Abstract
To study the effects of IL-1α in arthritis, we generated human IL-1α (hIL-1α). Transgenic mice expressed hIL-1α mRNA in various organs, had high serum levels of hIL-1α, and developed a severe polyarthritic phenotype at 4 weeks of age. Not only bone marrow cells but also synoviocytes from knee joints produced biologically active hIL-1α. Synovitis started 2 weeks after birth, and 8-week-old mice showed hyperplasia of the synovial lining layer, the formation of hyperplastic synovium (pannus) and, ultimately, destruction of cartilage. Hyperplasia of the synovial lining was due to the accumulation of macrophage-like cells expressing F4/80 molecules. hIL-1α was widely distributed in macrophage- and fibroblast-like cells of the synovial lining cells, as well as synovial fluid monocytes. T and B cells were rare in the synovial fluid, and analysis of marker expression suggests that synoviocytes were directly histolytic and did not act as antigen-presenting cells. In the joints of these mice, we found elevated levels of cells of the monocyte/macrophage and granulocyte lineages and of polymorphonuclear neutrophils (PMNs), most of which expressed Gr-1, indicating that they were mature, tissue-degrading PMNs. Cultured synoviocytes and PMNs from these animals overexpress GM-CSF, suggesting that the hematopoietic changes induced by IL-1 and the consequent PMN activation and joint destruction are mediated by this cytokine.
Introduction
IL-1 is an immunomodulatory and proinflammatory cytokine that possesses a wide spectrum of biological properties, including the stimulation of T and B lymphocytes, bone resorption, and pyrogenicity (1–4). IL-1 has also been implicated in the pathogenesis of chronic inflammatory joint diseases such as rheumatoid arthritis (RA). Elevated IL-1 levels have been identified in the synovial fluid, synovial membrane, and cartilage-pannus junction of arthritic joints from RA patients (5). Indeed, IL-1 triggers synovial cell proliferation and induces matrix metalloproteinase (MMP) production in vitro (6, 7), and intra-articular injections of IL-1 cause active synovitis and marked depletion of the cartilage matrix in several mammalian species in vivo (8, 9). Studies of collagen-induced arthritis (CIA) in mice have revealed that IL-1 plays an important role in the process of joint destruction, since the blocking of IL-1 activity can reduce the severity of synovitis and prevent cartilage and bone destruction (10, 11). Recent controversies in the field of inflammatory joint disease include the relative pathogenic contributions of IL-1 and TNF. Overexpression of TNF in mice results in the development of chronic arthritis with 100% phenotypic penetrance (12). However, when these mice were treated with anti–IL-1 receptor, the arthritis was completely prevented (13). Furthermore, in several mouse models of RA, IL-1 is reportedly the dominant cytokine in cartilage destruction, whereas TNF involvement is limited (14, 15). These lines of evidence allow us to speculate that IL-1 plays a central role in the joint destruction seen in RA patients and those with other related types of arthropathy.
On the other hand, the inflammatory process in several animal models of RA has been characterized by a T cell–driven immune response which contributes to the initiation and perpetuation of arthritis. However, the depletion of T cells using specific antibody against α/βTCR or CD4 did not attenuate the disease state in CIA, once arthritis was established (16, 17). Moreover, definitive evidence for T/B lymphocyte–independence in disease induction has recently been obtained using RAG-1–deficient mice (18). This suggests that antigen-nonspecific inflammation mediated by synovial fibroblast- or macrophage-derived cytokines, such as IL-1 and TNF, is more responsible for disease perpetuation than the antigen-specific immune response by T/B lymphocytes.
In the present study, we generated transgenic (Tg) mice which constitutively produce human IL-1α (hIL-1α). These mice exhibit chronic inflammatory polyarthritis with remarkable synovial proliferation, as well as cartilage and bone destruction. In particular, the recruitment of a considerable number of polymorphonuclear neutrophils (PMNs), macrophage-enriched synovium, and paucity of lymphocyte infiltration into the synovium were unique pathological features of these Tg mice. Flow cytometric analysis clearly indicated that hIL-1α overexpression in mice altered the distribution of hematopoietic cells through the production of GM-CSF, and increased the number of synovial macrophages and PMNs playing key roles in the development of chronic arthritis in our Tg mice.
Methods
Generation of Tg mice.
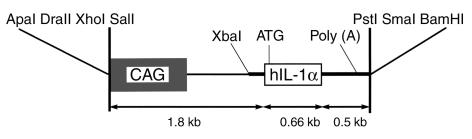
hIL-1α cDNA (Immunex Co., Seattle, Washington, USA) was excised with HindIII and HincII (Roche Diagnostics, Mannheim, Germany). This 660 bp HindIII/HincII restriction fragment was inserted into the EcoRI site of the third exon of the rabbit β-globin gene in the expression plasmid, pBsCAG-2. pBsCAG-2 possesses CAG containing the first intron of the chicken β-actin gene and a portion of the rabbit β-globin gene (comprising the second intron, third exon, and 3′ untranslated region). The resulting construct (designated CAG-IL-1α, Figure 1) was excised and used for microinjection into the pronuclei of fertilized 1-cell eggs from C57BL/6N × B6C3F1 mice. The resulting littermates were screened for incorporation of the transgene by Southern blot analysis after the extraction of DNA from tail biopsies. Seven founder (F0) mice were identified, and two of these exhibited polyarthritis (designated Tg1705 and Tg1706). These two F0 mice were used to establish lines. Tg1706 line mice were backcrossed with C3H/HeJ mice 6 to 8 times. Since both Tg mice lines were unable to breed normally, we performed in vitro fertilization to obtain offspring. Mice of the Tg1706 line (C3H background) were used for all experiments described.
Figure 1.
Structures of the CAG-IL-1α transgene. Shaded box indicates the CAG promoter; open box indicates the 660 bp HindIII/HincII restriction fragment of hIL-1α cDNA; thick line indicates rabbit β-globin gene; CAG, a gene encoding the cytomegalovirus enhancer, the chicken β-actin promoter and the first intron of chicken β-actin gene.
Reagents.
The following antibodies were used for flow cytometric analysis. Anti-CD16/32 (2.4G2) Ab was kindly provided by T. Tadakuma (National Defense Medical College). Anti-TCRαβ, anti–IL-2Rβ, anti-B220, anti-CD11b (Mac-1), anti–Ly-6G (Gr-1), anti-TCRγδ, anti-CD80 (B7.1), anti-CD54 (ICAM-1), and anti-CD86 (B7.2) mAb’s were purchased from PharMingen (San Diego, California, USA). Biotinylated anti-F4/80 (Cedarlane Laboratories Ltd., Hornby, Ontario, Canada), phycoerythrin-conjugated anti–hIL-1α (Becton Dickinson Immunocytometry Systems, San Jose, California, USA) and anti–I-Ak (Caltag Laboratories, Burlingame, California, USA) antibodies were also purchased. All mAb’s were used in the FITC-, phycoerythrin-, or biotin-conjugated form. Biotin-conjugated hamster IgG and phycoerythrin-conjugated mouse IgG (PharMingen) were used to rule out the nonspecific binding. Anti–hIL-1α polyclonal Ab (Endogen Inc., Woburn, Massachusetts, USA) was used for immunohistochemical analysis.
Cells and culture conditions.
Synovial specimens obtained from the knee joints of 8-week-old Tg mice were treated with 120 U/ml of Streptomyces sp.C-51 collagenase (Sanko Junyaku Co., Tokyo, Japan) at 37°C for 30 minutes. The dispersed synovial cells were allowed to adhere to dishes in DMEM (Life Technologies Inc., Rockville, Maryland, USA) with 10% FBS (Life Technologies Inc.). Bone marrow (BM) macrophages were obtained by flushing the femoral and tibial cavities and were allowed to adhere to dishes in DMEM with 10% FBS. After 16 hours, any nonadherent cells were discarded. The adherent cells were maintained in DMEM with 10% FBS, 5% horse serum, 0.01 mM sodium pyruvate, 50 mM 2-mercaptoethanol (Sigma Chemical Co., St. Louis, Missouri, USA), 100 U/ml penicillin and 100 μg/ml streptomycin, and 30% L cell–conditioned medium. After 1 week of culture, both cells were used for the experiments.
Northern blot analysis.
Total RNA was extracted from various tissues of Tg and non-Tg littermates using the guanidinium thiocyanate/cesium chloride centrifugation method (19). Ten micrograms of total RNA was fractionated on a 1.0% agarose/formaldehyde gel, blotted to Gene Screen Plus nylon membrane (Perkin Elmer Life Sciences, Boston, Massachusetts, USA) and hybridized to 32P-labeled probes generated with Megaprime DNA labeling kit (Amersham Life Sciences Inc., Arlington Heights, Illinois, USA). The probe used was a 660 bp HindIII/HincII fragment from mature hIL-1α (Immunex Co.). The hybridization signals were detected using an image analyzer (BAS 2000; Fuji Photo Film Co., Tokyo, Japan).
Immunoprecipitation and analysis of hIL-1α on SDS polyacrylamide gels.
Cultured synoviocytes and BM macrophages were kept in methionine/cysteine-free medium (Life Technologies Inc.) for 2 hours and the medium was replaced with freshly prepared appropriate deficient media containing 40 μCi/ml [35S] methionine/cysteine (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, United Kingdom). One set of the culture was washed with PBS after a 5-hour labeling period, and the cell lysates were extracted with the lysis buffer (25 mM TrisHCl (pH 7.5), 5 mM EDTA (pH 7.5), 250 mM NaCl, 1% Triton-X-100, 0.5 mM PMSF, 0.5 mM DTT). A second set of cultures was chased for an additional appropriate time in nonradioactive medium. The culture supernatants and the cell lysates were concentrated 10- to 20-fold with CENTRICON Centrifugal Concentrator (Millipore Corp., Bedford, Massachusetts, USA) and subjected to immunoprecipitation with anti–hIL-1α polyclonal antibodies (Endogen Inc.) using ImmunoPure Protein A IgG Orientation Kit (Pierce Chemical Co., Rockford, Illinois, USA) and prepared for electrophoresis on 12.5% SDS-polyacrylamide gels, fixed, treated with ENLIGHTNING (Perkin Elmer Life Sciences), and then dried and exposed to film for autoradiography at –80°C.
ELISA.
After 48 hours of culture, the supernatants of each cell culture were removed and the cell lysates were extracted using lysis buffer containing 1% Triton-X-100. The DNA in each cell culture was also measured using the method of Labarca and Paigen (20). Mouse GM-CSF levels in the supernatants, lysates, and sera were measured by ELISA (R&D Systems Inc., Minneapolis, Minnesota, USA). For quantitation, the data were standardized based on the respective DNA contents.
Bioassay for hIL-1α.
The bioactivity of the transgene-derived hIL-1α was measured as previously described (21). Briefly, D10.G4.1 (D10) cells (kindly provided by T. Tadakuma) were propagated in the presence of a 10% supernatant of concanavalin A–stimulated spleen cells. D10 cells (4 × 104) were added to 50 μl aliquots of 48-hour supernatants from Tg mice–derived synoviocytes and BM macrophages. Total volume was adjusted to 200 μl/well. The plates were incubated for 24 hours in the presence of 0.3 μCi/well [3H]thymidine (Perkin Elmer Life Sciences) during the last 4 hours. The IL-1α bioactivity of supernatants from the littermate controls was also measured to serve as control data. The neutralizing antibodies against either hIL-1α (5 U/ml; Endogen Inc.) or mouse IL-1α (5 U/ml; Genzyme Corp., Cambridge, Massachusetts, USA) were directly added to the bioassay in some experiments. The proliferation of D10 cells was determined by measuring the uptake of [3H] thymidine.
Immunohistochemistry.
Isolated knee joints were fixed in 4% paraformaldehyde and decalcified in 15% EDTA. The knees were snap frozen in liquid nitrogen, embedded in OCT-compound (Tissue Tek) and kept at –80°C until cryosectioning. Sections (6-μm-thick) were cut in a cryostat, air-dried, and then washed in PBS. The endogenous peroxidase was depleted by treatment with H2O2. Nonspecific Ab binding was prevented by incubation with appropriate normal serum. The sections were incubated with primary Ab against hIL-1α followed by sequential incubation with biotinylated secondary Ab. Ab binding was detected by reacting with fluorescein streptavidin (Vector Laboratories Inc., Burlingame, California, USA).
Flow cytometric analysis.
Synovial fluid, peripheral blood, inguinal lymph nodes, spleens, livers, and BMs were obtained from 8-week-old Tg mice, and cell suspensions were prepared. For the flow cytometric analysis, 0.5 × 106 to 1 × 106 cells were incubated with anti-CD16/32 (2.4G2) at saturation to block FCRII/III receptors, and then were stained with appropriately diluted biotin, FITC, and phycoerythrin(PE) Ab’s. Biotinylated reagents were developed with FITC- or PE-conjugated streptavidin (Becton Dickinson Immunocytometry Systems). The cells were then analyzed by FACScan (Becton Dickinson Biosciences, San Jose, California, USA). Dead cells were excluded by forward scatter, side scatter, and propidium iodide gating.
Statistical analysis.
Results are expressed as means ± SEM. Statistical comparisons were performed using Student’s t test for unpaired data. P values less than 0.05 were considered significant for all tests.
Results
Generation of hIL-1α Tg mice.
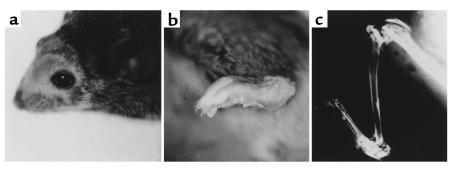
Out of seven F0 mice, two F0 mice (Tg1705 and Tg1706) that expressed sufficient levels of the transgene to exhibit phenotypic change were used to establish lines. The other five F0 mice displayed little expression of the transgene-derived mRNA and a minimum level of serum hIL-1α. The F0 and all offspring of both lines consistently exhibited a severe arthritic phenotype. Macroscopic findings of Tg mice included hair loss, body weight loss, and bilaterally symmetrical polyarthritis (Figure 2, a and b). The polyarthritis started with peripheral joint swelling in the ankles at 3–4 weeks of age and extended rapidly to the proximal joints including hip and shoulder joints, and cartilage destruction was ultimately observed by 8 weeks after birth. A complete loss of joint movement was observed at around 14 weeks of age. On the other hand, no significant changes were observed in the spine. Radiography of the knee joint confirmed complete loss of cartilage and severe erosion of subchondral bone (Figure 2c).
Figure 2.
Macroscopic findings of hIL-1α Tg mice (a female F3 mouse from Tg1706 line, 8 weeks old). (a) The mice spontaneously developed hair loss on the head. (b) The hind paw of Tg mice showed gross swelling of the ankle joint. (c) Radiograph of the knee joint showed ultimate destruction of cartilage and severe erosion of subchondral bone. A stress fracture had occurred in the proximal portion of the tibia possibly due to osteopenia.
Overexpression of hIL-1α was detected at both mRNA and protein levels, and the hIL-1α was biologically active.
Northern blot analysis demonstrated highV levels of hIL-1α mRNA expression in a wide variety of tissues, while a weaker signal was detected in the testis (Figure 3a). Serum hIL-1α levels of Tg mice were 108 ± 20 pg/ml on average as determined by specific ELISA.
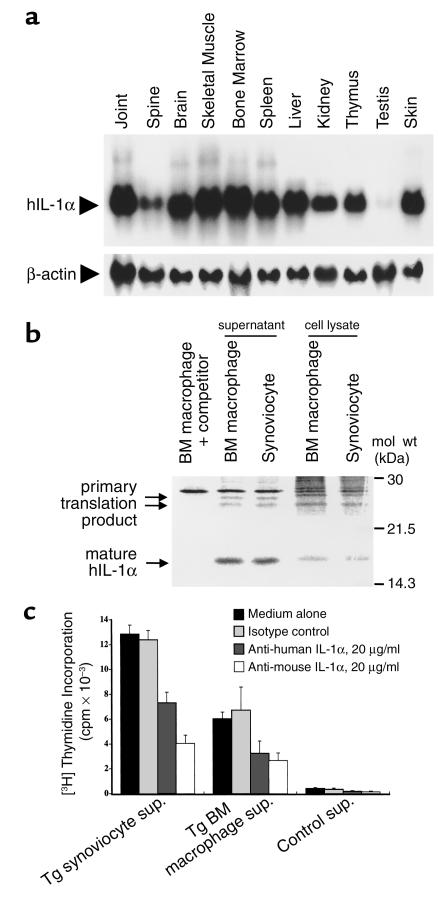
Figure 3.
(a) Northern blot analysis of hIL-1α mRNA from various tissues. Total RNA preparations from the knee joint, lumbar spine, brain, skeletal muscle, BM, spleen, liver, kidney, thymus, testis, and skin were analyzed. RNA (10 μg) was hybridized to an hIL-1α cDNA probe (upper panel). A mouse β-actin probe was used to control for qualitative and quantitative differences between the RNA preparations (lower panel). The results of a representative assay are shown. (b) SDS-PAGE and autoradiography of transgene-derived hIL-1α. Primary cultured synoviocytes and BM macrophages were pulsed with [35S]methionine/cysteine as outlined in Methods. After 5 hours, the cells were extensively washed and chased with cold media, for 2 hours for hIL-1α in cell lysates and for 12 hours for hIL-1α in culture supernatants. The cell lysates and supernatants were analyzed for radiolabeled hIL-1α by immunoprecipitation with anti–hIL-1α Ab. The immunoprecipitates were then subjected to SDS-PAGE and autoradiography. Two bands for high molecular weight hIL-1α and a band for low molecular weight hIL-1α were detected in both synoviocytes and BM macrophages. The specificity of the band was confirmed by a competition analysis in which the Ab was preblocked with recombinant hIL-1α (left lane). (c) Bioassay for transgene-derived hIL-1α. The culture supernatants were obtained from the synoviocytes or BM macrophages 48 hours after inoculation. D10 cells were cultured for 24 hours with or without a 25% vol/vol final concentration of the supernatants in culture wells and pulsed with [3H]TdR for the final 4 hours of incubation. [3H]TdR incorporation by the D10 cells was determined. The results are expressed as the mean ± SEM (n = 4). The specificity of IL-1 activity was confirmed by neutralizing with isotype-matched control Ig or anti–hIL-1α Ab or anti-mouse IL-1α Ab.
When we confirmed the production of hIL-1α by synoviocytes and BM macrophages at the protein level, as shown in Figure 3b, both cell types produced closely spaced 23 to 25 kDa high molecular weight hIL-1α and 17 kDa low molecular weight hIL-1α, which corresponded to the primary translation product of the transgene with a predicted molecular mass and a processed form of hIL-1α, respectively. The two different molecular weight forms of the primary translation product may be attributable to a number of posttranslational modifications within the NH2-terminal domain, including phosphorylation (22), mannosylation (23), and myristolation (24), and these posttranslational modifications may affect the intracellular distribution process.
We further examined the biological activity of transgene-derived hIL-1α because the transgene was of human origin. hIL-1α secreted into the supernatants by synoviocytes and BM macrophages stimulated proliferation of the IL-1–sensitive mouse T cell clone D10 (Figure 3c) and also induced synoviocyte proliferation (data not shown). Therefore, transgene-derived hIL-1α is biologically active. To rule out the possibility that such mitogenic activity was actually due to a minor contaminant in the preparation, we confirmed neutralization of IL-1 activity using specific Ab. Preincubation with neutralizing Ab’s against either human or mouse IL-1α before the bioassay resulted in a significant inhibition of D10 cell proliferation, suggesting that the bioactivity is due to either transgene-derived hIL-1α or subsequently induced mouse-derived IL-1.
PMNs and macrophage-like synoviocytes predominated within the joints of Tg mice.
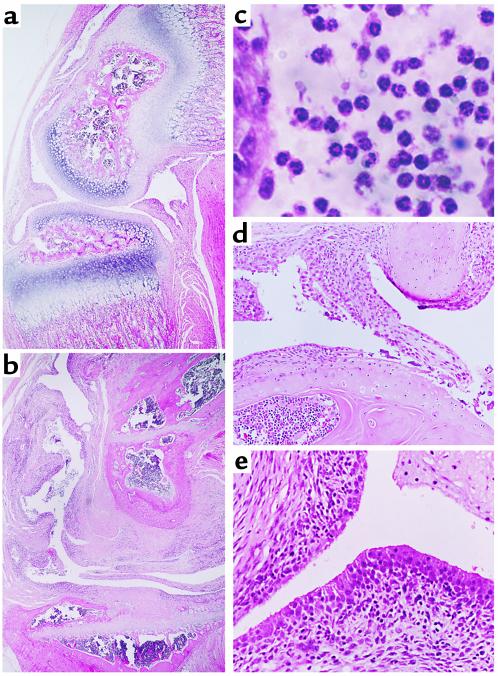
Histological analysis of the knee joints revealed synovial lining cell hyperplasia, loss of cartilage, bony erosion, fibrin deposits, and the formation of pannus-like tissues in 8-week-old mice, whereas the articular surface was still intact in 2-week-old mice (Figure 4, a and b). Higher magnification showed the majority of the cells infiltrating below and within the synovium to apparently be PMNs rather than mononuclear cells such as T/B lymphocytes (Figure 4c). Hyperplastic synovial tissue (pannus), which overgrows and invades the adjacent cartilage matrix, was frequently observed in the knee joints of Tg mice (Figure 4d). Hyperplasia of synovial lining cells was also demonstrated to be the result of accumulation of cells with a macrophage-like morphology (Figure 4e) and to resemble previously described type-A synoviocytes in rats (25) and in humans (26).
Figure 4.
The histological findings of knee joints from Tg mice. Almost intact articular cartilage with slight hyperplasia of the synovial lining layer was observed in a 2-week-old mouse (a), while destroyed cartilage was ultimately replaced by proliferative synovium, as seen in an 8-week-old mouse (b). Higher magnification of synovial fluid cells in an 8-week-old mouse shows PMN-dominant infiltration into the joint (c), cartilage/pannus junction with erosive changes in cartilage (d), and proliferation of synovial lining cells which have macrophage-like morphology (e). Magnifications: (a and b) ×50; (c) ×320; (d and e) ×200.
hIL-1α was mainly produced by synovial lining cells, which consisted mainly of monocyte/macrophage lineage cells.
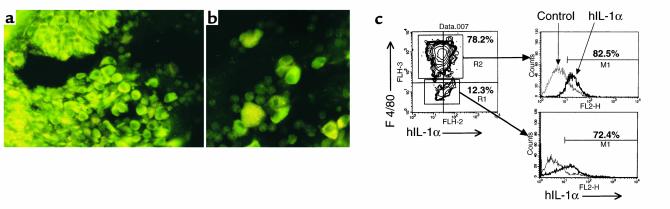
Immunostaining of hIL-1α in frozen knee joint sections revealed a large fraction of the synovial lining cells, which are morphologically identical to type-A synoviocytes, to be strongly stained with anti–hIL-1α Ab (Figure 5a). Large mononuclear cells in the synovial fluid, presumably indicating monocytes but not PMNs, also demonstrated intense staining (Figure 5b). There was no detectable staining of any cells in the synovia of littermate controls. In agreement with the data, flow cytometric analysis revealed that nearly 80% of the freshly isolated synoviocytes had the F4/80 antigen, and both F4/80+ and F4/80– synoviocytes spontaneously synthesize hIL-1α, as shown in Figure 5c. Actually, according to our in vitro immunoprecipitation analysis after a pulse-chase experiment, transgene-derived hIL-1α protein was preferentially synthesized in monocyte/macrophage and fibroblast lineage cells in the synovium and BM, despite the widespread hIL-1α mRNA expression illustrated in Figure 3a (data not shown). On the other hand, neither antigen-specific lymphocytes (Thy1.2+ T cells or B220+ B cells) nor potentially antigen-presenting cells (APCs; I-A+ CD80+/86+) were found in the synovium, whereas these cells were observed in the BM cavity (data not shown).
Figure 5.
Immunolocalization of transgene-derived hIL-1α. Immunostaining of hIL-1α in knee joint sections showed positive staining in proliferating synovial lining cells (a, ×200) and large mononuclear cells in synovial fluid (b, ×320). Freshly isolated synoviocytes from 8-week-old Tg mice were analyzed for F4/80 and hIL-1α expression by flow cytometry (c). The levels of intracellular hIL-1α after gating on F4/80+ cells or F4/80– cells are shown in the histograms. The percentage of cells producing hIL-1α is indicated. The data are representative of four separate experiments.
Phenotypic characteristics of freshly isolated synoviocytes from Tg mice.
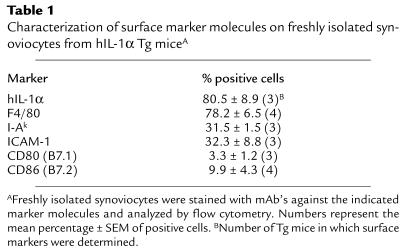
Based on our histological results, macrophage-like synoviocytes were the dominant population within the synovium, but they possessed few costimulatory molecules such as CD80 and CD86, as assessed by immunohistochemistry (data not shown). We further characterized the synoviocyte phenotype using flow cytometry. The expressions of CD80 and CD86 molecules were found to be low, whereas nearly one-third of the synoviocytes possessed ICAM-1 and I-Ak molecules (Table 1).
Table 1.
Characterization of surface marker molecules on freshly isolated synoviocytes from hIL-1α Tg miceA
hIL-1α overproduction affected the distribution of phenotypes of hematopoietic cells in various tissues.
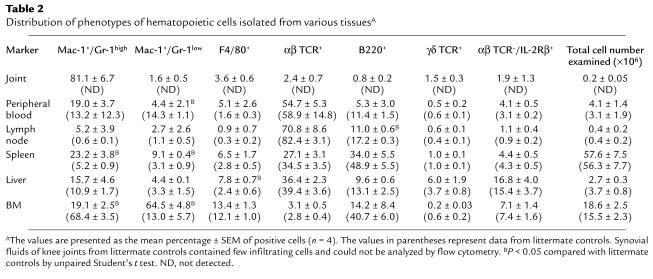
IL-1 has been shown to synergistically act on hematopoiesis at various levels with colony-stimulating factors (CSFs) and other cytokines (27). We therefore analyzed the hematopoietic effects of IL-1 on the distribution of leukocyte phenotypes in various tissues. Flow cytometric analysis revealed increased systemic leukopoiesis especially in Mac-1+/Gr-1+ neutrophils and F4/80+ macrophages (Table 2). A slight decrease in the proportion of B cells was observed in the peripheral lymphoid organs, though the total number of B cells remained constant because of an up to two- to threefold increase in the number of systemic leukocytes in Tg mice when standardized by body weight. Neutrophils are the most abundant cells in joints and they comprise approximately 80% of the total cell number, whereas both TCRαβ+ T cells and B220+ B cells comprise a much smaller proportion of cells. Since Gr-1 expression is a marker of neutrophil maturation based on the report by Hestdal et al. (28), we also analyzed the intensity of Gr-1 expression in neutrophils. Two-color flow cytometric analysis revealed that Mac-1+/Gr-1high mature neutrophils selectively infiltrate the joint, whereas the Gr-1 intensity of neutrophils was seen diffusely in other tissues (Table 2).
Table 2.
Distribution of phenotypes of hematopoietic cells isolated from various tissuesA
hIL-1α–induced GM-CSF may contribute to a higher proportion of neutrophils and monocyte-macrophage lineage cells.
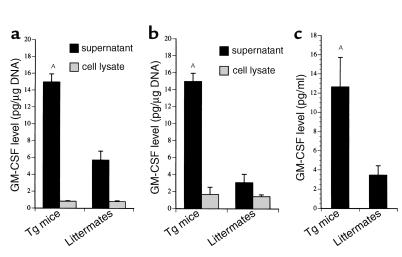
Macrophage-like synoviocytes and PMNs appeared to be the predominant cell populations in arthritic joints. We speculated that hIL-1α–induced GM-CSF acts on progenitor stem cells of the granulocyte and macrophage lineage. To account for this observation, we examined the induction of GM-CSF in Tg mice. As can be seen in Figure 6, the levels of GM-CSF in sera and supernatants from synoviocytes and BM macrophages were two- to threefold higher than those from littermates.
Figure 6.
GM-CSF levels in culture supernatants, cell lysates, and sera. Primary cultured synoviocytes and BM macrophages isolated from Tg mice or littermates were incubated with DMEM containing 10% FBS for 48 hours. These culture supernatants and cell lysates from BM macrophages (a) or synoviocytes (b) were assayed for GM-CSF production with specific ELISA. Sera from the mice, from which the culture supernatants and cell lysates had been obtained, were also assayed (c). The data are expressed as the mean ± SEM (n = 4). AP < 0.01 compared with littermate controls by unpaired Student’s t test.
Discussion
This is the first demonstration to our knowledge that overexpression of hIL-1α in mice results in chronic inflammatory arthritis characterized by synovial proliferation and the formation of pannus, which ultimately destroys articular cartilage. Earlier studies have clarified the arthritogenicity of IL-1 that an intra-articular injection of IL-1α in rabbits causes joint inflammation characterized by neutrophil infiltration and cartilage proteoglycan loss, but these arthritic changes are reversible and recovered by 9 days after the injection (9). Groves et al. have generated Tg mice overexpressing 17 kDa, a mature form of mouse IL-1α, using keratinocyte-specific K14 promoter to examine the role of IL-1α in cutaneous biology (29). As expected, these mice had inflammatory skin lesions but did not develop inflammatory arthritis despite a markedly increased serum IL-1α level. The Tg mice described here represent chronic polyarthritis with a 100% incidence under the control of the CAG promoter system. Actually, although the CAG promoter is known to direct ubiquitous transgene expression, transgene-derived hIL-1α protein was distributed preferentially in monocyte/macrophage and fibroblast lineage cells in the synovium and BM, which contributed to an arthritic phenotype and hematopoietic changes in our Tg mice. Such preferential distribution in these cells could be explained by a higher translational efficiency or a longer retention of hIL-1α protein in these cells (our unpublished data). The latter speculation is based on IL-1α being able to act as a membrane-associated form in these cells. As a consequence, transgene-derived hIL-1α was more abundantly distributed in cells capable of physiologically producing IL-1α than in cells which do not normally produce IL-1α.
Convincing evidence implicates lymphocytes in the pathogenesis of inflammatory arthritis in several RA models. The antigen-specific activation of T cells by functional APCs has been reported to play an important role in the pathogenesis of RA and several immune-driven arthritis models. However, in our Tg mice, few α/βTCR+ cells, B220+ cells, or CD80+/CD86+ functional APCs were observed within the joints during the course of arthritis. Freshly isolated synoviocytes were mostly CD80–CD86–, and one-third of the cells were ICAM-1+I-A+, whereas a large fraction of these synoviocytes were F4/80+, potentially APCs. These findings contrast strongly with the findings of CIA in which I-A+ macrophage-like synoviocytes predominated in the inflamed synovium (30). Similar effects of IL-1α have been reported in mouse epidermal Langerhans cells (31), thymic epithelial cells (32), and peripheral blood monocytes (33), in which IL-1α directly upregulated ICAM-1 and indirectly upregulated I-A through GM-CSF production, while neither CD80 nor CD86 was affected. Based on the evidence that the accessory function during antigen presentation is closely related to the levels of CD80 and CD86 (33, 34), synoviocytes in Tg mice may be insufficient to promote antigen presentation. Consequently, we speculated that hIL-1α may modulate synoviocyte (preferentially macrophage-like cells) functions leading to a tissue-destructive phenotype rather than having an antigen-presenting function.
IL-1 has been reported to affect hematopoiesis via an action on early progenitor stem cells in BM. IL-1 not only induces production of CSFs, but also stimulates the stem cell response to CSFs (35). Among the CSFs, GM-CSF is known to be a potent stimulator of granulocytes and macrophages and is reportedly involved in the pathogenesis of RA (36). Remarkably, in our Tg mice, hIL-1α induced GM-CSF production by synoviocytes and BM macrophages, possibly leading to a characteristic change in monocyte-macrophage lineage cells and PMNs, and also increasing their proportions, especially in joints. Interestingly, flow cytometric analysis revealed nearly 80% of PMNs infiltrating joints to be Gr-1high cells, a potentially tissue-degrading phenotype. The Gr-1 expression level has been reported to be directly proportional to neutrophil differentiation and IL-1 receptor expression (28), indicating that Gr-1high PMNs within joints may be one of the important responders to hIL-1α. Moreover, the data on freshly isolated synoviocytes were also noteworthy. Approximately 80% of the synoviocytes were F4/80+ cells, a rather high percentage as compared with CIA (34%; our unpublished data). Although the origin of tissue macrophages remains controversial, IL-1 overproduction seems to promote local macrophage proliferation through GM-CSF production, which is similar to the case of GM-CSF Tg mice (37). Otherwise, synovial macrophages were supplied by the BM via the peripheral circulation, since hematopoiesis in the BM should be upregulated because GM-CSF levels were high in the culture supernatants of BM macrophages and sera from Tg mice, as indicated in Figure 6.
In trials of RA therapy, special attention has been paid to the functional hierarchy between IL-1 and TNF. To date, TNF has been recognized as a primary cytokine and the TNF→IL-1 cascade is known to be important in the pathogenesis of RA. Interestingly, Northern blot analysis revealed high levels of TNF mRNA expression in synovia from our Tg mice (data not shown). Consistent with these data, IL-1 has been reported to induce TNF production in vitro and in vivo in rabbits and human PBMCs (38). Moreover, a recent study showed reduced TNF mRNA expression in the synovium after anti–IL-1 treatment for CIA in mice (11). Although we can never rule out the possibility that upregulated TNF expression results from an increased number of synovial macrophages serving as a source of TNF, in certain situations, IL-1 rather than TNF may be the primary cytokine.
The difference between IL-1 and TNF in terms of the pathogenic contribution to arthritis remains a matter of debate. It is difficult to clearly dissociate the roles of these two cytokines, because they share many biological activities. In fact, arthritis in TNF Tg mice can be prevented by blockade of the type I IL-1 receptor (13), suggesting that TNF-mediated pathology, for the most part, may take place via IL-1. We can, however, address the potential difference between the roles of two cytokines by comparing our hIL-1α Tg mice with TNF Tg mice. First, the cartilage breakdown in hIL-1α Tg mice appeared to be more rapid and drastic than that in TNF Tg mice. TNF Tg mice have shown relative preservation of cartilage despite advanced arthritis, even though they were crossed with DBA/1J mice in order to generate more exacerbated arthritis than that occurring in the original strain (39). In contrast, in hIL-1α Tg mice, articular cartilage completely disappeared and destruction reached subchondral bone by 8 weeks after birth, as shown in Figures 2c and 3a. This progressive cartilage destruction in hIL-1α Tg mice was consistent with the recent insight, obtained from experimental arthritis, that IL-1 plays a dominant role in cartilage destruction, whereas TNF plays a role in joint swelling in the early phase of arthritis (40). Second, although a paucity of lymphocyte infiltration into arthritic joints was also seen in TNF Tg mice, the proportion of neutrophils in synovial fluid and particularly that of monocyte-macrophage lineage cells in the synovium was markedly higher in hIL-1α Tg mice than in the TNF Tg mice, reported previously (39). These findings were extended to other organs as shown in Table 2 and were likely to be attributable to direct hematopoietic effects of hIL-1α. In contrast, TNF reportedly exerts an inhibitory effect rather than a stimulatory effect on hematopoiesis (41). These hIL-1α–induced hematopoietic changes resulted in accumulation of monocyte-derived cytokines and proteases in the synovium, including IL-1, TNF, IL-8, GM-CSF, and MMPs (our unpublished data), which contributed to the arthritic phenotype of our Tg mice.
Considerable interest in the relative roles of IL-1α and IL-1β in the disease process is also warranted. IL-1α and IL-1β possess essentially identical activities and potencies, and their relative pathogenic contributions during the evolution of arthritis remain to be fully elucidated. Both isoforms appear to play a pivotal role in cartilage destruction, while it has recently been reported that IL-1β plays a more dominant role in established CIA based on evidence of the marked inhibitory effects of anti–IL-1β treatment (11) or IL-1 converting enzyme (ICE) inhibitor (42). On the other hand, IL-1α is reportedly prominent in the early phase of arthritis and participates in the inhibition of proteoglycan synthesis (40). It must be noted that the precursor form of IL-1α, in contrast to that of IL-1β, is known to act as a membrane-associated form. We have confirmed not only the secreted form of IL-1α but also biologically active membrane-associated IL-1α (MA-IL-1) to be derived from a transgene in the membrane fraction of synoviocytes. Our preliminary data suggested that MA-IL-1 on the synoviocytes triggers the synoviocyte’s own proliferation in the early phase and cartilage degradation at the cartilage/pannus junction in a more established phase and that these MA-IL-1–mediated events occur through cell-to-cell interaction (i.e., juxtacrine).
In summary, hIL-1α overproduction in mice exhibited chronic polyarthritis with severe cartilage destruction. The arthritogenic capacity of IL-1α was attributed to the induction of other inflammatory cytokines, chemokines, and cartilage-degrading proteases and changing the hematopoietic distribution in favor of monocyte-macrophage lineage cells and neutrophils. Our data clearly demonstrate a pivotal role for IL-1 during the evolution of arthritis and support the good clinical results obtained in anti–IL-1 therapy against RA.
Acknowledgments
We are grateful to the late Masayuki Shinmei (Department of Orthopaedic Surgery, National Defense Medical College) for the planning of this investigation. We also thank Takushi Tadakuma (Department of Parasitology, National Defense Medical College) for providing anti-CD16/32 Ab and D10 cells used in this study.
References
- 1.Rosenwasser LM, Dinarello CA, Rosenthal AS. Adherent cell function in murine T-lymphocyte antigen recognition. IV. Enhancement of murine T-cell antigen recognition by human leukocytic pyrogen. J Exp Med. 1979;150:709–714. doi: 10.1084/jem.150.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipsky PE, Thompson PA, Rosenwasser LJ, Dinarello CA. The role of interleukin 1 in human B cell activation: inhibition of B cell proliferation and the generation of immunoglobulin-secreting cells by an antibody against human leukocyte pyrogen. J Immunol. 1983;130:2708–2714. [PubMed] [Google Scholar]
- 3.Gowen M, Wood DD, Ihrie EJ, McGuire MKB, Russell RGG. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983;306:378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA, Wolff SM. Molecular basis of fever in humans. Am J Med. 1982;72:799–819. doi: 10.1016/0002-9343(82)90548-4. [DOI] [PubMed] [Google Scholar]
- 5.Chu CQ, et al. Detection of cytokines at the cartilage/pannus junction in patients with rheumatoid arthritis: implications for the role of cytokines in cartilage destruction and repair. Br J Rheumatol. 1992;31:653–661. doi: 10.1093/rheumatology/31.10.653. [DOI] [PubMed] [Google Scholar]
- 6.Mizel SB, Dayer J-M, Krane SM, Mergenhagen SE. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (interleukin-1) Proc Natl Acad Sci USA. 1981;78:2474–2477. doi: 10.1073/pnas.78.4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dayer J-M, de Rochemonteix B, Burrus B, Demczuk S, Dinarello CA. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986;77:645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van de Loo AAJ, van den Berg WB. Effects of murine recombinant interleukin 1 on synovial joints in mice: measurements of patellar cartilage metabolism and joint inflammation. Ann Rheum Dis. 1990;49:238–245. doi: 10.1136/ard.49.4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pettipher ER, Higgs GA, Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci USA. 1986;83:8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wooley PH, et al. The effect of an interleukin-1 receptor antagonist protein on type II collagen-induced arthritis and antigen-induced arthritis in mice. Arthritis Rheum. 1993;36:1305–1314. doi: 10.1002/art.1780360915. [DOI] [PubMed] [Google Scholar]
- 11.Joosten LAB, Helsen MMA, van de Loo FAJ, van den Berg WB. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice: a comparative study using anti-TNFα, anti-IL-1α/β, and IL-1ra. Arthritis Rheum. 1996;39:797–809. doi: 10.1002/art.1780390513. [DOI] [PubMed] [Google Scholar]
- 12.Keffer J, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Probert L, Plows D, Kontogeorgos G, Kollias G. The type I interleukin-1 receptor acts in series with tumor necrosis factor (TNF) to induce arthritis in TNF-transgenic mice. Eur J Immunol. 1995;25:1794–1797. doi: 10.1002/eji.1830250647. [DOI] [PubMed] [Google Scholar]
- 14.Joosten LAB, et al. IL-1αβ blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-α blockade only ameliorates joint inflammation. J Immunol. 1999;163:5049–5055. [PubMed] [Google Scholar]
- 15.van Lent PLEM, et al. Major role for interleukin-1 but not for tumor necrosis factor in early cartilage damage in immune complex arthritis in mice. J Rheumatol. 1995;22:2250–2258. [PubMed] [Google Scholar]
- 16.Yoshino S, Cleland LG. Depletion of α/β T cells by a monoclonal antibody against the α/β T cell receptor suppresses established adjuvant arthritis, but not established collagen-induced arthritis in rats. J Exp Med. 1992;175:907–915. doi: 10.1084/jem.175.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hom JT, Butler LD, Riedl PE, Bendele AM. The progression of the inflammation in established collagen-induced arthritis can be altered by treatments with immunological or pharmacological agents which inhibit T cell activities. Eur J Immunol. 1998;18:881–888. doi: 10.1002/eji.1830180608. [DOI] [PubMed] [Google Scholar]
- 18.Plows D, Kontogeorgos G, Kollias G. Mice lacking mature T and B lymphocytes develop arthritic lesions after immunization with type II collagen. J Immunol. 1999;162:1018–1023. [PubMed] [Google Scholar]
- 19.Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 20.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 21.Villacres-Eriksson M, Bergstrom-Mollaoglu M, Kaberg H, Lovgren K, Morein B. The induction of cell-associated and secreted IL-1 by iscoms, matrix or micelles in murine splenic cells. Clin Exp Immunol. 1993;93:120–125. doi: 10.1111/j.1365-2249.1993.tb06507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beuscher HU, Nickells MW, Colten HR. The precursor of interleukin-1α is phosphorylated at residue serine 90. J Biol Chem. 1988;263:4023–4028. [PubMed] [Google Scholar]
- 23.Brody DT, Durum SK. Membrane IL-1: IL-1α precursor binds to the plasma membrane via a lectin-like interaction. J Immunol. 1989;143:1183–1187. [PubMed] [Google Scholar]
- 24.Stevenson FT, Bursten SL, Fanton C, Locksley RM, Lovett DM. The 31-kDa precursor of interleukin-1α is myristolated on specific lysines within the 16-kDa N-terminal propiece. Proc Natl Acad Sci USA. 1993;90:7245–7249. doi: 10.1073/pnas.90.15.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caulfield JP, Hein A, Dynesius-Trentham R, Trentham DE. Morphologic demonstration of two stages in the development of type II collagen-induced arthritis. Lab Invest. 1982;46:321–343. [PubMed] [Google Scholar]
- 26.Kobayashi I, Ziff M. Electron microscopic studies of the cartilage-pannus junction in rheumatoid arthritis. Arthritis Rheum. 1975;18:475–483. doi: 10.1002/art.1780180507. [DOI] [PubMed] [Google Scholar]
- 27.Bagby GC., Jr Interleukin-1 and hematopoiesis. Blood Rev. 1989;3:152–161. doi: 10.1016/0268-960x(89)90012-x. [DOI] [PubMed] [Google Scholar]
- 28.Hestdal K, et al. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991;147:22–28. [PubMed] [Google Scholar]
- 29.Groves RW, Mizutani H, Kieffer JD, Kupper TS. Inflammatory skin disease in transgenic mice that express high levels of interleukin 1α in basal epidermis. Proc Natl Acad Sci USA. 1995;92:11874–11878. doi: 10.1073/pnas.92.25.11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmdahl R, Jonsson R, Larsson P, Klareskog L. Early appearance of activated CD4+ T lymphocytes and class II antigen-expressing cells in joints of DBA/1 mice immunized with type II collagen. Lab Invest. 1988;58:53–60. [PubMed] [Google Scholar]
- 31.Chang C-H, Furue M, Tamaki K. Selective regulation of ICAM-1 and major histocompatibility complex class I and II molecule expression on epidermal Langerhans cells by some of the cytokines released by keratinocytes and T cells. Eur J Immunol. 1994;24:2889–2895. doi: 10.1002/eji.1830241146. [DOI] [PubMed] [Google Scholar]
- 32.Galy AHM, Spits H. IL-1, IL-4, and IFN-γ differentially regulate cytokine production and cell surface molecule expression in cultured human thymic epithelial cells. J Immunol. 1991;147:3823–3830. [PubMed] [Google Scholar]
- 33.Scheinecker C, et al. Initiation of the autologous mixed lymphocyte reaction requires the expression of costimulatory molecules B7-1 and B7-2 on human peripheral blood dendritic cells. J Immunol. 1998;161:3966–3973. [PubMed] [Google Scholar]
- 34.Larsen CP, Ritchie SC, Pearson TC, Linsley PS. Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J Exp Med. 1992;176:1215–1220. doi: 10.1084/jem.176.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oppenheim JJ, et al. Interleukin-1 enhances survival of lethally irradiated mice treated with allogeneic bone marrow cells. Blood. 1989;74:2257–2263. [PubMed] [Google Scholar]
- 36.Kotake S, et al. Detection of myeloid precursors (granulocyte/macrophage colony forming units) in the bone marrow adjacent to rheumatoid arthritis joints. J Rheumatol. 1992;19:1511–1516. [PubMed] [Google Scholar]
- 37.Metcalf D, Elliott MJ, Nicola NA. The excess numbers of peritoneal macrophages in granulocyte-macrophage colony-stimulating factor transgenic mice are generated by local proliferation. J Exp Med. 1992;175:877–884. doi: 10.1084/jem.175.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikejima T, Okusawa S, Ghezzi P, van der Meer JW, Dinarello CA. Interleukin-1 induces tumor necrosis factor (TNF) in human peripheral blood mononuclear cells in vitro and a circulating TNF-like activity in rabbits. J Infect Dis. 1990;162:215–223. doi: 10.1093/infdis/162.1.215. [DOI] [PubMed] [Google Scholar]
- 39.Butler DM, et al. DBA/1 mice expressing the human TNF-α transgene develop a severe, erosive arthritis. J Immunol. 1997;159:2867–2876. [PubMed] [Google Scholar]
- 40.van den Berg WB. Joint inflammation and cartilage destruction may occur uncoupled. Springer Semin Immunopathol. 1998;20:149–164. doi: 10.1007/BF00832004. [DOI] [PubMed] [Google Scholar]
- 41.Gasparetto C, et al. Effects of interleukin-1 on hematopoietic progenitors: evidence of stimulatory and inhibitory activities in a primate model. Blood. 1989;74:547–550. [PubMed] [Google Scholar]
- 42.Ku G, Faust T, Lauffer LL, Livingston DJ, Harding MW. IL-1β converting enzyme inhibition blocks progression of type II collagen-induced arthritis in mice. Cytokine. 1996;8:377–386. doi: 10.1006/cyto.1996.0052. [DOI] [PubMed] [Google Scholar]