Abstract
The Saethre-Chotzen syndrome is characterized by premature fusion of cranial sutures resulting from mutations in Twist, a basic helix-loop-helix (bHLH) transcription factor. We have identified Twist target genes using human mutant calvaria osteoblastic cells from a child with Saethre-Chotzen syndrome with a Twist mutation that introduces a stop codon upstream of the bHLH domain. We observed that Twist mRNA and protein levels were reduced in mutant cells and that the Twist mutation increased cell growth in mutant osteoblasts compared with control cells. The mutation also caused increased alkaline phosphatase and type I collagen expression independently of cell growth. During in vitro osteogenesis, Twist mutant cells showed increased ability to form alkaline phosphatase-positive bone-like nodular structures associated with increased type I collagen expression. Mutant cells also showed increased collagen synthesis and matrix production when cultured in aggregates, as well as an increased capacity to form a collagenous matrix in vivo when transplanted into nude mice. In contrast, Twist mutant osteoblasts displayed a cell-autonomous reduction of osteocalcin mRNA expression in basal conditions and during osteogenesis. The data show that genetic deletion of Twist causing reduced Twist dosage increases cell growth, collagen expression, and osteogenic capability, but inhibits osteocalcin gene expression. This provides one mechanism that may contribute to the premature cranial ossification induced by deletion of the bHLH Twist domain in Saethre-Chotzen syndrome.
Introduction
The Saethre-Chotzen syndrome (ACS III) is an inherited autosomal dominant syndrome characterized by craniosynostosis, facial asymmetry, hypertelorism, and brachydactyly (1). Craniosynostosis is the most striking anomaly and is characterized by premature fusion of cranial sutures. This genetic syndrome has been ascribed recently to dominant heterozygotic mutations in Twist gene (2, 3), a basic helix-loop-helix (bHLH) transcription factor (4). The function of bHLH transcription factors depends on the basic DNA-binding region and HLH structure that allows HLH monomers to interact and form a functional dimer that can recognize and bind to DNA motifs called E-boxes (4). Not surprisingly, in the Saethre-Chotzen syndrome, most reported mutations were found to involve the bHLH domain of the Twist gene (5). Developmental studies in Drosophila showed that Twist is specifically expressed in mesodermal and cranial neural crest cells (6) and plays a key role in mesoderm formation and myogenesis through activation of downstream genes (7–11). In the mouse, the expression pattern of Twist suggests that this transcription factor regulates genes involved in the specification and the differentiation of cranial mesenchyme (12, 13). Accordingly, Twist was found to control murine cranial tube morphogenesis, and mesodermal, muscle, and neuronal cell differentiation (14–19). Twist is expressed in mesodermal-derived osteoblast precursors in vivo and in calvaria-derived murine osteoblast precursor cells in vitro (20–22), suggesting that Twist may serve as a regulator of osteoblast differentiation. However, the role of Twist in intramembranous ossification and the cellular and molecular abnormalities induced by Twist mutations on target genes in osteoblasts have not been identified.
We showed previously that the premature ossification of cranial sutures in Apert craniosynostosis characterized by mutations in fibroblast growth factor receptor-2 (FGFR-2) involves activation of osteoblast differentiation genes such as alkaline phosphatase (ALP), α(1)I collagen (COLIA1), and osteocalcin (OC) (23–25). Therefore, in this study, we investigated the possibility that these genes may be target genes for Twist in Saethre-Chotzen syndrome. We show here that genetic deletion of the bHLH domain in Twist inhibits OC expression and increases ALP, type I collagen expression and synthesis and matrix deposition during in vitro osteogenesis, which indicates that the premature cranial suture ossification in Saethre-Chotzen syndrome is secondary to alteration of these osteoblast differentiation genes.
Methods
Subjects, bone samples, and mutation analysis.
Our previous studies established that human calvaria cell cultures are useful tools in determining the mechanisms involved in normal and pathologic osteogenesis in craniosynostosis (23–26). In this study, we obtained calvaria bone samples at the coronal suture levels from one infant, aged 3.5 months, with clinical evidence of Saethre-Chotzen syndrome. Normal calvaria bone samples at equivalent areas were obtained from one normal age-matched infant who underwent local reconstruction of the skull unrelated to bone diseases (27). This subject was representative of control subjects of the same age. Previous studies showed a minimal variation in calvaria cell proliferation or differentiation parameters within controls of the same age (27).
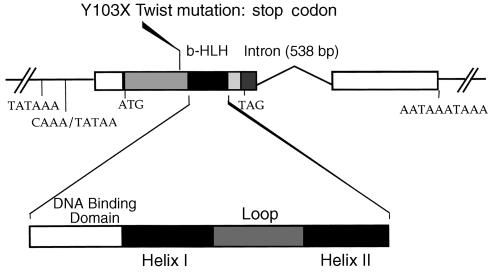
All processes were done according to the French ethical committee recommendations. The bone samples were sectioned into two parts and used for histological or cell culture studies. For mutation analyses, blood samples were collected and genomic DNA was extracted from lymphocytes by SDS lysis, according to standard procedures (2). Twist mutations were screened by fluorometric sequencing or endonuclease digestion (2). The mutation found (Y103X) in the patient with Saethre-Chotzen syndrome introduces a stop codon, leading to truncated protein without functional bHLH domain (Figure 1).
Figure 1.
Schematic representation of the Twist gene showing that the Y103X non-sense mutation introduces a stop codon leading to truncated protein without functional bHLH domain.
Calvaria cell cultures.
Calvaria cells were obtained from mutant and normal bone samples as described previously (23, 27). Briefly, the samples were dissected into small fragments, washed extensively with PBS to remove marrow cells, treated with 0.25% collagenase (type I; Sigma Chemical Co., St. Louis, Missouri, USA) for 2 hours at 37°C, and washed in DMEM supplemented with glutamine (292 mg/l), 10% heat-inactivated FCS, and antibiotics (100 IU/ml penicillin and 100 μg/ml streptomycin). Cells were then collected by centrifugation, and cultured in DMEM with 10% FCS until confluence. This method yields a cell population with characteristics of preosteoblastic cells without other cell types in the cultures (23–27). Cells isolated from the mutant subject expressed the Y103X Twist mutation as found by PCR and restriction enzyme analyses. Both calvaria cell populations obtained from the mutant and normal subjects were immortalized in order to determine further the cellular phenotype induced by the mutation. Immortalization was performed similarly in the two cell types using a plasmid containing the SV-40 large T antigen, as described (28). All immortalized normal (Nl) and mutant Twist (M-Tw) cells showed genomic functional insertion of the large T antigen as determined by immunocytochemistry and RT-PCR analysis (data not shown). Only 1 clone out of 13 in Nl cells clearly differed from others in terms of ALP activity, and this clone was not included in the pooled population in order to avoid mixed stages of differentiation. A similar approach was used for mutant cells: M-Tw clones (n = 13) that were homogeneous with regard to ALP activity were pooled for study.
Analysis of cell growth.
Cell growth of Nl and M-Tw cells was evaluated by DNA synthesis and cell counting, as described (29). Briefly, cells plated at 104 cells/cm2 were cultured in DMEM with 10% FCS for 7 days. Cells were incubated with 0.8 μCi/well of [3H]-thymidine on days 1 to 6. [3H]-thymidine incorporation into DNA was determined in four aliquots by liquid-scintillation counting, and total DNA synthesis (cumulative peak of [3H]-thymidine incorporation into DNA) was determined (29). In parallel cultures, cells were counted and reported as cell number per square centimeter.
Analysis of osteoblast markers.
Because preliminary studies indicated that the mutation affected cell growth, we analysed the expression of osteoblast differentiation marker genes independently of cell growth in a first series of experiments. Nl and M-Tw cells were plated at the same cell density (2 × 104 cells/cm2) and cultured in DMEM with 10% FCS until they reached exactly the same stage of confluence (preconfluence and confluence stages obtained at 2 and 4 days of culture, respectively). At these two time points, the expression of mRNA for Twist, ALP, COLIA1, and OC was determined by RT-PCR analysis, and Twist protein levels were determined by Western blot analysis as described below.
A second series of experiments was conducted to analyze the effect of the mutation on osteoblast phenotype in culture conditions that promote cell differentiation and matrix synthesis. Nl and M-Tw cells were cultured at the same cell density (20 × 104 cells/cm2) for up to 4 to 8 days in the presence of 25 μg/ml ascorbic acid to allow cell differentiation and matrix synthesis. In these conditions the cells formed nodular structures, which were histochemically stained for ALP activity. The plates were rinsed with cold PBS, fixed in 70% ethanol at 4°C, and incubated for 1 hour at 37°C with naphthol AS-BI phosphate in Tris buffer (pH 8.5) in the presence of fast red violet LB salt. The number of ALP+ nodules formed at 8 days by Nl and M-Tw cells per square centimeter was evaluated under the microscope at ×125. In the same culture conditions, the expression of Twist and early and late osteoblast differentiation marker genes (ALP, COLIA1, OC) was determined in Nl and M-Tw cells at 4, 6, and 8 days of culture using RT-PCR analysis as described below.
To further determine the phenotype induced by the mutation, a third series of experiments was conducted using histological and biochemical analyses. Nl and M-Tw cells were cultured in aggregates, conditions that allow matrix formation and osteogenesis in human calvaria cell cultures (23, 26). The cells were cultured at high cell density (5 × 105 cells/aggregate) on bacteriological-grade dishes in the presence of ascorbic acid (25 μg/ml) to form aggregates. After 14 days, the aggregates were harvested, fixed in 70% ethanol, and embedded undecalcified in glycolmetacrylate (30). Thin (5-μm) histologic sections were stained with Goldner trichrome to visualize matrix deposition (30). ALP activity in cells was determined by a colorimetric assay (BioMérieux SA, Marcy Etoile, France), and collagen type 1 synthesized by the cells was determined by the level of collagen type 1 carboxy-terminal propeptide (P1CP) released into the medium, which reflects type I collagen synthesis, using a specific radioimmunoassay (Orion Diagnostics, Espoo, Finland). The results were normalized for protein content.
Western blot analysis.
Twist protein levels were determined in confluent Nl and M-Tw cells by Western blot analysis. Nuclear cell extracts were prepared as described (31). The protein content of supernatants was determined using the DC Protein Assay (Bio-Rad Laboratories Inc., Hercules, California, USA). Proteins were subjected to SDS-PAGE using 4–15% gel (Bio-Rad Laboratories Inc.) for detection of Twist, and β-actin was used as internal control for protein loading. Proteins were transferred onto PVDF membranes (Hybond-P; Amersham Pharmacia, Saclay, France) in buffer containing 20% methanol. The membranes were incubated overnight with 1% blocking buffer (Roche Diagnostics, Meylan, France) in TBS (50 mM Tris HCl, 150 mM NaCl) containing 0.1% Tween-20, and then incubated with either affinity-purified goat polyclonal Ab raised against a peptide corresponding to amino acids 172-188 mapping at the COOH-terminal domain of human Twist (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA), or rabbit mAb raised against β-actin (Sigma Chemical Co.). The membranes were then washed twice with TBS/0.1% Tween-20 and 0.5% blocking buffer, and incubated for 1 hour with horseradish peroxidase–conjugated (HRP-conjugated) secondary Ab’s. After three washes in TBS/0.1% Tween-20, the signal was visualized with ECL Plus blotting substrate (Amersham Pharmacia).
RT-PCR analysis.
Analysis of mRNA for Twist and osteoblast markers was performed in preconfluent and confluent Nl and M-Tw cells and, during the course of the formation of nodules, in the culture conditions described above. The expression of Twist, ALP, COLIA1, and OC mRNAs was analysed by semiquantitative RT-PCR. Optimization of RT-PCR results was carried out by generating saturation curves of RT-PCR products of each gene and of GAPDH against cycle number (0–35 cycles) (25). We chose the same cycle number (24 cycles) for all genes, except for ALP (30 cycles), in which the amplification was linear. Nl and M-Tw cells were washed with PBS and lysed with Extract-All reagent (Eurobio, Les Ulis, France) according to the manufacturer’s instructions. Three micrograms of total cellular RNA from each sample was reverse transcribed, and the cDNA samples were then divided and amplified using the specific primers described previously (20 pmol/tube) for ALP, COLIA1, OC, and GAPDH (25). Primers for Twist were sense: 5′-TCTTACGAGGAGCTGCAGAC-3′ and antisense: 5′-TATCCAGCTCCAGAGTCTCT-3′ and were designed to amplify a sequence including the bHLH domain. Southern blot analyses were performed by running aliquots of amplified cDNA on 1.2% agarose gel followed by transfer onto nylon membrane according to the manufacturer’s protocol. Hybridization of blots was carried out overnight at 50°C with (γ32P)ATP-labeled internal primers. Membranes were washed twice in 2 × SSC/0.1% SDS at room temperature for 10 minutes, once in 0.1 × SSC/0.1% SDS at 50°C for 10 minutes, then exposed to x-ray films. Autoradiographic signals were quantified using a scanner densitometer. The signal for each gene was corrected for GAPDH.
Matrix formation in vivo.
The matrix formation by Nl and M-Tw cells was also determined in vivo. Cell suspensions of Nl and M-Tw cultures were prepared by repeated dispersion through a 20-gauge needle; 107 cells were resuspended in 2.5 ml DMEM plus 10% FCS and added to 3-mm porous hydroxyapatite (HA) ceramic cubes (CRITT, Maubeuge, France). After a slight vacuum to release air pockets from the ceramic, the HA cubes were placed overnight at 37°C to allow cell attachment. The cubes were then implanted subcutaneously (4–6 HA cubes per mouse) in the back of athymic mice (homozygote CD-1 nu/nu, 4-week-old female; Charles River, St. Aubin Les Elbeuf, France) kept under a controlled environment. Controls consisted of HA cubes implanted without cells. After 4 and 8 weeks of implantation, the HA samples were removed, fixed in 70% ethanol, and embedded decalcified in methylmethacrylate (32). Longitudinal sections (5-μm thick) of plastic-embedded bone samples were stained with Goldner trichrome to identify the collagenous matrix (32). The matrix formed along the HA material was visualized on Goldner trichrome–stained sections, measured by histomorphometric methods (33) using an integrating ocular mounted on an Olympus microscope (×250), and expressed as the percentage of osteoid volume per total implant volume.
Statistics.
The data were expressed as the mean ± SEM. Differences between the mean values were analysed using the statistical package super-ANOVA (Macintosh; Abacus Concepts Inc., Berkeley, California, USA) with a P value less than 0.05 considered to be of minimal significance.
Results
The Y103X Twist mutation reduces Twist dosage in mutant calvaria cells.
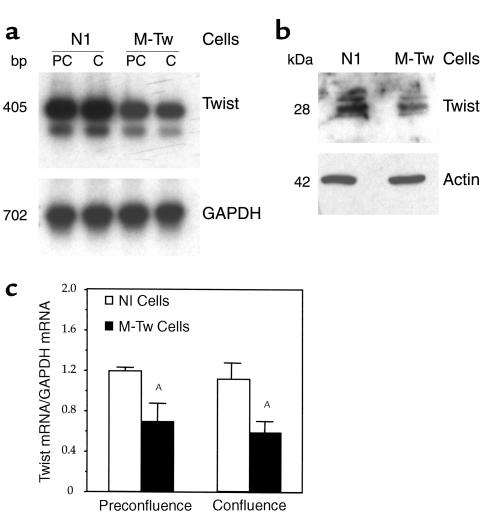
We first determined the effect of the Y103X mutation on the expression of Twist mRNA and protein levels. Figure 2a shows that Twist mRNA were expressed in Nl and M-Tw cells cultured at preconfluence and confluence. Quantitative analysis of mRNA levels corrected for GAPDH showed that the levels of Twist mRNA were about 40% lower in M-Tw cells compared with Nl cells (Figure 2c). Furthermore, Western blot analysis showed that the level of Twist protein recognized by a COOH-terminal Ab was reduced in M-Tw cells, as predicted from the deletion of the COOH-terminal domain of the protein induced by the mutation (Figure 2b). These data show that the mutation decreases Twist mRNA and protein levels, indicating that Twist dosage is reduced in mutant cells.
Figure 2.
Reduced expression of Twist mRNA and Twist protein in mutant calvaria cells. Control (Nl) and mutant (M-Tw) calvaria cells were cultured at preconfluence (PC; 2 days) or confluence (C; 4 days), and Twist mRNA and protein levels were determined. (a) Total cellular RNA was reverse transcribed and cDNA amplified by PCR to examine the expression of Twist or GAPDH transcripts followed by Southern blot analysis. (b) At confluence (4 days), equal aliquots of nuclear cell lysates were examined for Twist protein by using Western blot analysis probed with a polyclonal affinity-purified COOH-Twist Ab or a monoclonal β-actin Ab and detected by enhanced chemiluminescence. (c) Densitometric analysis of Twist mRNA levels after correction for GAPDH (mean ± SEM of three experiments, AP < 0.05 vs. Nl cells).
Reduced Twist dosage increases human calvaria cell growth.
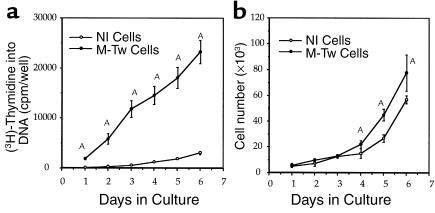
The effect of the Twist mutation causing reduced Twist dosage was then determined on calvaria cell proliferation. DNA synthesis was higher in M-Tw cells compared with Nl cells at 1 to 6 days of culture (Figure 3a). Cell counting showed that the growth rate of M-Tw cells was twofold greater than in Nl cells (Figure 3b), confirming the change in DNA synthesis. These data indicate that the Y103X Twist mutation causing reduced Twist dosage results in increased calvaria osteoblastic cell proliferation in vitro.
Figure 3.
Reduced Twist dosage increases cell growth in mutant osteoblasts. Control (Nl) and mutant (M-Tw) calvaria cells with the Y103X Twist mutation were plated at the same cell density (20 × 104 cells/cm2), and DNA synthesis and cell counts were determined at time intervals. The time-course analysis shows that both DNA synthesis (a) and cell number (b) were increased in M-Tw cells compared with Nl cells. Data are the mean ± SEM of four cultures. AP < 0.01 vs. Nl cells.
Reduced Twist dosage inhibits OC expression.
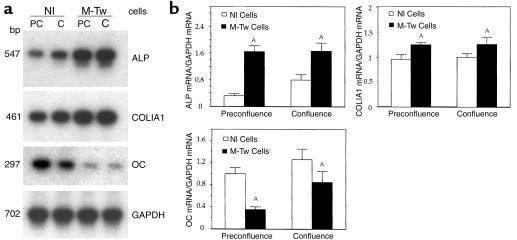
To study the effect of the Twist mutation on cell differentiation independent of cell growth, early and late osteoblast marker genes were determined in Nl and M-Tw cells cultured at similar density conditions (preconfluence or confluence). In these conditions, Twist mRNA levels were reduced in M-Tw cells compared with Nl cells cultured at both preconfluence or confluence (Figure 2). This was associated with significant changes in the expression of osteoblast marker genes. ALP mRNA levels were markedly increased (twofold to threefold) in M-Tw cells compared with Nl cells at both preconfluence and confluence (Figure 4, a and b). COLIA1 mRNA levels were also significantly increased in mutant cells compared with Nl cells cultured at the same density (Figure 4, a and b). In contrast, M-Tw cells showed a marked decrease in OC mRNA levels compared with Nl cells (Figure 4a). Quantitative analyses of gene expression confirmed that OC mRNA transcripts levels were twofold to threefold lower in M-Tw cells compared with Nl cells (Figure 4b). Similar results were found at preconfluence and confluence, confirming the cell growth–independent alteration of OC expression in M-Tw cells. Thus, the Y103X Twist mutation causing reduced Twist dosage in human calvaria cells is associated with increased ALP and COLIA1, but reduced OC gene expression, independently of cell growth.
Figure 4.
Reduced Twist dosage decreases OC expression in calvaria osteoblasts. Control (Nl) and mutant (M-Tw) calvaria cells were cultured until they reached preconfluence (PC; 2 days) or confluence (C; 4 days), and the expression of osteoblast marker genes was determined. (a) Total cellular RNA was reverse transcribed and cDNA amplified by PCR to examine the expression of ALP, COLIA1, OC, or GAPDH transcripts followed by Southern blot analysis. (b) Densitometric analysis of mRNA levels was performed after correction for GAPDH (mean ± SEM of three experiments; AP < 0.05 vs. Nl cells).
Reduced Twist dosage increases osteogenesis in vitro.
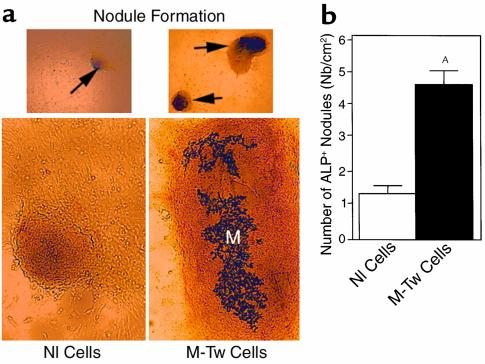
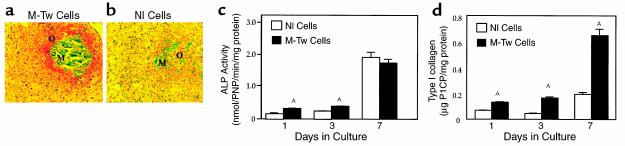
We then performed in vitro analyses to determine the effect of the Y103X Twist mutation on matrix formation by calvaria osteoblasts during in vitro osteogenesis. When cultured in the presence of ascorbic acid that favors matrix formation and osteogenesis in vitro (20), M-Tw and Nl cells rapidly produced ALP+ nodule-like structures. However, M-Tw cells produced more and larger nodules than Nl cells during the same period of time (8 days) (Figure 5a). The quantitative analysis showed that M-Tw cells produced fourfold more nodules than Nl cells (Figure 5b), demonstrating the increased in vitro osteogenic capability of M-Tw cells. To further assess the capacity of mutant cells to form a collagenous matrix in vitro, M-Tw cells were cultured in aggregates, conditions that favor osteogenesis in human calvaria cell cultures (23, 25). In these conditions, M-Tw cells formed a large amount of calcified and uncalcified matrix in aggregates at 14 days of culture (Figure 6a). In contrast, Nl cells only formed small matrix areas (Figure 6b). Biochemical analysis performed in parallel aggregate cultures showed that ALP activity was increased in M-Tw cell cultures compared with Nl cells during the first 3 days of culture in aggregates (Figure 6c). The increased in vitro osteogenesis was also associated with a marked increase in type I collagen synthesis, as revealed by P1CP levels (Figure 6d). This confirms that reduced Twist dosage enhances these osteoblast markers in mutant cells during in vitro osteogenesis, and increases collagenous matrix formation by M-Tw cells during osteogenesis in vitro.
Figure 5.
Reduced Twist dosage increases the formation of nodular structures by mutant calvaria osteoblasts. Control (Nl) and mutant (M-Tw) calvaria cells were plated at the same density in the presence of ascorbic acid to induce the formation of nodules. (a) Nodule formation (arrows in upper panel; ×125) and matrix formation (M in lower panel; ×250) is shown. The number of ALP+ nodules was determined at 8 days of culture (b). Data are the mean ± SEM of three to four cultures. AP < 0.01 vs. Nl cells.
Figure 6.
Reduced Twist dosage increases in vitro osteogenesis by mutant calvaria cells. Control (Nl) and mutant (M-Tw) calvaria cells were cultured in aggregates. Histological 5-μm-thick sections stained with Goldner trichrome showed increased amount of osteoid (O) and calcified matrix (M) formed by M-Tw cells (a) compared with Nl cells (b) at 14 days of culture (×250). (c) Increased ALP activity and (d) type I collagen synthesis, determined by P1CP levels in M-Tw cells compared with Nl cells cultured in aggregates. Data are the mean ± SEM of three to four cultures. AP < 0.05 vs Nl cells.
Reduced Twist dosage increases matrix synthesis in vivo.
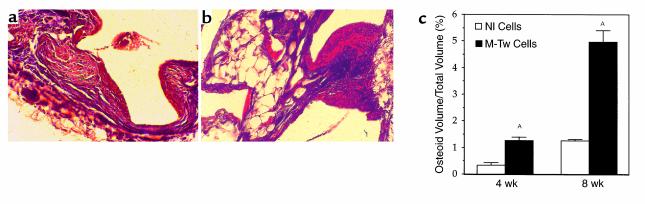
To further characterize the osteogenic capacity of Twist mutant cells in vivo, we analysed the matrix-formation capacity of M-Tw cells cultured on porous HA ceramics that were implanted in nude mice. In these conditions, the matrix formed by cells along the implanted HA can be detected histologically, whereas the HA ceramic is no longer visible due to the decalcification process (Figure 7). The histologic analysis after 8 weeks of implantation showed an increased amount of osteoid tissue formed by M-Tw cells along the HA material (Figure 7b), whereas osteoid formation by Nl cells was weak (Figure 7a). HA material implanted without cells did not show matrix formation (not shown). Histomorphometric analysis of the osteoid matrix showed that M-Tw cells formed fourfold more osteoid matrix than normal cells at 4 and 8 weeks of implantation (Figure 7c). This shows that the Twist mutation causing reduced Twist dosage induces increased bone matrix formation in vivo, confirming the in vitro analysis.
Figure 7.
Increased matrix production by Twist mutant calvaria cells in vivo. Control (Nl) and mutant (M-Tw) calvaria cells were preincubated on HA, which was implanted subcutaneously in nude mice. After 4–8 weeks, the material was embedded, 5-μm-thick sections were stained with Goldner trichrome, and the amount of osteoid matrix formed along HA was quantified. HA ceramic implants were no longer visible after the decalcification process. M-Tw cells (b) formed an increased osteoid amount (red) deposited along HA, compared with Nl cells (a) (×250). (c) Histomorphometric analysis showed that the amount of matrix formed by M-Tw cells was greater than in control Nl cells (mean ± SEM; AP < 0.05 vs. Nl cells).
Reduced Twist dosage alters osteoblast marker genes during in vitro osteogenesis.
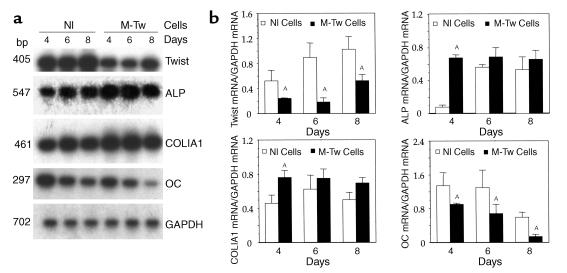
To further identify the effect of the Twist mutation on osteoblast marker genes, we investigated the kinetics of expression of Twist and osteoblast marker genes during in vitro osteogenesis. M-Tw and Nl cells were cultured in conditions to form nodular structures for up to 8 days, and mRNA levels were determined. As shown in Figure 8, Twist mRNA levels remained lower in M-Tw cells than in Nl cells during in vitro osteogenesis (Figure 8, a and b). In these culture conditions, ALP mRNA levels were initially increased at 4 days in M-Tw cells compared with Nl cells (Figure 8, a and b). Thereafter, ALP mRNA levels were similar in M-Tw and Nl cells at 6 to 8 days, when the cells formed nodules. Similarly, COLIA1 mRNA levels were initially increased in M-Tw cells compared with Nl cells and then did not differ significantly (Figure 8, a and b). In contrast, OC mRNA levels were markedly decreased in M-Tw cells compared with Nl cells throughout the 4 to 8 days of culture, i.e., during nodule formation (Figure 8, a and b). Thus, the Twist mutation causing reduced Twist dosage initially alters ALP and COLIA1 expression and permanently decreases OC gene expression in mutant osteoblasts during in vitro osteogenesis. Overall, the results show that the phenotype induced by the Y103X Twist mutation causing Twist haploinsufficiency in human calvaria osteoblasts is characterized by increased cell proliferation, reduced OC expression, and increased type I collagen synthesis and osteogenesis in vitro and in vivo, indicating that the premature cranial suture ossification in Saethre-Chotzen syndrome is secondary to alteration of these osteoblast differentiation genes.
Figure 8.
Reduced Twist dosage differentially affects osteoblast marker genes during in vitro osteogenesis. Control (Nl) and mutant (M-Tw) calvaria cells were plated at the same cell density to favor cell differentiation and formation of nodular structures. (a) Total cellular RNA was reverse transcribed and cDNA amplified by PCR to examine the expression of Twist, ALP, COLIA1, OC, or GAPDH transcripts followed by Southern blot analysis. (b) Densitometric analysis of mRNA levels after correction for GAPDH (mean ± SEM of three experiments; AP < 0.05 vs. Nl cells).
Discussion
The premature fusion of cranial sutures in the Saethre-Chotzen syndrome is associated with mutations in the Twist gene (2, 3). However, the effects of Twist mutations on the cellular phenotype and the target genes for Twist in human calvaria osteoblasts have not been identified. In this study, we have determined the phenotype engendered by deletion of the bHLH domain in the Twist gene in vitro and in vivo and identified target genes in mutant human calvaria osteoblasts.
Most mutations in the Saethre-Chotzen syndrome were found to affect the Twist bHLH domain (2, 3). Because this bHLH functional domain is essential in the transactivating function of bHLH transcription factors (4), we studied the cellular phenotype induced by the Y103X mutation causing deletion of the entire bHLH domain. Consistent with the Y103X mutation introducing a stop codon, we found decreased Twist protein levels in mutant cells. The Twist loss of function associated with bHLH deletion may also be associated with protein degradation (34). In addition, we found that the mutation decreased the levels of Twist mRNA. Thus, the phenotype induced by the Y103X Twist mutation in human calvaria cells results from reduced Twist dosage. We then determined the alterations of cell growth and osteoblast gene markers induced by Twist loss of function. Reduction of Twist dosage in mutant calvaria osteoblasts induced a twofold increase in mutant cell growth, suggesting that Twist acts as a repressor of cell proliferation in human calvaria osteoblasts. The dissociation between the rate of cell proliferation and number of mutant and control cells also suggests that the rate of apoptosis may differ in the two cell types (35). The molecular mechanisms involved in the effect of the mutation on cell growth are not known yet. Experiments in Drosophila suggest that D-Twist may affect the expression of a FGFR homologue, DFR1 (36). Twist was also suggested to regulate the expression of FGFRs during craniofacial development in the mouse (37), suggesting an implication of FGFRs in the cellular phenotype induced by Twist. We found no alteration in the mRNA and protein expression of FGFR-1 in M-Tw cells compared with Nl cells (data not shown). The mechanisms that may relay other FGFRs to Twist in human calvaria osteoblasts remain to be identified.
We then determined whether the reduced Twist dosage induced by the Y103X Twist mutation in Saethre-Chotzen syndrome induces alterations of osteoblast marker genes and matrix synthesis during in vitro and in vivo osteogenesis. The mutation initially increased ALP mRNA levels and ALP activity independently of cell growth in the monolayer and during in vitro osteogenesis, indicating that Twist controls ALP expression and activity in human calvaria osteoblasts. Moreover, the Twist mutation promoted the formation of bone-like nodular structures in vitro and increased type I collagen synthesis and the amount of matrix deposition during in vitro osteogenesis. The implantation experiments in nude mice confirmed the increased capability of Twist mutant osteoblasts to form a collagenous matrix in vivo. Taken together, our data indicate that Twist loss of function induced by deletion of the bHLH domain results in increased bone matrix deposition by mutant osteoblasts in vivo and in vitro. This phenotype is relevant to the human disease since ex vivo histological analysis of the coronal suture in the same patient with Saethre-Chotzen syndrome revealed increased deposition of collagen fibrils at the subperiosteal level (data not shown). These findings indicate that the increase in matrix bone formation induced by the Twist mutation in calvaria osteoblasts may contribute to the premature cranial ossification in the Saethre-Chotzen syndrome.
The Twist mutation did not affect osteoblast marker genes similarly. Although we found that the reduced Twist dosage increased ALP and COLIA1 expression during osteogenesis in vitro, the Twist mutation reduced expression of OC in human calvaria osteoblasts. This effect of the mutation was unrelated to cell proliferation, since the expression of these genes was consistently altered in mutant cells independently of cell growth. Moreover, this OC defect was cell autonomous and intrinsic to osteoblasts that express OC. Although recent transfection studies in osteosarcoma cells indicate that overexpression of Twist may inhibit osteoblast differentiation (38), the expression of OC, a master osteoblast marker gene, was not determined. The present study provides, we believe for the first time, therefore, the first genetic evidence that deletion of the Twist bHLH domain in human mutant calvaria osteoblasts alters the expression of the osteoblast marker gene OC in addition to promoting osteogenesis. These results are consistent with previous genetic evidence showing that OC-deficient mice develop a phenotype characterized by increased bone formation (39). Several molecular mechanisms may be involved in the alteration of OC expression induced by the Twist Y103X mutation. Deletion of the bHLH domain may restrain appropriate dimerization with bHLH or E proteins (34), thus preventing binding of the dimeric proteins to E boxes in target promoters and subsequent transactivation of tissue-specific genes (4). This appears unlikely since Twist does not bind E boxes in the OC promoter (G. Karsenty, personal communication). Twist has been shown to dimerize with E proteins and to inhibit myogenic regulatory factors such as MEF2 in muscle cells (15, 17). However, we did not find alteration of MEF2 expression in M-Tw human calvaria cells (data not shown). Our preliminary data indicate that the expression of Cbfa1/Runx2, a transcription factor that plays an important role in the regulation of osteoblast marker genes (40), is decreased in M-Tw cells (41). However, this cannot explain the increased collagenous matrix formation induced by the mutation, and other mechanisms using other regulatory proteins may also be at play in mutant human calvaria cells. Finally, it is noteworthy that the cellular phenotype induced by Twist deletion in Saethre-Chotzen syndrome differs from that caused by FGFR-2 mutations in Apert craniosynostosis. While Twist bHLH deletion increases ALP and COLIA1 and reduces OC expression in human calvaria osteoblasts, activating FGFR-2 mutations cause increased ALP, COLIA1, and OC expression in Apert osteoblasts. Moreover, cell growth is not altered in FGFR-2 mutant human osteoblasts (23, 24). Thus, the premature cranial ossification in the two human syndromes appear to arise from distinct phenotypic abnormalities in calvaria osteoblastic cell growth and differentiation.
In summary, the present study shows, we believe for the first time, that genetic deletion of Twist bHLH domain causing reduced Twist dosage alters cell proliferation and increases type I collagen expression and osteogenic capability of human calvaria osteoblasts in vitro and in vivo. Furthermore, the increased bone formation induced by Twist haploinsufficiency is associated with inhibition of OC gene expression, which may provide one mechanism to explain the premature cranial ossification induced by deletion of the bHLH Twist domain in Saethre-Chotzen syndrome.
Acknowledgments
We wish to thank J. Bonaventure (Institut National de la Santé et de la Recherche Médicale Unité 393, Hôpital Necker-Enfants Malades, Paris, France) for the mutation analysis, D. Renier (Department of Neurosurgery, Hôpital Necker-Enfants Malades, Paris, France) for providing human calvaria samples, and M. M. Descamps (CRITT, Maubeuge, France) for providing hydroxyapatite samples.
Footnotes
This work has been presented in part at the 21st meeting of the American Society for Bone and Mineral Research in St. Louis, Missouri, USA, in October, 1999.
References
- 1.Reardon W, Winter RM. Saethre-Chotzen syndrome. Am J Med Genet. 1994;31:393–396. doi: 10.1136/jmg.31.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Ghouzzi V, et al. Mutations of the TWIST gene in the Saethre-Chotzen syndrome. Nat Genet. 1997;15:42–46. doi: 10.1038/ng0197-42. [DOI] [PubMed] [Google Scholar]
- 3.Howard TD, et al. Mutations in TWIST, a basic helix-loop-helix transcription factor, in Saethre-Chotzen syndrome. Nat Genet. 1997;15:36–41. doi: 10.1038/ng0197-36. [DOI] [PubMed] [Google Scholar]
- 4.Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Ghouzzi V, et al. Mutations within or upstream of the basic helix-loop-helix domain of the TWIST gene are specific to Saethre-Chotzen syndrome. Eur J Hum Genet. 1999;7:27–33. doi: 10.1038/sj.ejhg.5200240. [DOI] [PubMed] [Google Scholar]
- 6.Thisse B, Stoetzel C, Gorostiza-Thisse C, Perrin-Schmitt F. Sequence of the twist gene and nuclear localization of its protein in endomesodermal cells of early Drosophila embryos. EMBO J. 1988;7:2175–2183. doi: 10.1002/j.1460-2075.1988.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thisse B, el Messal M, Perrin-Schmitt F. The twist gene: isolation of a Drosophila zygotic gene necessary for the establishment of dorsoventral pattern. Nucleic Acids Res. 1987;15:3439–3453. doi: 10.1093/nar/15.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leptin M. Twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 1991;5:1568–1576. doi: 10.1101/gad.5.9.1568. [DOI] [PubMed] [Google Scholar]
- 9.Baylies MK, Bate M. Twist: a myogenic switch in Drosophila. Science. 1996;272:1481–1484. doi: 10.1126/science.272.5267.1481. [DOI] [PubMed] [Google Scholar]
- 10.Cripps RM, Olson EN. Twist is required for muscle template splitting during adult Drosophila myogenesis. Dev Biol. 1998;203:106–115. doi: 10.1006/dbio.1998.9040. [DOI] [PubMed] [Google Scholar]
- 11.Anant S, Roy S, VijayRaghavan K. Twist and Notch negatively regulate adult muscle differentiation in Drosophila. Development. 1998;125:1361–1369. doi: 10.1242/dev.125.8.1361. [DOI] [PubMed] [Google Scholar]
- 12.Wolf C, et al. The M-twist gene of Mus is expressed in subsets of mesodermal cells and is closely related to the Xenopus X-twi and the Drosophila twist genes. Dev Biol. 1991;143:363–373. doi: 10.1016/0012-1606(91)90086-i. [DOI] [PubMed] [Google Scholar]
- 13.Stoetzel C, Weber B, Bourgeois P, Bolcato-Bellemin AL, Perrin-Schmitt F. Dorso-ventral and rostro-caudal sequential expression of M-twist in the postimplantation murine embryo. Mech Dev. 1995;51:251–263. doi: 10.1016/0925-4773(95)00369-x. [DOI] [PubMed] [Google Scholar]
- 14.Chen ZF, Behringer RR. Twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995;9:686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- 15.Olson EN, Perry M, Schulz RA. Regulation of muscle differentiation by the MEF2 family of MADS box transcription factors. Dev Biol. 1995;172:2–14. doi: 10.1006/dbio.1995.0002. [DOI] [PubMed] [Google Scholar]
- 16.Rohwedel J, Horak V, Hebrok M, Fuchtbauer EM, Wobus AM. M-twist expression inhibits mouse embryonic stem cell-derived myogenic differentiation in vitro. Exp Cell Res. 1995;220:92–100. doi: 10.1006/excr.1995.1295. [DOI] [PubMed] [Google Scholar]
- 17.Spicer DB, Rhee J, Cheung WL, Lassar AB. Inhibition of myogenic bHLH and MEF2 transcription factors by the bHLH protein Twist. Science. 1994;272:1476–1480. doi: 10.1126/science.272.5267.1476. [DOI] [PubMed] [Google Scholar]
- 18.Hebrok M, Wertz K, Fuchtbauer EM. M-twist is an inhibitor of muscle differentiation. Dev Biol. 1994;165:537–544. doi: 10.1006/dbio.1994.1273. [DOI] [PubMed] [Google Scholar]
- 19.Hebrok M, Fuchtbauer A, Fuchtbauer EM. Repression of muscle-specific gene activation by the murine Twist protein. Exp Cell Res. 1997;232:295–303. doi: 10.1006/excr.1997.3541. [DOI] [PubMed] [Google Scholar]
- 20.Kellermann O, Buc-Caron MH, Marie PJ, Lamblin D, Jacob F. An immortalized osteogenic cell line derived from mouse teratocarcinoma is able to mineralize in vivo and in vitro. J Cell Biol. 1990;110:123–132. doi: 10.1083/jcb.110.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawaguchi N, DeLuca HF, Noda M. Id gene expression and its suppression by 1,25-dihydroxyvitamin D3 in rat osteoblastic osteosarcoma cells. Proc Natl Acad Sci USA. 1992;89:4569–4572. doi: 10.1073/pnas.89.10.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray SS, et al. Expression of helix-loop-helix regulatory genes during differentiation of mouse osteoblastic cells. J Bone Miner Res. 1992;7:1131–1138. doi: 10.1002/jbmr.5650071004. [DOI] [PubMed] [Google Scholar]
- 23.Lomri A, et al. Increased calvaria cell differentiation and bone matrix formation induced by fibroblast growth factor receptor 2 mutations in Apert syndrome. J Clin Invest. 1998;101:1310–1317. [PMC free article] [PubMed] [Google Scholar]
- 24.Lemonnier J, et al. The Ser252Trp fibroblast growth factor receptor-2 (FGFR-2) mutation induces PKC-independent downregulation of FGFR-2 associated with premature calvaria osteoblast differentiation. Exp Cell Res. 2000;256:158–167. doi: 10.1006/excr.2000.4820. [DOI] [PubMed] [Google Scholar]
- 25.Lemonnier, J., et al. 2001. Role of N-Cadherin and protein kinase C in osteoblast gene activation induced by the S252W fibroblast growth factor receptor 2 mutation in Apert craniosynostosis. J. Bone Miner. Res. In press. [DOI] [PubMed]
- 26.De Pollak C, Renier D, Hott M, Marie PJ. Increased bone formation and osteoblastic cell phenotype in premature cranial ossification (craniosynostosis) J Bone Miner Res. 1996;11:401–407. doi: 10.1002/jbmr.5650110314. [DOI] [PubMed] [Google Scholar]
- 27.De Pollak C, Arnaud E, Renier D, Marie PJ. Age-related changes in bone formation, osteoblastic cell proliferation and differentiation during postnatal osteogenesis in human calvaria. J Cell Biochem. 1997;64:128–139. [PubMed] [Google Scholar]
- 28.Lomri A, Fromigue O, Hott M, Marie PJ. Genomic insertion of the SV-40 large T oncogene in normal adult human trabecular osteoblastic cells induces cell growth without loss of the differentiated phenotype. Calcif Tissue Int. 1999;64:394–401. doi: 10.1007/pl00005821. [DOI] [PubMed] [Google Scholar]
- 29.Marie PJ, de Vernejoul MC, Connes D, Hott M. Decreased DNA synthesis by cultured osteoblastic cells in eugonadal osteoporotic men with defective bone formation. J Clin Invest. 1991;88:1167–1172. doi: 10.1172/JCI115418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hott M, Marie PJ. Glycol methacrylate as an embedding medium for bone. Stain Technol. 1987;62:51–57. doi: 10.3109/10520298709107965. [DOI] [PubMed] [Google Scholar]
- 31.Schreiber E, Matthias P, Muller MM, Schuffner W. Rapid detection of octamer binding proteins with “mini-extracts” prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baron, R., Vignery, A., Neff, L., Silverglate, A., and Maria, A.S. 1983. Processings of undecalcified bone specimens for bone histomorphometry. In Bone histomorphometry. R.R. Recker, editor. CRC Press Inc. Boca Raton, Florida, USA. 13–36.
- 33.Parfitt AM, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 34.El Ghouzzi V, et al. Saethre-Chotzen mutations cause TWIST protein degradation or impaired nuclear location. Hum Mol Genet. 2000;9:813–819. doi: 10.1093/hmg/9.5.813. [DOI] [PubMed] [Google Scholar]
- 35.Yousfi M, Marie PJ, Lasmoles F. Deletion of the basic helix-loop-helix domain in Twist increases apoptosis in osteoblasts in the Saethre-Chotzen craniosynostosis. J Bone Miner Res. 2000;15:S207. (Abstr.) [Google Scholar]
- 36.Shishido E, Higashijima S, Emori Y, Saigo K. Two FGF-receptor homologues of Drosophila: one is expressed in mesodermal primordium in early embryos. Development. 1993;117:751–761. doi: 10.1242/dev.117.2.751. [DOI] [PubMed] [Google Scholar]
- 37.Rice DP, et al. Integration of FGF and TWIST in calvarial bone and suture development. Development. 2000;127:1845–1855. doi: 10.1242/dev.127.9.1845. [DOI] [PubMed] [Google Scholar]
- 38.Lee MS, Lowe GN, Strong DD, Wergedal JE, Glackin CA. TWIST, a basic helix-loop-helix transcription factor, can regulate the human osteogenic lineage. J Cell Biochem. 1999;75:566–577. doi: 10.1002/(sici)1097-4644(19991215)75:4<566::aid-jcb3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 39.Ducy P, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 40.Karsenty G. Role of Cbfa1 in osteoblast differentiation and function. Semin Cell Dev Biol. 2000;11:343–346. doi: 10.1006/scdb.2000.0188. [DOI] [PubMed] [Google Scholar]
- 41.Yousfi M, et al. The Y103X mutation in twist, a bHLH transcription factor, alters human calvaria osteoblastic cell proliferation, expression of CBFA1/OSF2 and osteogenesis in the Saethre-Chotzen syndrome. J Bone Miner Res. 1999;14:S223. (Abstr.) [Google Scholar]