [DOI] [PubMed] [Google Scholar]
- 36.Noto Y, Sato T, Kudo M.et al The relationship between salivary biomarkers and state‐trait anxiety inventory score under mental arithmetic stress: a pilot study. Anesth Analg 20051011873–1876. [DOI] [PubMed] [Google Scholar]
- 37.Nater U M, La Marca R, Florin L.et al Stress‐induced changes in human salivary alpha‐amylase activity–associations with adrenergic activity. Psychoneuroendocrinology 20063149–58. [DOI] [PubMed] [Google Scholar]
- 38.Van Stegeren A, Rohleder N, Everaerd W.et al Salivary alpha amylase as marker for adrenergic activity during stress: effect of betablockade. Psychoneuroendocrinology 200631137–141. [DOI] [PubMed] [Google Scholar]
- 39.Takai N, Yamaguchi M, Aragaki T.et al Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Arch Oral Biol 200449963–968.This paper found that salivary amylase was increased more rapidly and significantly than salivary cortisol in response to acute psychological stress. [DOI] [PubMed] [Google Scholar]
- 40.Chatterton R T, Jr, Vogelsong K M, Lu Y C.et al Salivary alpha‐amylase as a measure of endogenous adrenergic activity. Clin Physiol 199616433–448. [DOI] [PubMed] [Google Scholar]
- 41.Koh D, Yong Y, Ng V.et al Stress, mucosal immunity, upper respiratory tract infections and sickness absence. J Occup Environ Med 200244987–988. [DOI] [PubMed] [Google Scholar]
- 42.Mestecky J. Saliva as a manifestation of the common mucosal immune system. Ann N Y Acad Sci 1993694184–194. [DOI] [PubMed] [Google Scholar]
- 43.Mackinnon L T, Hooper S. Mucosal (secretory) immune system responses to exercise of varying intensity and during overtraining. Int J Sports Med 199415S179–S183. [DOI] [PubMed] [Google Scholar]
- 44.Miletic I D, Schiffman S S, Miletic V D.et al Salivary IgA secretion rate in young and elderly persons. Physiol Behav 199660243–248. [DOI] [PubMed] [Google Scholar]
- 45.Hucklebridge F, Clow A, Evans P. The relationship between salivary secretory immunoglobulin A and cortisol: neuroendocrine response to awakening and the diurnal cycle. Int J Psychophysiol 19983169–76.This paper reported the inverse relationship between salivary sIgA and salivary cortisol in their diurnal cycle. [DOI] [PubMed] [Google Scholar]
- 46.Ng V, Koh D, Chan G.et al Are salivary IgA and lysozyme biomarkers of stress among nurses? J Occup Environ Med 199941920–927. [DOI] [PubMed] [Google Scholar]
- 47.Ng V, Koh D, Chan G.et al Stress and salivary IgA among female nurses. Proceedings of an International Expert Meeting on Women at Work . 1997, Research Report 20196–203.
- 48.Ng V, Koh D, Mok B.et al Stressful life events of dental students and salivary immunoglobulin A. Int J Immunopathol Pharmacol . 2004;17: 2,S49–S56. [DOI] [PubMed]
- 49.Jolles P, Jolles J. What is new in lysozyme research? Always a model system, today as yesterday. Mol Cell Biochem 198463165–189. [DOI] [PubMed] [Google Scholar]
- 50.Perera S, Uddin M, Hayes J A. Salivary lysozyme: a noninvasive marker for the study of the effects of stress of natural immunity. Int J Behav Med 19974170–178. [DOI] [PubMed] [Google Scholar]
- 51.P'an A Y. Lead levels in saliva and in blood. J Toxicol Environ Health 19817273–280. [DOI] [PubMed] [Google Scholar]
- 52.Omokhodion F O, Cockford G W. Lead in sweat and its relationship to salivary and urinary levels in normal healthy subjects. Sci Tot Environ 1991103113–122. [DOI] [PubMed] [Google Scholar]
- 53.Fung H L, Yaffe, Mattar M E.et al Blood and saliva lead levels in children. Clin Chim Acta 197561423–424. [DOI] [PubMed] [Google Scholar]
- 54.DiGergorio G J, Ferko A P, Sample R G.et al Lead and δ–aminolevulinic acid concentrations in human parotids saliva. Toxicol Appl Pharmacol 197427491–493. [DOI] [PubMed] [Google Scholar]
- 55.Liu P. (Study on comparison of the determination of lead concentrations in saliva and blood). Zhonghua Yu Fang Yi Xue Za Zhi 19912530–32. [PubMed] [Google Scholar]
- 56.Thaweboon S, Thaweboon B, Veerapradist W. Lead in saliva and its relationship to blood in the residents of Klity Village in Thailand. Southeast Asian J Trop Med Public Health 2005361576–1579. [PubMed] [Google Scholar]
- 57.Barbosa F, Jr, Correa Rodrigues M H, Buzalaf M R.et al Evaluation of the use of salivary lead levels as a surrogate of blood lead or plasma lead levels in lead exposed subjects. Arch Toxicol 200680633–637. [DOI] [PubMed] [Google Scholar]
- 58.Smith L W, Borzelleca J F. Excretion of cadmium and mercury in rat saliva. Toxicol Appl Pharmacol 198054134–140. [DOI] [PubMed] [Google Scholar]
- 59.White M A, O'Hagan S A, Wright A L.et al The measurement of salivary cadmium by electrothermal atomic absorption spectrophotometry and its use as a biological indicator of occupational exposure. J Expos Anal Environ Epidemiol 19922195–206. [PubMed] [Google Scholar]
- 60.Gervais L, Lacasse Y, Brodeur J.et al Presence of cadmium in the saliva of adult male workers. Toxicol Lett 1981863–66. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez M, Banderas J A, Baez A.et al Salivary lead and cadmium in a young population residing in Mexico City. Toxicol Lett 19979355–64. [DOI] [PubMed] [Google Scholar]
- 62.Bramer S L, Kallungal B A. Clinical considerations in study designs that use continine as a biomarker. Biomarkers 20038187–203.This excellent paper reviews the metabolism of nicotine to cotinine, methods to assess exposure to tobacco smoke and recommend standards for clinical studies using cotinine. [DOI] [PubMed] [Google Scholar]
- 63.Bernett J T, McGuffey J E, Morrison M A.et al Comparison of serum and salivary cotinine measurements by a sensitive high‐performance liquid chromatography‐tandem mass spectrometry method as an indicator of exposure to tobacco smoke among smokers and nonsmokers. J Anal Toxicol 200024333–339. [DOI] [PubMed] [Google Scholar]
- 64.Feyerabend C, Bryant A E, Jarvais M J.et al Determination of cotinine in biological fluids of non‐smokers by packed column gas‐liquid chromatography. J Pharm Pharmacol 198638917–919. [DOI] [PubMed] [Google Scholar]
- 65.Etzel R A. A review of the use of saliva cotinine as marker of tobacco smoke exposure. Prev Med 199019190–197. [DOI] [PubMed] [Google Scholar]
- 66.Allwright S, Paul G, Greiner B.et al Legislation for smoke‐free workplaces and health of bar workers in Ireland: before and after study. BMJ 20053311117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brigham J, Gross J, Stitzer M L.et al Effects of a restricted work‐site smoking policy on employees who smoke. Am J Pub Health 199484773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Broder I, Pilger C, Corey P. Environment and well‐being before and following smoking ban in office buildings. Can J Public Health 199384254–258. [PubMed] [Google Scholar]
- 69.Derr R L, Cameron S J, Golden S H. Pre‐analytic considerations for the proper assessment of hormones of the hypothalamic‐pituitary axis in epidemiological research. Eur J Epidemiol 199621217–226. [DOI] [PubMed] [Google Scholar]
- 70.Lamond N, Dorrian J, Roach G D.et al The impact of a week of simulated night work on sleep, circadian phase, and performance. J Occup Environ Med 200360e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swift R. Direct measurement of alcohol and its metabolites. Addiction 200398S73–S80. [DOI] [PubMed] [Google Scholar]
- 72.Wolff K, Farrell M, Marsden J.et al A review of biological indicators of illicit drug use, practical considerations and clinical usefulness. Addiction 1999941279–1298. [DOI] [PubMed] [Google Scholar]
- 73.Ng D P K, Koh D, Choo S.et al Effect of storage conditions on the extraction of PCR‐quality genomic DNA from saliva. Clin Chim Acta 2004343191–194. [DOI] [PubMed] [Google Scholar]
- 74.Quinque D, Kittler R, Kayser M.et al Evaluation of saliva as a source of human DNA for population and association studies. Anal Biochem 2006353272–277. [DOI] [PubMed] [Google Scholar]
- 75.Shirtcliff E A, Granger D A, Schwartz E.et al Use of salivary biomarkers in biobehavioural research: cotton‐based collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology 200126165–173.This paper found that cotton‐based saliva sampling can raise levels of sex steroid hormones but reduce sIgA levels. [DOI] [PubMed] [Google Scholar]
- 76.Strazdins L, Meyerkort S, Brent V.et al Impact of saliva collection methods on sIgA and cortisol assays and acceptability to participants. J Immunol Methods 2005307167–171. [DOI] [PubMed] [Google Scholar]
- 77.Hibel L C, Granger D A, Kivlighan K T.et al Individual differences in salivary cortisol: associations with common over‐the‐counter and prescription medication status in infants and their mothers. Horm Behav 200650293–300. [DOI] [PubMed] [Google Scholar]
- 78.Granger D A. Integrating salivary biomarkers into behavioural medicine research: practical aspects of study design, sample collection and assay. Presented at the Annual Meeting for the Society for Behavioral Medicine, Nashville 2000
- 79.Ng V, Koh D, Fu Q.et al Effects of storage time on stability of salivary immunoglobulin A and lysozyme. Clin Chim Acta 2003338131–134.This paper illustrates the importance of analysing salivary biomarkers within recommended storage times for biomarker stability. [DOI] [PubMed] [Google Scholar]
- 80.Ng D P K, Koh D, Choo S.et al Saliva as a viable alternative source of human genomic DNA in genetic epidemiology. Clin Chem Acta 200636781–85. [DOI] [PubMed] [Google Scholar]