Abstract
Aims
To examine the association of hourly time lagged concentration of ambient particulate matter and death due to stroke.
Methods
Mortality data for five years (January 1990 to December 1994) were obtained from the Ministry of Health, Labour, and Welfare of Japan. Data were used only if the deceased was 65 years old or older at the time of death, if death was attributed to intracerebral haemorrhage or ischaemic stroke, and if the deceased lived in one of 13 major urban areas. Hourly mean concentrations of PM7, NO2, and photochemical oxidants were measured at monitoring stations in the 13 areas. Time stratified case‐crossover analysis was used to examine the data for evidence of triggering stroke mortality.
Results
The 1‐hour mean concentration of PM7 measured about 2 hours before death was associated with the risk of death due to intracerebral haemorrhage from April to September (odds ratio = 2.40, 95% CI 1.48 to 3.89, for exposure to PM7 of more than 200 μg/m3 (threshold)). The higher risk was independent of the 24‐hour mean concentration of PM7. PM7 was not associated with death due to ischaemic stroke.
Conclusions
Transiently high concentrations of PM7 are associated with death due to intracerebral haemorrhage. Air quality standards or guidelines for particulate matter should be based not only on 24‐hour mean concentrations, but also on hourly data.
Keywords: air pollution, cerebral haemorrhage, mortality
Air pollution is associated with cardiovascular mortality and morbidity, and its effects can be seen very soon after exposure.1,2,3,4 Inhaled particles can be detected in blood as early as 1 minute after they are inhaled, can remain at their maximum level in blood for up to 60 minutes,4 and may decrease vagal tone.3 Outside the laboratory, the effects of such exposures on cardiovascular morbidity and mortality might be relatively difficult to study when the indices of exposure are 24‐hour mean concentrations. Transiently high concentrations of air pollution have been observed in hourly data,5 but their effects on cardiovascular events have been measured only rarely. Non‐laboratory studies of associations between brief air pollution events and cardiovascular health are few and their results are inconsistent: Peters et al found that the risk of myocardial infarction (MI) was higher within 2 hours after exposure to high concentrations of fine particles,6 but in two studies no association was found between hourly concentrations of ambient particulate matter and onset of MI.7,8 To look for links between air pollution and other cardiovascular events, we collected and analysed hourly air pollution data and records of deaths due to ischaemic and haemorrhagic stroke.
Methods
Subjects
The study was done in the 13 urban areas designated, on the basis of population size, as major cities by the government of Japan (Chiba, Fukuoka, Hiroshima, Kawasaki, Kitakyushu, Kobe, Kyoto, Nagoya, Osaka, Sapporo, Sendai, the Tokyo metropolitan area, and Yokohama). Mortality data for the period January 1990 to December 1994, coded according to the 9th revision of the International Classification of Diseases (ICD‐9), were obtained from the Ministry of Health, Labour, and Welfare of Japan. Subjects were 65 years old or older when they died, and the cause of death was intracerebral haemorrhage (ICD‐9 code 431), and ischaemic stroke (ICD‐9 code 434.) Cause of death was defined by the patient's consulting physician. When a patient dies, the physician records the disease that was the main cause of death and the time of the event, and prepares the death certificate. In this study, the source of data was from death certificates. Approval by an ethics committee was not sought because all the data were obtained from anonymous statistical registers.
Exposure assessments
Suspended particulate matter (SPM) is defined under the Japanese Air Quality Standard as any particle collected with an upper 100% cut‐off point of 10 μm aerodynamic diameter. The 50% cut‐off diameter for SPM is assumed to be approximately 7 μm; therefore, we referred to this variable as PM7.9 In Japan, 1‐hour and 24‐hour mean concentrations of PM7 are measured and recorded at government managed monitoring stations. We used data from one centrally located monitoring station in each study area. Hourly concentrations of PM7, nitrogen dioxide (NO2), and photochemical oxidants (Ox) from January 1990 to December 1994 were used. Hourly temperature and relative humidity data for each area were obtained from the Japan Meteorological Agency.
Statistical methods
We used time stratified case‐crossover analysis, a technique for assessing brief changes in risk associated with transient exposures.10,11 Case‐crossover analyses require exposure data for cases only. They can be regarded as a special type of case‐control study in which each case serves as his or her own control. This design has the advantage of controlling for potential confounding caused by fixed individual characteristics, such as sex, race, diet, and age. “Time stratified” indicates the method by which the control periods were chosen. Specifically, we stratified time into months to select days of control periods that fell on the same day of the week within the same month as the date of death (day of the index period). For example, if death occurred on 18 November, the three control days were 4, 11, and 25 November. The control periods were also matched by time (clock hour) of index periods. Therefore, this approach also controls for long term trends, seasonality, day of the week, and circadian rhythm. The merits of case‐crossover designs in studies of health effects of air pollution have been discussed in detail by Schwartz.12
We separated risks associated with transiently high concentrations of pollutants from those associated with 24‐hour mean concentrations. If 24‐hour concentrations of air pollutants can be resolved into hourly concentrations, the association between exposure levels and health status could be represented as follows:
Yt = a + b(WtXt + Wt−1Xt−1 + Wt−2 Xt−2 + … +Wt−pXt−p) + et (equation 1)
where Y is the index of health status (in this study, Y was either 0 or 1; 1 indicated death), and the subscript “t” is the hour during which death occurred. The subscripts “1,2…p” indicate the number of hours before the hour of death, also called “lag”. Xt, Xt−1, Xt−2…Xt−p are concentrations of air pollutants during the hour of death (Xt) and during previous hours. Wt, Wt−1, Wt−2…Wt−p are weights that reflect the relative effects of current and lagged pollution levels. This is a “restricted distributed lag” model.13 The effects of air pollutants might be estimated by such a model, but assigning the appropriate weights is difficult because there is not enough biological information. The 24‐hour mean concentration of air pollutants could be thought of as a cumulative exposure index, assuming that p ranges from 0 to 23 (0 ⩽ p < 24) and that Wt, Wt−1, Wt−2…Wt−p are all set at 1. However, such assumptions can result in a model that is insensitive to some short term changes in concentrations of air pollutants. Because air pollution events that are not reflected in 24‐hour mean concentrations could have important physiological effects, we therefore analysed data on hourly concentrations of air pollutants independently from the data on 24‐hour mean concentrations. We analysed the hourly air‐pollution data as a dichotomous variable for several reasons: to focus on concentrations above the national standards, to look for transient (hourly) phenomena, and to minimise effects of multicollinearity between 1‐hour and 24‐hour values (as these are serially correlated).
First, we examined associations between 24‐hour mean concentrations of air pollutants and the risk of death due to stroke. These concentrations are subject specific values averaged over the 24 hours before the index death time. We estimated odds ratios (ORs) of death caused by stroke for 30 μg/m3 differences in PM7, 10 ppb differences in NO2, and 10 ppb differences in Ox in a multi‐pollutant model adjusted for 24‐hour mean temperature and relative humidity (Model A). Using Model A, we also calculated ORs of death due to intracerebral haemorrhage (ICD‐9: 431) and ischaemic stroke (ICD‐9: 434) for each 30 μg/m3 increase in various lagged 24‐hour mean concentrations of PM7. These results are adjusted for 24‐hour Ox, 24‐hour NO2, 24‐hour temperature, and 24‐hour relative humidity. Second, we evaluated the associations between brief (i.e. hourly) exposures to highly concentrated air pollution and the risk of death due to stroke, adjusted for 24‐hour mean concentrations of air pollutants. In this analysis, we estimated ORs of death caused by stroke for exposure indices of 1‐hour high concentrations of PM7, NO2, and Ox, adjusted for 24‐hour mean concentrations of PM7, NO2, Ox, temperature, and relative humidity (Model B). The exposure indices of 1‐hour mean concentrations were changed to dichotomous variables: thresholds defining “high” concentrations were set at the Japanese National Air Quality Standards for hourly levels of PM7 (200 μg/m3) and Ox (60 ppb), and at double the 24‐hour mean value of the standard for NO2 (80 ppb).
While the time‐of‐death data included both the hour and the minute of death, only the hour was used. Exposure to PM7 during the hour of death was defined as exposure with a time lag of zero hours (lag0), exposure during the previous hour was defined as exposure with a lag of 1 hour (lag1), etc. Lagged hour exposures of up to 5 hours were examined.
We also estimated ORs of death caused by stroke for 30 μg/m3 differences in hourly PM7 adjusted for temperature and relative humidity to examine the hourly lag structure. In this analysis, lagged hour exposures of up to 48 hours were examined. These were pooled (warmer months and colder months) analyses with single lags assessed in 48 models.
We examined deaths due to ischaemic stroke and intracerebral haemorrhage separately. All models took into consideration the effects of season and of unusually high and low temperatures: modified effects were examined by using a two level indicator variable for the warmer months (April to September) and the colder months (October to March). Unusually high and low temperatures were defined as less than the 1st centile, and greater than the 99th centile. The PHREG procedures of SAS (release 8.2, SAS Institute, Inc., Cary, NC, USA) were used to perform the conditional logistic regression. All tests were two tailed, and alpha was set at 0.05.
Results
We studied 63 724 deaths: 17 354 due to intracerebral haemorrhage and 46 370 due to ischaemic stroke (table 1). Of the 17 345 deaths due to intracerebral haemorrhage, 7972 occurred in warmer months (April to September) and 9382 occurred in colder months (October to March). Of the 46 370 deaths due to ischaemic stroke, 21 894 took place in the warmer months and 24 476 in the colder months. Because photochemical oxidants are generated mainly during the summer (table 1), the correlation structure among air pollutants differed between the warmer and colder months. In particular, the correlation between PM7 and Ox was positive in the warmer months and negative in the colder months (table 2). Of the 63 724 deaths (index periods), 374 (intracerebral haemorrhage, 110; ischaemic stroke, 264), 382 (intracerebral haemorrhage, 115; ischaemic stroke, 267), and 380 (intracerebral haemorrhage, 117; ischaemic stroke, 263) occurred in people who had been exposed to high concentrations of PM7 during the hour of death (lag0, lag1, and lag2, respectively). Of the 110, 115, and 117 intracerebral haemorrhage deaths, 66, 68, and 69, respectively, were in Tokyo; 16, 17, and 19, respectively, were in Yokohama; and 16, 17, and 16, respectively, were in Kawasaki. On the other hand, there were 203 208 control periods. Of these 203 208 control periods, 1109 (intracerebral haemorrhage, 320; ischaemic stroke, 789), 1066 (intracerebral haemorrhage, 281; ischaemic stroke, 785), and 1044 (intracerebral haemorrhage, 296; ischaemic stroke, 748) occurred in high concentrations of PM7 during the hour of death (lag0, lag1, and lag2, respectively).
Table 1 Summary of statistics of population, number of deaths, hourly mean concentration of air pollutants, and hourly mean temperature and relative humidity in the 13 major urban areas studied.
| Population in 1990 (in thousands) | Number of deaths, 1990–94 (65 years or older) | Mean concentration of air pollutants, temperature, and relative humidity in warmer months (April to September), 1990–94 | Mean concentration of air pollutants, temperature, and relative humidity in colder months (October to March), 1990–94 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All age | 65 years or older | ICH | IS | Temp | RH | PM7* | NO2 | Ox | Temp | RH | PM7* | NO2 | Ox | |
| ICD‐9: 431 | ICD‐9: 434 | °C | % | μg/m3 | ppb | ppb | °C | % | μg/m3 | ppb | ppb | |||
| Sapporo | 1672 | 152 | 595 | 2140 | 16.4 | 70.2 | 20.6 | 27.0 | 15.8 | 2.4 | 68.6 | 21.0 | 33.7 | – |
| Sendai | 918 | 80 | 421 | 1486 | 18.5 | 75.1 | 33.1 | 14.8 | 25.5 | 6.9 | 66.5 | 24.2 | 17.9 | 23.8 |
| Chiba | 829 | 61 | 384 | 983 | 21.5 | 74.3 | 41.9 | 16.9 | 22.3 | 10.4 | 63.1 | 54.5 | 27.4 | 14.4 |
| Tokyo | 8164 | 911 | 6337 | 16786 | 22.1 | 67.8 | 43.0 | 31.4 | 19.8 | 10.7 | 57.5 | 55.8 | 38.4 | 14.1 |
| Yokohama | 3220 | 278 | 1458 | 3856 | 21.5 | 74.4 | 44.1 | 36.9 | 13.3 | 10.4 | 62.5 | 51.5 | 44.6 | 10.7 |
| Kawasaki | 1174 | 94 | 1252 | 2854 | 21.5 | 74.4 | 57.4 | 31.9 | 18.3 | 10.4 | 62.5 | 62.2 | 40.5 | 12.3 |
| Nagoya | 2155 | 222 | 1600 | 4276 | 22.3 | 68.3 | 45.2 | 19.3 | 24.4 | 9.8 | 64.7 | 44.0 | 30.2 | 14.9 |
| Kyoto | 1461 | 185 | 965 | 2440 | 22.5 | 66.2 | 33.3 | 22.7 | 27.3 | 9.7 | 67.7 | 28.4 | 26.5 | 18.4 |
| Osaka | 2624 | 306 | 625 | 1686 | 23.3 | 65.1 | 46.4 | 30.5 | 21.4 | 11.0 | 62.2 | 41.9 | 35.9 | 13.9 |
| Kobe | 1477 | 169 | 1675 | 4494 | 22.3 | 70.6 | 38.3 | 20.1 | 29.5 | 10.3 | 65.1 | 29.8 | 20.3 | 24.7 |
| Hiroshima | 1086 | 107 | 648 | 1563 | 22.8 | 69.3 | 44.4 | 19.9 | 25.9 | 10.3 | 67.5 | 36.2 | 24.1 | 17.0 |
| Kitakyushu | 1026 | 130 | 753 | 2351 | 22.7 | 72.9 | 39.3 | 20.7 | 21.6 | 11.3 | 66.6 | 28.2 | 24.3 | 20.7 |
| Fukuoka | 1237 | 113 | 641 | 1455 | 22.7 | 72.9 | 37.5 | 25.6 | 19.8 | 11.3 | 66.6 | 33.9 | 30.4 | 19.3 |
| Total | 27043 | 2,808 | 17354 | 46370 | 21.5 | 70.4 | 40.3 | 24.4 | 21.9 | 9.4 | 64.7 | 39.4 | 30.3 | 17 |
*Defined under the Japanese Air Quality Standard as any particles collected with an upper 100% cut‐off point of 10 μm aerodynamic diameter. The 50% cut‐off diameter for the particles is assumed to be approximately 7 μm (PM7).
ICH, intracerebral haemorrhage; IS, ischaemic stroke; Temp, temperature; RH, relative humidity; Ox, photochemical oxidants.
Table 2 Coefficients of correlation between hourly measured concentrations of PM7 and NO2, photochemical oxidants (Ox), temperature, and relative humidity.
| Variable | Range of correlation coefficients* | |
|---|---|---|
| Warmer months (April–September) | Colder months (October–March) | |
| PM7† and NO2 | 0.46 to 0.63 | 0.42 to 0.79 |
| PM7† and Ox‡ | −0.14 to 0.20 | −0.36 to −0.14 |
| PM7† and temperature | −0.09 to 0.28 | 0.04 to 0.28 |
| PM7† and relative humidity | −0.11 to 0.15 | 0.01 to 0.34 |
*Ranges of correlation coefficient for all 13 areas studied, 1990–94.
†Defined under the Japanese Air Quality Standard as any particles collected with an upper 100% cut‐off point of 10 μm aerodynamic diameter. The 50% cut‐off diameter for the particles is assumed to be approximately 7 μm (PM7).
‡Photochemical oxidants.
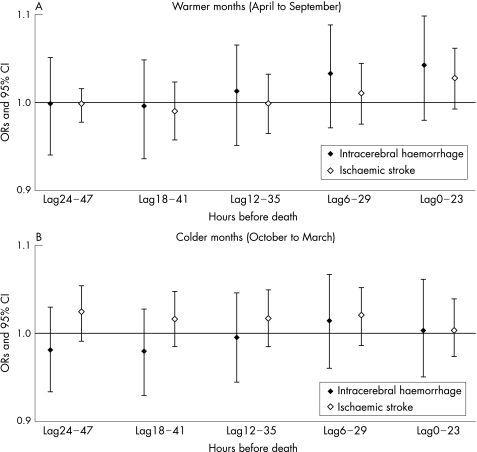
The 24‐hour mean concentration of PM7 was not significantly associated with the risk of death due to stroke (table 3). We did not find any significant association when we estimated the ORs of death due to intracerebral haemorrhage and ischaemic stroke for each 30 μg/m3 increase of various lagged 24‐hour mean concentrations of PM7 (fig 1). However, data presented in table 3 and fig 1 revealed that there were positive associations of both death from intracerebral haemorrhage and death from ischaemic stroke with the 24‐hour mean PM7 (lag0). The 24‐hour mean temperature and relative humidity were associated with the risk of death due to stroke. Compared to the reference temperature of 15–22°C, the odds ratio (OR) of death due to ischaemic stroke associated with extreme heat (more than 30°C) in the warmer months was 1.133 (95% CI 1.002 to 1.280), and the OR of death due to intracerebral haemorrhage associated with cold (0–8°C) in the colder months was 1.225 (95% CI 1.082 to 1.386) (table 3).
Table 3 Association between 24‐hour mean concentrations of air pollutants and death due to stroke (Model A).
| Variables included in the multi‐pollutants model* | Intracerebral haemorrhage | Ischaemic stroke | ||||||
|---|---|---|---|---|---|---|---|---|
| (ICD‐9 code 431) | (ICD‐9 code 434) | |||||||
| OR | (95% CI) | OR | (95% CI) | |||||
| Warmer months (April to September) | ||||||||
| 24‐hour PM7† | Increment | 30 μg/m3 | 1.041 | (0.984–1.102) | 1.027 | (0.993–1.062) | ||
| 24‐hour NO2 | Increment | 10 ppb | 0.995 | (0.955–1.037) | 0.995 | (0.971–1.019) | ||
| 24‐hour Ox ‡ | Increment | 10 ppb | 0.996 | (0.961–1.033) | 0.975 | (0.955–0.996)¶ | ||
| Temperature§ | ||||||||
| <0°C (0–1%) | – | (–) | – | (–) | ||||
| 0–8°C (1–25%) | 1.253 | (0.938–1.674) | 1.074 | (0.904–1.275) | ||||
| 8–15°C (25–50%) | 1.054 | (0.948–1.172) | 0.993 | (0.931–1.059) | ||||
| 15–22°C (50–75%) | Reference | 1.000 | 1.000 | |||||
| 22–30°C (75–99%) | 0.940 | (0.865–1.023) | 0.993 | (0.945–1.043) | ||||
| >30°C (99–100%) | 1.153 | (0.937–1.420) | 1.133 | (1.002–1.280)¶ | ||||
| RH‡ | Increment | 10% | 0.961 | (0.881–1.048) | 0.931 | (0.884–0.981)¶ | ||
| Colder months (October to March) | ||||||||
| 24‐hour PM7† | Increment | 30 μg/m3 | 1.005 | (0.951–1.061) | 1.005 | (0.973–1.039) | ||
| 24‐hour NO2 | Increment | 10 ppb | 1.025 | (0.976–1.077) | 1.000 | (0.971–1.030) | ||
| 24‐hour Ox ‡ | Increment | 10 ppb | 1.010 | (0.950–1.033) | 1.006 | (0.970–1.044) | ||
| Temperature§ | ||||||||
| <0°C (0–1%) | 0.498 | (0.199–1.247) | 0.974 | (0.666–1.425) | ||||
| 0–8°C (1–25%) | 1.225 | (1.082–1.386)¶ | 1.058 | (0.984–1.138) | ||||
| 8–15°C (25–50%) | 1.098 | (0.987–1.221) | 1.037 | (0.974–1.104) | ||||
| 15–22°C (50–75%) | Reference | 1.000 | 1.000 | |||||
| 22–30°C (75–99%) | 0.819 | (0.668–1.006) | 0.933 | (0.830–1.048) | ||||
| >30°C (99–100%) | – | (–) | – | (–) | ||||
| RH‡ | Increment | 10% | 0.935 | (0.866–1.009) | 0.981 | (0.936–1.028) | ||
*Variables included in the model are 24‐hour PM7, 24‐hour Ox, 24‐hour NO2, 24‐hour temperature, and 24‐hour relative humidity.
†PM7: Defined under the Japanese Air Quality Standard as any particles collected with an upper 100% cut‐off point of 10 μm aerodynamic diameter. The 50% cut‐off diameter for the particles is assumed to be approximately 7 μm (PM7).
‡Ox, photochemical oxidants; RH, relative humidity.
§Categorised by quartile and to examine extreme temperature highest 1% and lowest 1% were also categorised.
¶p<0.05.
Figure 1 Odds ratios of death due to intracerebral haemorrhage (ICD‐9: 431) and ischaemic stroke (ICD‐9: 434) for each 30 μg/m3 increase in various lagged 24‐hour mean concentrations of PM7. These results are adjusted for 24‐hour photochemical oxidants (Ox), 24‐hour NO2, 24‐hour temperature, and 24‐hour relative humidity.
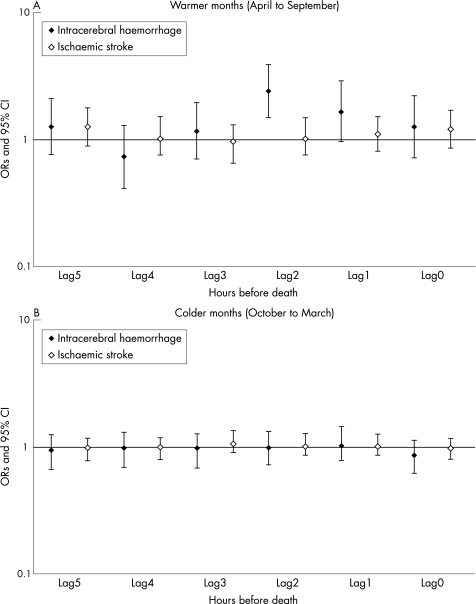
The 1‐hour mean concentration of PM7 was associated with the risk of death due to intracerebral haemorrhage (with time lag2) during the warmer months (threshold: 200 μg/m3, OR = 2.397, 95% CI 1.476 to 3.892) (table 4, fig 2). In contrast, the 1‐hour mean concentration of PM7 was not associated with death due to ischaemic stroke (table 4, fig 2). When we analysed the data for each city, ORs for death due to intracerebral haemorrhage (with time lag2) during warmer months with a more than 200 μg/m3 1‐hour mean concentration of PM7 were 2.683 (95% CI 1.335 to 5.392) in Tokyo, 1.105 (95% CI 0.284 to 4.294) in Yokohama, and 7.845 (95% CI 1.498 to 41.072) in Kawasaki. The 1‐hour mean concentrations of gaseous pollutants were not associated with death due to ischaemic or haemorrhagic stroke (data not shown).
Table 4 Odds ratios of death due to intracerebral haemorrhage (ICD‐9: 431) and ischaemic stroke (ICD‐9: 434) for exposures measured as 24‐hour and 1‐hour mean concentrations of pollutants estimated jointly (2 hours before death: lag2) (Model B).
| Variables included in the multi‐pollutants model* | Intracerebral haemorrhage | Ischaemic stroke | ||||
|---|---|---|---|---|---|---|
| (ICD‐9 code 431) | (ICD‐9 code 434) | |||||
| OR | (95% CI) | OR | (95% CI) | |||
| Warmer months (April to September) | ||||||
| 1‐hour lag2 PM7† | Threshold | 200 μg/m3 | 2.397 | (1.476–3.892)¶ | 1.051 | (0.750–1.472) |
| 1‐hour lag2 NO2 | Threshold | 80 ppb | 1.050 | (0.796–1.386) | 1.042 | (0.873–1.243) |
| 1‐hour lag2 Ox ‡ | Threshold | 60 ppb | 1.009 | (0.854–1.193) | 0.969 | (0.874–1.074) |
| 24‐hour PM7† | Increment | 30 μg/m3 | 1.019 | (0.960–1.082) | 1.018 | (0.983–1.055) |
| 24‐hour NO2 | Increment | 10 ppb | 0.996 | (0.954–1.040) | 1.001 | (0.976–1.026) |
| 24‐hour Ox ‡ | Increment | 10 ppb | 1.000 | (0.962–1.039) | 0.979 | (0.957–1.001) |
| Temperature § | ||||||
| <0°C (0–1%) | – | (–) | – | (–) | ||
| 0–8°C (1–25%) | 1.286 | (0.958–1.726) | 1.077 | (0.903–1.286) | ||
| 8–15°C (25–50%) | 1.039 | (0.931–1.159) | 0.994 | (0.930–1.062) | ||
| 15–22°C (50–75%) | Reference | 1.000 | 1.000 | |||
| 22–30°C (75–99%) | 0.947 | (0.869–1.033) | 0.985 | (0.936–1.037) | ||
| >30°C (99–100%) | 1.204 | (0.972–1.492) | 1.110 | (0.978–1.260) | ||
| RH ‡ | Increment | 30% | 0.958 | (0.876–1.048) | 0.939 | (0.890–0.991)¶ |
| Colder months (October to March) | ||||||
| 1‐hour lag2 PM7† | Threshold | 200 μg/m3 | 0.970 | (0.712–1.322) | 1.040 | (0.855–1.265) |
| 1‐hour lag2 NO2 | Threshold | 80 ppb | 0.995 | (0.802–1.233) | 0.982 | (0.856–1.125) |
| 1‐hour lag2 Ox‡ | Threshold | 60 ppb | 0.874 | (0.391–1.955) | 0.984 | (0.645–1.501) |
| 24‐hour PM7† | Increment | 30 μg/m3 | 1.015 | (0.958–1.075) | 1.003 | (0.968–1.039) |
| 24‐hour NO2 | Increment | 10 ppb | 1.019 | (0.968–1.074) | 1.004 | (0.973–1.036) |
| 24‐hour Ox‡ | Increment | 10 ppb | 1.000 | (0.938–1.066) | 1.012 | (0.975–1.052) |
| Temperature§ | ||||||
| <0°C (0–1%) | 0.509 | (0.203–1.280) | 0.919 | (0.616–1.373) | ||
| 0–8°C (1–25%) | 1.222 | (1.075–1.388)¶ | 1.061 | (0.984–1.144) | ||
| 8–15°C (25–50%) | 1.099 | (0.985–1.226) | 1.038 | (0.972–1.107) | ||
| 15–22°C (50–75%) | Reference | 1.000 | 1.000 | |||
| 22–30°C (75–99%) | 0.783 | (0.632–0.970)¶ | 0.935 | (0.829–1.054) | ||
| >30°C (99–100%) | – | (–) | – | (–) | ||
| RH‡ | Increment | 30% | 0.924 | (0.853–1.000) | 0.983 | (0.937–1.032) |
*Variables included in the model are 1‐hour PM7 (lag2), 1‐hour NO2 (lag2), 1‐hour Ox (lag2), 24‐hour PM7, 24‐hour Ox, 24‐hour NO2, 24‐hour temperature, and 24‐hour relative humidity.
†PM7: Defined under the Japanese Air Quality Standard as any particles collected with an upper 100% cut‐off point of 10 μm aerodynamic diameter. The 50% cut‐off diameter for the particles is assumed to be approximately 7 μm (PM7).
‡Ox, photochemical oxidants; RH, relative humidity.
§Categorised by quartile and to examine extreme temperature highest 1% and lowest 1% were also categorised.
¶p<0.05.
Figure 2 Odds ratios of death due to intracerebral haemorrhage (ICD‐9: 431) and ischaemic stroke (ICD‐9: 434) for exposure to various lagged 1‐hour mean concentrations of PM7. Threshold is 200 μg/m3. Results are adjusted for 1‐hour NO2, 1‐hour photochemical oxidants (Ox), 24‐hour PM7, 24‐hour Ox, 24‐hour NO2, 24‐hour temperature, and 24‐hour relative humidity.
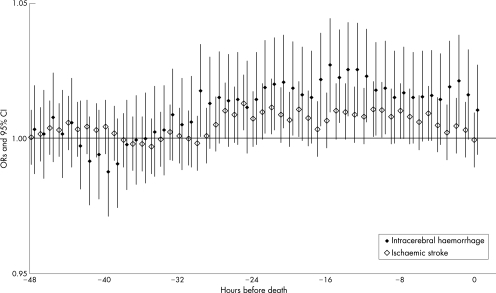
With respect to the hourly lag structure, we observed significant positive associations between death due to intracerebral haemorrhage and PM7 from 2 to 23 hour lags, except for lags of 4–9, 18, and 19 hours in pooled (warmer months and colder months) analysis (fig 3).
Figure 3 Lag structure of the association between 1‐hour PM7 and death due to intracerebral haemorrhage (ICD‐9: 431). The odds ratios of death due to intracerebral haemorrhage are shown for exposure to various lagged 1‐hour mean concentration of PM7. Increment is 30 μg/m3. Results are adjusted for 24‐hour temperature, and 24‐hour relative humidity. These were pooled (warmer months and colder months) analyses with single lags assessed in 48 models.
Discussion
In this study, the 24‐hour mean concentration of PM7 was not significantly associated with the risk of death due to either intracerebral haemorrhage or ischaemic stroke (table 3). Moreover, we did not find any significant association when we estimated the ORs of death due to intracerebral haemorrhage and ischaemic stroke for each 30 μg/m3 increase in various lagged 24‐hour mean concentrations of PM7 (fig 1). However, data presented in table 3 revealed positive associations of both death for intracerebral haemorrhage and death for ischaemic stroke with 24‐hour mean PM7; the positive association between 24‐hour PM and stroke had the same tendency as shown in previous studies, regardless of whether findings were statistically significant.14,15,16,17 Analysing the data on air‐pollution for hourly intervals, we found that a high concentration of PM7 was associated with a higher risk of death due to intracerebral haemorrhage during the warmer months. This association with 1‐hour mean concentration was independent of the 24‐hour mean concentration. Death due to ischaemic stroke was not associated with the 1‐hour mean concentration of PM7. Hong et al14 found that the relative risk of death due to haemorrhagic stroke, but not ischaemic stroke, was associated with the concentration of total suspended particles on the day of death, although the sizes of the particles measured probably differed. Moreover, other recent studies of particulate matter and stroke also found that haemorrhagic stroke was more sensitive to particulate matter than was ischaemic stroke.15,16 However, another recent epidemiological study on stroke shows that for ischaemic stroke an interquartile range increase in PM10 was associated with a 1.03% (95% CI 0.04% to 2.04%) increase in admissions on the same day; such an association was not shown for haemorrhagic stroke.17 The results were different for different cities; for example, there is a positive association between hospital admissions for haemorrhagic stroke and PM10 in Minneapolis and a negative association in Chicago. We believe that the association between haemorrhagic stroke and ambient particulate matter remains controversial and that further epidemiological studies are needed to confirm the association. We also examined the simple lagged structure of hourly PM7 and death due to haemorrhagic stroke, and observed significant associations with 2 hour to 23 hour lags, except for 4–9, 18, and 19 hour lags (fig 3).
Extreme temperatures are important triggers of stroke and the pattern of association differs between ischemia and haemorrhage. We found a negative association between the 24‐hour mean temperature and death due to ischaemic stroke in the colder months, and also a positive association between high temperature and death due to intracerebral haemorrhage in the warmer months.
The finding that exposure to PM7 is associated with the risk of death due to intracerebral haemorrhage but not ischaemic stroke is noteworthy. One possibility is that the interval from stroke onset to death is much longer, or much more variable, for ischaemic stroke than for intracerebral haemorrhage. Another possibility involves the effects of inhaled particles on blood pressure and the fact that hypertension is a risk for intracerebral haemorrhage. Laboratory findings suggest that exposure to fine particles can increase blood pressure.18 In other studies, more untreated hypertensive patients had cerebral haemorrhage than had other types of stroke,19 and blood pressure is more strongly associated with haemorrhagic stroke than with ischaemic stroke.20 High concentrations of PM7 might increase blood pressure and thus increase the risk of fatal intracerebral haemorrhage. Further studies might explore whether this risk is greatest in people with pre‐existing hypertension.
Peters et al6,21 showed that transient exposures can be important environmental triggers of cardiovascular events. They found that the onset of MI is linked to hourly exposure to particulate matter,6 and also to hourly exposure to vehicular traffic.21 With regard to death due to intracerebral haemorrhage, by using hourly data we were able to detect associations between such deaths and transiently high concentrations of PM7—associations that might not have been observed had we used only the daily mean values. For example, using the data for the Tokyo metropolitan area, we found that during the five years covered by this study there were 443 hours in which the concentration of PM7 was over the 1‐hour air quality standard, and that 49 of those hours (11%) occurred on days when the 24‐hour mean concentration of PM7 was within the air quality standard for 24‐hour periods. Thus, the connection between air pollution and the higher risk of death due to stroke in Tokyo on those days, and during those hours, would never have been noticed if 24‐hour data alone had been collected. Preventing particulate matter associated death due to stroke might require basing air quality standards not only on 24‐hour mean concentrations, but also on hourly data. (If hourly data are not available, one might predict that stricter standards for 24‐hour mean concentrations would have a similar effect.) Advice to people in vulnerable groups, and even pharmacological interventions, might help to protect the public from stroke related death associated with transient exposures to ambient particles.
In other studies, the events of interest were the time of onset of MI,6,7,8 the day of death due to stroke,14 and the days of hospital with a diagnosis of stroke.15,16,17 However, we focused this study only on the time of stroke attributed death. Many stroke patients do not die immediately after the stroke. Our results might indicate that transiently high concentrations of PM7 trigger death among frail persons who had recently had a stroke. Further studies might explore whether deaths due to other causes are also associated with transient air polluting events, and whether hourly concentrations of air pollutants are also associated with stroke onset.
We did not obtain data on the subjects' time related behaviours that are risk factors for stroke, such as smoking or heavy exercise. However, we believe that associations between such risk factors and concentrations of air pollutants are unlikely, and therefore they would not confound the association between air pollution and stroke.
Main messages
In urban Japan, the risk of death due to intracerebral haemorrhage was associated with exposure to high concentrations of particulate matter about 2 hours before death.
The higher risk was independent of the 24‐hour mean concentration of particulate matter.
Policy implications
To help prevent death due to intracerebral haemorrhage, standards for concentrations of ambient particulate matter should be based not only on 24‐hour mean concentrations, but also on hourly data.
We observed an unexpected association between the 24‐hour mean concentration of Ox and ischaemic stroke death in warmer months (table 3). Ox are a secondary pollutant generated from NO2, NO, or hydrocarbons and are generated actively in summer. Therefore, we believe it is difficult to assess the exact effect of Ox on health.
Misclassification of exposure to air pollution is a potential source of error. For example, a death that occurred within the Tokyo metropolitan area (which is 621 square kilometres) but far from the monitoring station might have been unrelated to the PM7 concentration at that station. We expected any such exposure misclassification to be non‐differential with respect to death, and to bias the estimated associations towards null because concentrations of air pollutants measured at monitoring stations in the same city are highly correlated.
Conclusion
Death due to intracerebral haemorrhage was associated with exposure to high concentrations of PM7 about 2 hours before death, especially from April to September (in Japan). The increase in risk was independent of the 24‐hour mean concentration of PM7. To help prevent stroke related death due to air pollution, air quality standards for particulate matter should be based not only on 24‐hour mean concentrations, but also on hourly data.
Footnotes
Funding: this work was supported in part by the Ministry of the Environment, Japan
Competing interests: none
References
- 1.Dockery D W. Epidemiologic evidence of cardiovascular effects of particulate air pollution. Environ Health Perspect 2001109(suppl 4)483–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Air quality guidelines for Europe. WHO Reg Publ Eur Ser. 2000;(91):v–x, 1–273 [PubMed]
- 3.Gold D R, Litonjua A, Schwartz J.et al Ambient pollution and heart rate variability. Circulation 20001011267–1273. [DOI] [PubMed] [Google Scholar]
- 4.Nemmar A, Hoet P H, Vanquickenborne B.et al Passage of inhaled particles into the blood circulation in humans. Circulation 2002105411–414. [DOI] [PubMed] [Google Scholar]
- 5.Shoji H, Tsukatani T. Statistical model of air pollutant concentration and its application to the air quality standards. Atmos Environ 19737485–501. [Google Scholar]
- 6.Peters A, Dockery D W, Muller J E.et al Increased particulate air pollution and the triggering of myocardial infarction. Circulation 20011032810–2815. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan J, Sheppard L, Schreuder A.et al Relation between short‐term fine‐particulate matter exposure and onset of myocardial infarction. Epidemiology 20051641–48. [DOI] [PubMed] [Google Scholar]
- 8.Peters A, Klot S, Heier M.et al Air pollution, personal activities, and onset of myocardial infarction in a case‐crossover study. Particulate Air Pollution and Nonfatal Cardiac Events. Health Effects Institute. 2005, Number 124: 1–66, Available: http://www.healtheffects.org/Pubs/Report124.pdf [accessed 10 October 2005] [PubMed]
- 9.US EPA Air Quality Criteria for Particulate Matter. US Environmental Protection Agency, 2004. Available: http://cfpub2.epa.gov/ncea/cfm/recordisplay.cfm?deid = 87903 [accessed 16 February 2005]
- 10.Maclure M. The case‐crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 1991133144–153. [DOI] [PubMed] [Google Scholar]
- 11.Levy D, Lumley T, Sheppard L.et al Referent selection in case‐crossover analyses of acute health effects of air pollution. Epidemiology 200112186–192. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz J. The effects of particulate air pollution on daily deaths: a multi‐city case crossover analysis. Occup Environ Med 200461956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pope C A, 3rd, Schwartz J. Time series for the analysis of pulmonary health data. Am J Respir Crit Care Med 1996154(6 pt 2)S229–S233. [DOI] [PubMed] [Google Scholar]
- 14.Hong Y C, Lee J T, Kim H.et al Air pollution: a new risk factor in ischemic stroke mortality. Stroke 2002332165–2169. [DOI] [PubMed] [Google Scholar]
- 15.Tsai S S, Goggins W B, Chiu H F.et al Evidence for an association between air pollution and daily stroke admissions in Kaohsiung, Taiwan. Stroke 2003342612–2616. [DOI] [PubMed] [Google Scholar]
- 16.Yang C Y, Chen Y S, Chiu H F.et al Effects of Asian dust storm events on daily stroke admissions in Taipei, Taiwan. Environ Res 20059979–84. [DOI] [PubMed] [Google Scholar]
- 17.Wellenius G A, Schwartz J, Mittleman M A. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among Medicare beneficiaries. Stroke 2005362549–2553. [DOI] [PubMed] [Google Scholar]
- 18.Chang C C, Hwang J S, Chan C C.et al Effects of concentrated ambient particles on heart rate, blood pressure, and cardiac contractility in spontaneously hypertensive rats. Inhal Toxicol 200416421–429. [DOI] [PubMed] [Google Scholar]
- 19.Tsementzis S A, Gill J S, Hitchcock E R.et al Diurnal variation of and activity during the onset of stroke. Neurosurgery 198517901–904. [DOI] [PubMed] [Google Scholar]
- 20.Jain S, Namboodri K K, Kumari S.et al Loss of circadian rhythm of blood pressure following acute stroke. BMC Neurol 200441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters A, von Klot S, Heier M.et al Exposure to traffic and the onset of myocardial infarction. N Engl J Med 20043511721–1730. [DOI] [PubMed] [Google Scholar]