Osteopontin (OPN) is a phosphorylated acidic glycoprotein that has been implicated in a number of physiological and pathological events, including maintenance or reconfiguration of tissue integrity during inflammatory processes. As such, it is required for stress-induced bone remodeling and certain types of cell-mediated immunity. It also acts in dystrophic calcification, coronary restenosis, and tumor cell metastasis. An RGD-containing protein, OPN exists both as an immobilized ECM molecule in mineralized tissues and as a cytokine in body fluids; it is not a significant part of typical nonmineralized ECM.
OPN can engage a number of receptors, including the integrins αv(β1, β3, or β5) and (α4, α5, α8, or α9)β1, and it may also be a ligand for certain variant forms of CD44, specifically v6 and/or v7, but possibly only in conjunction with a β1 integrin (1). These receptors directly or indirectly activate cellular signaling pathways, allowing OPN to mediate cell-matrix, and possibly cell-cell, interactions. Several studies have demonstrated that OPN delivers a prosurvival, antiapoptotic signal to the cell. Here, we argue that OPN influences cellular functions in a unique manner, by mimicking key aspects of an ECM signal outside the confines of the ECM. We will explore this idea by reviewing recent data concerning OPN signaling and the consequences of OPN deficiency in several settings, notably inflammatory processes involving immune cells and bone cells.
OPN-integrin interactions: consequences of cleavage by thrombin
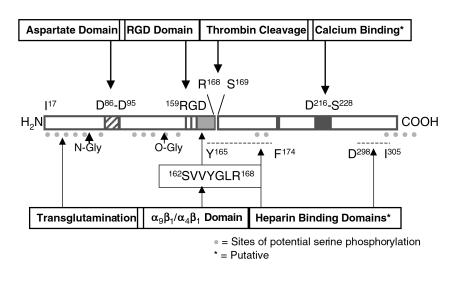
Figure 1 illustrates some of the features of the OPN molecule. The presence of a conserved thrombin cleavage site suggests that certain physiological processes employing OPN depend upon its cleavage by thrombin. Some of these adhesive interactions involve the RGD sequence, which is found in various ECM proteins and binds directly to many integrins. Both RGD-dependent and RGD-independent OPN-receptor interactions are modulated by thrombin cleavage of OPN. For instance, thrombin-cleaved OPN, but not intact OPN, can support RGD-dependent migration of melanoma cells (2). Likewise, K562 erythroleukemia cells bind via activated α5β1 to the RGD sequence in thrombin-cleaved OPN. A non–RGD-dependent interaction with α9β1 offers yet another example: only after cleavage by thrombin can human OPN interact with α9β1 via the sequence SVVYGLR, which is located between the RGD sequence and the thrombin cleavage site (3). This binding motif is also responsible for the RGD-independent binding of the J6 T-cell line to activated α4β1, but in the latter case, cleavage by thrombin is not required for binding of OPN by activated integrin (4). Adhesion of B lymphocytes via αvβ3 also occurs via a cryptic binding site masked in intact OPN, and TPA-activated B lymphocytes attach more effectively to thrombin-cleaved OPN than to full-length OPN (5). In contrast, binding of activated platelets via αvβ3 to the RGD sequence occurs to an equivalent extent with full-length or thrombin-cleaved OPN.
Figure 1.
Some features of the OPN protein. Indicated sites of O-glycosylation and phosphorylation are intended to be representative; both vary (phosphorylation in particular) with the source of the protein. Numbering of the amino acids is based on the human protein, and the signal sequence is not illustrated.
Senger and colleagues (6) demonstrated that interaction of thrombin-cleaved OPN with αvβ3 mediates endothelial cell migration during angiogenesis. They showed that VEGF/vascular permeability factor not only induces OPN and αvβ3 expression in microvascular endothelial cells but also stimulates cleavage of OPN by thrombin. As a result of thrombin cleavage, the receptor binding sites found on the NH2-terminal side of intact OPN are separated from interacting sites on the COOH-terminal side, thus exposing cryptic binding sites. These structural changes in the protein would be expected also to alter the signal transduction pathways stimulated in the cell, and indeed, the resulting OPN fragments are strongly chemotactic for the endothelial cells and may help promote new blood vessel formation. Because thrombin cleavage unmasks alternate cryptic sites, we suggest that the intact protein is a better mimic of ECM-generated signals than the cleaved protein.
Another role for thrombin-mediated cleavage of OPN may be seen in the coordination of inflammation with blood coagulation. The coagulation cascade is active at sites of inflammation, where thrombin appears to be activated. The level of procoagulant activity may influence the severity of inflammation, perhaps mediated by OPN. For example, mouse strains that are deficient in procoagulant activity exhibit decreased granuloma formation during delayed-type hypersensitivity (DTH) reactions (7). Heparin, which inactivates thrombin, also inhibits DTH responses in humans and rats, in part, perhaps, because it blocks the cleavage of OPN.
In addition to proteolytic cleavage, OPN-receptor interactions may also be determined at the transcriptional and posttranslational levels, since three splice variants have been identified and OPN is subject to both phosphorylation and glycosylation at multiple sites; some studies suggest that specific forms of OPN may have distinct functions (for reviews, see refs. 8, 9).
The emerging role of CD44 variants as OPN receptors
CD44, a cell surface glycoprotein that serves as an adhesion molecule in cell-substrate or cell-cell interactions, is strongly upregulated in acute and chronic inflammation. Its ligands include OPN and the ECM molecules hyaluronic acid and chondroitin sulfate, all of which can inhibit the cell-cell interactions that lead to macrophage fusion (10). The widely expressed standard form of this transmembrane protein is CD44s, but a number of splice variants are known that differ in the combinations of additional exons represented in their extracellular region. These CD44 isoforms serve diverse functions. CD44v6 expression on multiple myeloma cells is increased in the bone marrow microenvironment, where it aids in the homing and adhesion of the cells (11). The CD44v7 variant isoform appears to mediate inflammatory bowel disease (12). In an experimental colitis model, a reduction in the initial inflammatory response in CD44v7-null mice correlates with increased cell apoptosis in the inflamed mucosa, and it has been suggested that upregulation of CD44v7 in response to CD40 ligation protects leukocytes from activation-induced cell death. It is particularly intriguing that CD44v6 and CD44v7, which can both act in DTH reactions, appear to be the principal isoforms able to bind OPN (1). These data suggest that the region encoded by v6/v7 promotes effector lymphocyte survival, thus prolonging inflammatory processes. We propose therefore that OPN is an activator of the CD44v6/v7 survival signal — a signal that may also be delivered by the ECM, for instance by hyaluronic acid.
In an effort to identify antiapoptotic genes, Lin and colleagues found that OPN is induced in hematopoietic cells by IL-3 and GM-CSF signaling, both of which are dependent upon a common β subunit shared by the IL-3 and GM-CSF receptors (13). They then showed that recombinant OPN can synergize with GM-CSF to promote the survival and growth of IL-3–dependent mouse bone marrow cells and the pro–B cell line Ba/F3. This effect seems to involve paracrine or autocrine signaling by OPN through CD44, since it can be blocked using antibodies to CD44, but not to the αv integrin. The survival pathway activated by OPN in Ba/F3 cells does not involve activation of NF-κB, as has been reported in endothelial cells (see ref. 8).
OPN functions in inflammation and immunity
Recent research has defined a role for OPN in regulating inflammatory cell accumulation and function at sites of inflammation and repair (reviewed in ref. 7). A variety of inflammatory mediators and growth factors, including IL-1, TNF-α, and PDGF, stimulate OPN transcription, often via activation of protein kinase C (reviewed in ref. 14). While the exact role of OPN in immune responses in vivo is unclear, it appears to be critical for macrophage recruitment and production of certain cytokines during cell-mediated immunity. Other studies suggest that OPN exerts anti-inflammatory effects and influences tissue repair at sites of inflammatory responses.
OPN is widely expressed by a variety of inflammatory cells in culture, including T cells, macrophages, and NK cells (reviewed in refs. 14, 15). It was identified as early T-cell activation gene-1 (Eta-1), whose mRNA transcript is abundant in mouse T cells activated by concanavalin-A. Expression of OPN is enhanced in a variety of inflammatory processes, ranging from infection of macrophages by mycobacteria to the granulomas of tuberculosis to atherosclerosis. In particular, extensive OPN expression is found in T cells and macrophages in granulomatous diseases such as sarcoidosis.
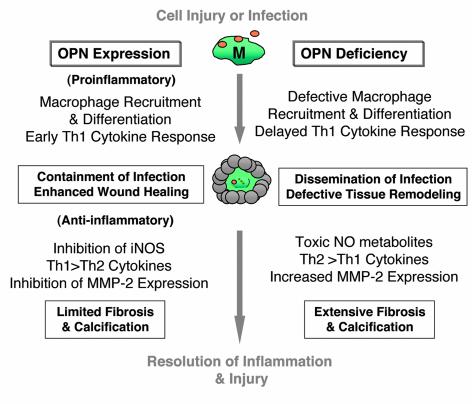
As diagrammed in Figure 2, OPN has both pro- and anti-inflammatory actions. As a proinflammatory agent, it is chemotactic for, supports adhesion of, and modulates the function of T cells and monocytes/macrophages. OPN induces chemotaxis and haptotaxis of T cells and macrophages in vitro, functioning as a typical chemoattractant (15). OPN injected subcutaneously results in the accumulation of macrophages at the site of injection. Interestingly, a similar cutaneous response to subcutaneous injection of the formyl-peptide fMLP is associated with significant expression of OPN by recruited macrophages and can be inhibited by pretreatment with anti-OPN antibodies (cited in ref. 7), suggesting that, in addition to its own direct chemoattractant effects, OPN can facilitate macrophage migration to other chemoattractants. The basis of this latter effect is unknown, but, as discussed below, recent studies suggest that OPN interacts directly with the intracellular machinery of cell migration and modulates the expression of matrix metalloproteinases required for movement through the ECM. These findings reveal an essential role for OPN in macrophage motility.
Figure 2.
Pro- and anti-inflammatory actions of OPN in cell injury and infection. The left side of the diagram summarizes proinflammatory and anti-inflammatory events believed to be regulated by OPN. The right side summarizes known or predicted events in the OPN-null mouse. The center illustrates the wounding of the epithelium or endothelium, which is followed by macrophage-mediated containment of an infecting agent and, finally, repair of the injury. MMP-2, matrix metalloproteinase 2; M, macrophage. See text for further details.
Polarization of Th cells to the Th1 or Th2 phenotypes, a critical aspect of cell-mediated immunity, is influenced by production of early cytokines, including OPN, which interacts with integrins and CD44 to enhance Th1 and inhibit Th2 cytokine expression. Ashkar et al. (16) report that in cultured mouse peritoneal macrophages, OPN-integrin interaction induces IL-12, a cytokine that drives Th1 responses. In the same cells, interaction of OPN with CD44 prevents LPS-stimulated production of the Th2 cytokine IL-10 (16). The integrin-mediated response, but not the CD44-mediated response, requires that OPN be phosphorylated. O’Regan et al. (17) show that OPN can enhance T cell–dependent IL-12 production from human PBMCs, in part via its ability to regulate CD3-induced expression of IFN-γ and CD40L by T cells. Coupled with the in vivo data described below, these results suggest that OPN acts as a Th1 cytokine and is important in early Th1 responses.
OPN exerts an anti-inflammatory effect by inhibiting the expression of nitric oxide (NO). In vitro, OPN downregulates inducible NO synthase (iNOS) and reduces NO production by macrophages and kidney tubule epithelial cells (8, 18). During sepsis, OPN expression is increased in the vasculature, where it attenuates iNOS activity and blocks the production of NO metabolites (19). NO stimulates expression of OPN, which, in turn, inhibits iNOS transcription and reduces NO production, thus establishing an autoregulatory loop (20).
Both in rheumatoid arthritis and, to a lesser extent, in osteoarthritis, OPN expression is elevated in the synovial fluid of the joints (21, 22), where it represses production of NO and prostaglandin E2. In the inflamed joint, macrophages are present in abundance, but only some of them express OPN. Among the agents that might be responsible for increased OPN expression are NO and IL-1. In rheumatoid arthritis, OPN is expressed predominantly by synovial fibroblasts attached to the cartilage at sites of invasion. Proinflammatory actions of OPN include its ability to stimulate collagenase 1 (matrix metalloproteinase 1) expression and activate invasive behavior of macrophages and articular chondrocytes. In addition, however, OPN may act in an anti-inflammatory fashion, by virtue of its ability to inhibit production of the proinflammatory mediators NO and prostaglandin E2, and may thus reduce the extent of cartilage damage and help maintain tissue integrity.
Compared with wild-type mice, OPN-null mice have a defective Th1 response and are more sensitive to infection by Herpes simplex and Listeria monocytogenes (16). Although DTH, as assessed in the footpad following ocular infection with H. simplex, appeared to be impaired in one set of studies, Bonvini et al. (23) found no such effect. In the latter study, mice were immunized with rabbit IgG to induce anti–globular basement membrane nephritis; subsequent challenge to the footpad resulted in comparable responses from both wild-type and OPN-null animals. These conflicting results suggest that some but not all cellular immune reactions are dependent upon OPN.
OPN is expressed in human and murine granulomatous responses of diverse etiology (15, 16), and work with OPN-null mice suggests that this protein is required for functional granuloma formation. Macrophages from these mutant mice fail to form skin granulomas when challenged intradermally with polyvinyl pyrrolidone or to accumulate normal levels of macrophages in pulmonary granulomas. Mycobacterium bovis bacille Calmette-Guérin proliferates more aggressively in granulomas and in macrophages from OPN-null mice than in wild-type controls, indicating that OPN helps blunt the course of the infection (24). Similarly, in humans with a defective IFN-γ receptor 1, OPN expression in mycobacterial granulomas is impaired. In these patients, as in OPN-null mice, mycobacterial infection takes a much more severe course (25).
OPN in tissue and bone remodeling
Mice deficient in OPN exhibit aberrant wound healing, characterized by normal wound strength but abnormal macrophage debridement and abnormal maturation of collagen bundles (26). OPN-null mice also exhibit less macrophage infiltration and collagen deposition in the kidney in a model of interstitial renal fibrosis (reviewed in ref. 8). The progressive hypertrophy of rat pulmonary arteries in organ culture, resulting from the induction of tenascin-C by matrix metalloproteinases and consequent enhanced smooth muscle cell proliferation, can be reversed by inhibition of metalloproteinase activity (27). Further, apoptosis of the smooth muscle cells, which results from the inhibition of matrix metalloproteinase activity, is suppressed by OPN, suggesting that OPN suppresses fibrosis following inflammation, perhaps because of its ability to support cell survival. In addition, as in mineralized tissues, the presence of OPN may be a pivotal regulator of dystrophic calcification, a deleterious consequence of inflammatory remodeling.
Injury to the endothelial lining of the vasculature may lead to pathological calcification. During inflammation resulting from such injury, growth factors and inflammatory mediators released by platelets induce leukocyte invasion and the proliferation of resident cells. Various cytokines, OPN included, are upregulated by smooth muscle cells and macrophages (reviewed in ref. 7). Although tissue integrity is generally restored, occasionally the process becomes pathological, resulting in excessive cell proliferation and the deposition of an ECM that in time becomes calcified. What, if any, contribution OPN might make to this process remains to be established, but since OPN can function as a negative regulator of calcification (9), it is possible that calcification of atherosclerotic lesions is augmented in its absence.
Although it is not required for normal bone formation and development, the presence of OPN on the bone surface is critical for the remodeling of mature bone. The abundance and distribution of aspartate and phosphorylated serine residues in OPN cause it to bind strongly to the calcium phosphate crystals in mineralized tissues and to inhibit crystal growth (9, 28). Certain functions of OPN require it to be phosphorylated, a fact of interest because OPN phosphorylation may be controlled by extracellular phosphatases and kinases. Extracellular phosphate induces OPN expression in the osteoblast-like MC3T3 cells (29). This regulation may be a control mechanism that ties an increase in OPN expression to the cessation of osteoblast proliferation and the onset of differentiation, events that coincide with the induction of alkaline phosphatase. Induction by phosphate could also account for high levels of OPN expression in osteoclasts involved in resorbing bone matrix and solubilizing bone mineral.
Using an OPN-null mouse model (8), Noda and colleagues (30) have shown that ovariectomized mice do not lose bone mineral to nearly the same extent as control animals. Four weeks after ovariectomy, the wild-type mice had lost 58% of their trabecular bone volume in the proximal tibia, while the OPN-null mice had lost only 12%. Resorption of ectopic bone is also substantially impaired in the absence of OPN (31). Calvaria bone discs from wild-type mice implanted intramuscularly in wild-type mice are resorbed much more rapidly than bone discs from OPN-null mice implanted in OPN-null mice. Vascularization of the implanted bone discs and the number of adherent osteoclasts are also much reduced in the absence of OPN. In a tail-suspension model of disuse osteoporosis, the OPN-null femur is resistant to the loss of bone mineral, and these mice do not exhibit the reduction in bone formation rate seen in wild-type, tail-suspended mice, suggesting that they are defective not just in osteoclast function, but also in the coupling of mechanical stress to osteoblast function (32).
OPN signaling and CD44
Osteoclasts share their lineage with macrophages, and both cell types are specialized for resorption, producing and responding to many of the same factors, including OPN. Osteoclast motility on bone is impaired in the absence of OPN (33), apparently as a result of decreased surface expression of CD44 and the absence of an association between the actin-binding protein gelsolin and mDia1, a Rho effector protein and transcriptional activator that mediates some of the effects of serum response factor. This association normally occurs during the formation of podosomes and is responsible for the αvβ3-mediated activation by OPN of gelsolin-associated Src, which in turn results in enhanced phosphatidylinositol 3-kinase activity and actin filament formation.
The involvement of CD44 is intriguing in light of the evidence that OPN found in migrating fibroblasts can associate with CD44 and the ERM (ezrin, radixin, moesin) proteins just inside the plasma membrane, notably at the leading edge in filopodia-like structures (34). This perimembranous distribution is distinct from the perinuclear and punctate cytoplasmic staining pattern seen in nonmigrating cells, which is presumed to represent OPN protein in the secretory pathway. The ERM proteins mediate interactions between the plasma membrane and cortical actin filaments, regulating formation of surface structures such as microvilli, filopodia, and membrane ruffles. Their activity is controlled by phosphatidylinositol 4,5-bisphosphate and by phosphorylation by various tyrosine and serine/threonine kinases. The CD44/OPN/ERM complex appears necessary for cell migration, since fibroblasts from CD44-null or OPN-null mice exhibit impaired migration and attach less efficiently to hyaluronan-coated beads. Intracellular OPN fails to localize to the perimembranous regions in the absence of CD44, suggesting that CD44 is required for OPN to form the perimembranous complex. The perinuclear and perimembranous localizations appear to be mutually exclusive, but it remains unclear whether the perimembranous pool of OPN is free in the cytoplasm or contained within secretory vesicles.
Regulation of OPN expression in bone
In some bone cell types OPN expression is strongly enhanced by mechanical stimuli (35). In cultured osteoblastic cells, OPN is expressed early during differentiation, and expression is maintained at a high level throughout the later stages of in vitro mineralization. OPN is also produced by osteoblasts involved in endochondral ossification during development. However, in vivo osteoinduction in mouse alveolar bone during tooth movement results in downregulation of OPN in osteoblasts that are stimulated to differentiate and synthesize matrix by mechanical loading (36–38). A study of experimental tooth movement in rats showed a rapid enhancement of OPN expression in osteocytes and osteoblasts on the pressure (compression) side followed by osteoclast recruitment and bone resorption (39).
Figure 3 shows the effect of mechanical loading on OPN expression in three mouse cell types in the region where the tooth root is anchored to bone. In a steady-state situation, in situ hybridization (shown in Figure 3a) reveals low and modest levels of OPN mRNA in cementoblasts and osteoblasts, respectively. In osteoblasts at sites of bone formation (Figure 3b), OPN mRNA is downregulated, whereas it is upregulated within the layer of cells on the bone surface in resorption sites (Figure 3c), perhaps as a result of increased local free phosphate. Similarly, OPN synthesis in human femoral heads occurs in osteoblasts engaged in de novo bone formation, but not adaptive bone remodeling (40). To date, OPN is the only known osteoblast-associated gene whose expression is reduced in mature, bone matrix–producing osteoblasts; expression of other markers (such as osteocalcin, alkaline phosphatase, type I collagen, and bone sialoprotein) is enhanced (36, 37).
Figure 3.
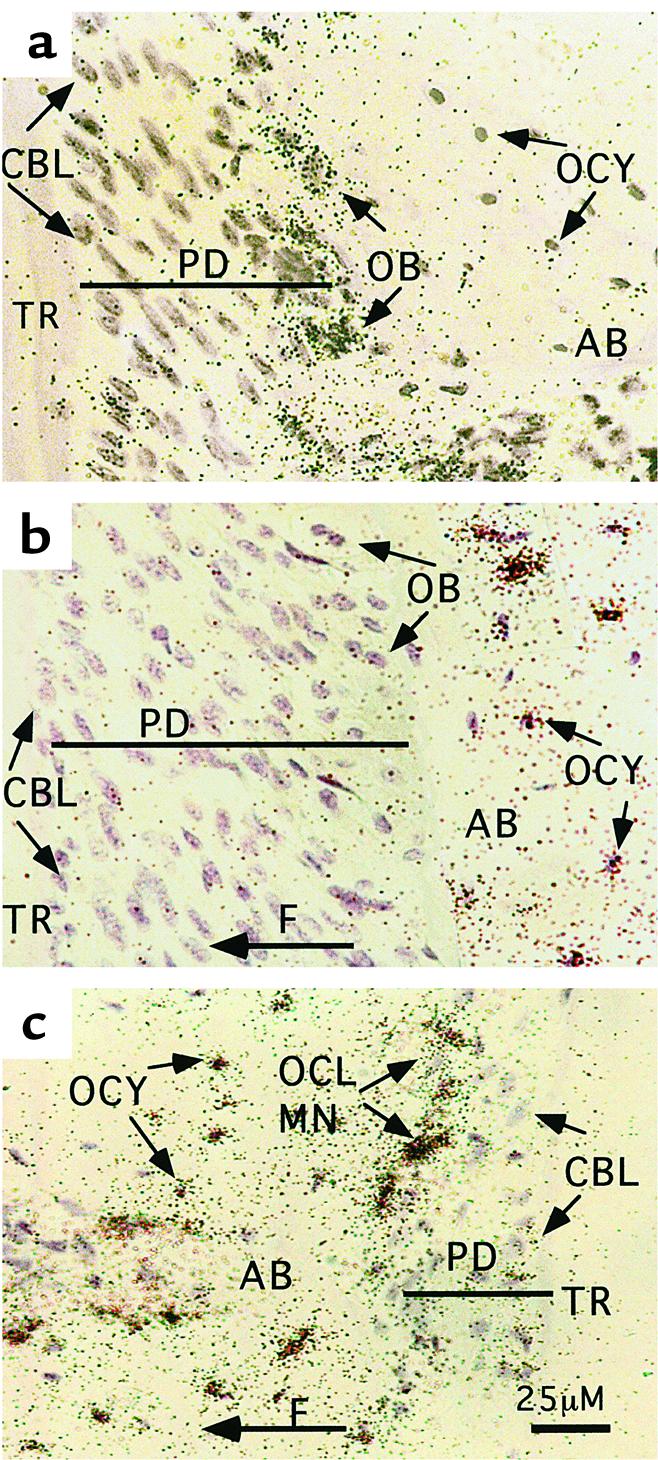
OPN mRNA expression during tooth relocation as assessed by in situ hybridization with an OPN mRNA probe in sections of a mouse dentoalveolar complex. (a) A control, untreated site. The tooth was moved for 3 days by a controlled orthodontic force, resulting in bone formation in distal periodontal sites (b) and direct resorption in mesial sites (c) of the first molar. Following relocation, the OPN mRNA hybridization signal decreased in periodontal osteoblasts in bone formation sites (b, right side), and increased in cells (osteoblasts, osteoclasts, and their precursors) lining alveolar bone surface in resorption sites (c). The mechanically induced OPN hybridization signal is present in alveolar osteocytes within the bone adjacent to both resorption and formation sites (b, c). A control sense OPN probe did not yield any signal above background. F, direction of orthodontic force and tooth movement; TR, tooth root; PD, periodontium; AB, alveolar bone; OB, osteoblasts; OCY, osteocytes; CBL, cementoblasts; OCL and MN, osteoclasts and their mononuclear precursors. Note that the PD is enlarged in b, where the TR is being pulled away from the AB, but that it is reduced in c, where the PD is being compressed between the TR and the AB.
OPN mRNA levels are augmented, possibly by mechanical strain, in osteocytes in sites of both bone formation and bone resorption (Figure 3, b and c). This effect parallels inhibition of expression in the osteoblasts in bone formation sites, and also stimulation of expression in osteoclasts and mononuclear cells in resorption sites. These results are consistent with a reciprocally coordinated regulation of OPN in the two cell types as a mechanism for coupling of bone resorption and formation (38). The simultaneous increase in OPN mRNA expression in bone osteocytes adjacent to both resorption and formation sites suggests that OPN exerts a reciprocal paracrine effect on cells on the bone surface, leading to stimulation and repression of its synthesis in osteoclasts and osteoblasts, respectively. The dendritic network between osteocytes and bone lining cells may help to integrate these opposing effects. Additional mechanisms, possibly including mineral exposure, may be necessary to determine which phase of the remodeling cycle — resorption or formation — will prevail on a particular area of bone surface exposed to an OPN-mediated signal initiated by osteocytes.
Summary
OPN is a multifunctional cytokine and adhesion protein that contains an integrin-binding RGD sequence and additional sequences that interact with CD44v6/7 or other adhesive receptors. Its expression is increased in response to early proinflammatory cytokines and to mechanical strain in bone. The function of the secreted protein may be altered by extracellular enzymes, including thrombin and kinases. The study of OPN-null mice has revealed roles for OPN in a broad range of homeostatic (bone remodeling, tissue debridement) and pathologic (cellular immunity, wound healing, cancer metastasis) processes. While these processes seem disparate, they are linked by several common themes, including enhanced expression of OPN in response to stress or tissue injury, and stimulation of cell motility and cell survival pathways via interactions of OPN with adhesive receptors.
OPN is chemotactic for various cell types, notably monocytes/macrophages, which are attracted to sites of infection and inflammation. It is essential for cell-mediated immunity and a normal Th1 cytokine response during granuloma formation. OPN serves both to attach bone cells to bone matrix and to generate intracellular signals essential for normal osteoclast motility on bone; it may mediate osteocyte recognition of bone strain. OPN activates intracellular signaling pathways and regulates gene expression as a consequence of its interactions with its various receptors. The best-characterized is the integrin-stimulated FAK-Src-Rho pathway, which alters gelsolin function and podosome formation in osteoclasts. Identification and dissection of the signal transduction pathways and their targets are complicated by the fact that OPN can engage more than one type of receptor on the cell. For this reason, it is important to ascertain which receptors are in play in any given experimental system.
There is compelling evidence that soluble OPN can in a variety of situations help cells survive an otherwise lethal insult. Remarkably, this survival signaling is mediated by receptors that are generally considered to be receptors for ECM components. We suggest that OPN delivers an antiapoptotic “ECM-like” signal via multiple ligand-receptor interactions to cells, both adherent and nonadherent.
Acknowledgments
Research in the authors’ laboratories has been generously supported by grants from NIH (ES-06897 and AR-44434 to D.T. Denhardt, P50-HL56386 and HL-63339 to J.S. Berman, HL-04343 to A.W. O’Regan, and DE-11005 to D. Pavlin), and from the Japanese Ministry of Education to M. Noda. Sincere apologies to those whose papers have not been cited because of space constraints.
References
- 1.Katagiri YU, et al. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999;59:219–226. [PubMed] [Google Scholar]
- 2.Smith LL, Giachelli CM. Structural requirements for α9β1-mediated adhesion and migration to thrombin-cleaved OPN. Exp Cell Res. 1998;242:351–360. doi: 10.1006/excr.1998.4108. [DOI] [PubMed] [Google Scholar]
- 3.Yokosaki Y, et al. The integrin α9β1 binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J Biol Chem. 1999;274:36328–36334. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- 4.Barry ST, Ludbrook SB, Murrison E, Horgan CMT. Analysis of the α4β1 integrin-osteopontin interaction. Exp Cell Res. 2000;258:342–351. doi: 10.1006/excr.2000.4941. [DOI] [PubMed] [Google Scholar]
- 5.Helluin O, et al. The activation state of αvβ3 regulates platelet and lymphocyte adhesion to intact and thrombin-cleaved osteopontin. J Biol Chem. 2000;275:18337–18343. doi: 10.1074/jbc.M001529200. [DOI] [PubMed] [Google Scholar]
- 6.Senger DR, et al. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the αvβ3 integrin, osteopontin, and thrombin. Am J Pathol. 1996;149:293–305. [PMC free article] [PubMed] [Google Scholar]
- 7.O’Regan A, Berman JS. Osteopontin: a key cytokine in cell-mediated and granulomatous inflammation. Int J Exp Pathol. 2000;81:373–390. doi: 10.1046/j.1365-2613.2000.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rittling SR, Denhardt DT. Osteopontin (OPN) function in pathology: lessons from OPN-deficient mice. Exp Nephrol. 1999;7:103–113. doi: 10.1159/000020591. [DOI] [PubMed] [Google Scholar]
- 9.Sodek J, Ganss T, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- 10.Sterling H, Saginario C, Vignery A. CD44 occupancy prevents macrophage multinucleation. J Cell Biol. 1998;143:837–847. doi: 10.1083/jcb.143.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asosingh K, et al. In vivo induction of insulin-like growth factor-1 receptor and CD44v6 confers homing and adhesion to murine multiple myeloma cells. Cancer Res. 2000;60:3096–3104. [PubMed] [Google Scholar]
- 12.Wittig BM, Johansson B, Zöller M, Schwärzler C, Günthert U. Abrogation of experimental colitis correlates with increased apoptosis in mice deficient for CD44 variant exon 7 (CD44v7) J Exp Med. 2000;191:2053–2063. doi: 10.1084/jem.191.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin YH, et al. Coupling of osteopontin and its cell surface receptor CD44 to the cell survival response elicited by interleukin-3 or granulocyte-macrophage colony-stimulating factor. Mol Cell Biol. 2000;20:2734–2742. doi: 10.1128/mcb.20.8.2734-2742.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denhardt DT, Noda M. Osteopontin expression and function: role in bone remodeling. J Cell Biochem Suppl. 1998;30–31:92–102. [PubMed] [Google Scholar]
- 15.O’Regan AW, et al. Osteopontin is associated with T cells in sarcoid granulomas and has T cell adhesive and cytokine-like properties in vitro. J Immunol. 1999;162:1024–1031. [PubMed] [Google Scholar]
- 16.Ashkar S, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 17.O’Regan AW, Hayden JM, Berman JS. Osteopontin augments CD3-mediated interferon-γ and CD40 ligand expression by T cells, which results in IL-12 production from peripheral blood mononuclear cells. J Leukoc Biol. 2000;68:495–502. [PubMed] [Google Scholar]
- 18.Tian JY, et al. Regulation of NO synthesis induced by inflammatory mediators in RAW264.7 cells: collagen prevents inhibition by osteopontin. Cytokine. 2000;12:450–457. doi: 10.1006/cyto.1999.0634. [DOI] [PubMed] [Google Scholar]
- 19.Scott JA, et al. Osteopontin inhibits inducible nitric oxide synthase activity in rat vascular tissues. Am J Physiol. 1998;275:H2258–H2265. doi: 10.1152/ajpheart.1998.275.6.H2258. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi F, Takahashi K, Maeda K, Tominaga S, Fukuchi Y. Osteopontin is induced by nitric oxide in RAW264.7 cells. IUBMB Life. 2000;49:217–221. doi: 10.1080/713803614. [DOI] [PubMed] [Google Scholar]
- 21.Petrow PK, et al. Expression of osteopontin mRNA and protein in rheumatoid arthritis. Arthritis Rheum. 2000;43:1597–1605. doi: 10.1002/1529-0131(200007)43:7<1597::AID-ANR25>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Attur MG, et al. Osteopontin: an intrinsic inhibitor of inflammation in cartilage. Arthritis Rheum. 2001;44:578–584. doi: 10.1002/1529-0131(200103)44:3<578::AID-ANR106>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Bonvini JM, et al. Lack of in vivo function of osteopontin in experimental anti-GBM nephritis. J Am Soc Nephrol. 2000;11:1647–1655. doi: 10.1681/ASN.V1191647. [DOI] [PubMed] [Google Scholar]
- 24.Nau GJ, et al. Attenuated host resistance against Mycobacterium bovis BCG infection in mice lacking osteopontin. Infect Immun. 1999;67:4223–4230. doi: 10.1128/iai.67.8.4223-4230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nau GJ, et al. Osteopontin expression correlates with clinical outcome in patients with mycobacterial infection. Am J Pathol. 2000;157:37–42. doi: 10.1016/S0002-9440(10)64514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liaw L, et al. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cowan KN, Jones PL, Rabinovitch M. Elastase and matrix metalloproteinase inhibitors induce regression, and tenascin-C antisense prevents progression, of vascular disease. J Clin Invest. 2000;105:21–34. doi: 10.1172/JCI6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jono S, Peinado C, Giachelli CM. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J Biol Chem. 2000;275:20197–20203. doi: 10.1074/jbc.M909174199. [DOI] [PubMed] [Google Scholar]
- 29.Beck GR, Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci USA. 2000;97:8352–8357. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshitake H, Rittling SR, Denhardt DT, Noda M. Osteopontin-deficient mice are resistant to ovariectomy-induced bone resorption. Proc Natl Acad Sci USA. 1999;96:8156–8160. doi: 10.1073/pnas.96.14.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asou Y, et al. Osteopontin facilitates angiogenesis, accumulation of osteoclasts and resorption of ectopic bone. Endocrinology. 2001;142:1325–1332. doi: 10.1210/endo.142.3.8006. [DOI] [PubMed] [Google Scholar]
- 32.Ishijima M, et al. Enhancement of osteoclastic bone resorption and suppression of osteoblastic bone formation in response to reduced mechanical stress do not occur in the absence of osteopontin. J Exp Med. 2001;193:399–404. doi: 10.1084/jem.193.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chellaiah MA, et al. The molecular mechanisms of osteoclast dysfunction associated with osteopontin deficiency: the failure of Rho stimulation of mDia1. J Bone Miner Res. 2000;15(Suppl. 1):S396. (Abstr.) [Google Scholar]
- 34.Zohar R, et al. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J Cell Physiol. 2000;184:118–130. doi: 10.1002/(SICI)1097-4652(200007)184:1<118::AID-JCP13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 35.Klein-Nulend J, Roelofsen J, Semeins CM, Bronckers ALJJ, Burger EH. Mechanical stimulation of osteopontin mRNA expression and synthesis in bone cell cultures. J Cell Physiol. 1997;170:174–181. doi: 10.1002/(SICI)1097-4652(199702)170:2<174::AID-JCP9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 36.Pavlin D, Dove SB, Zadro R, Gluhak-Heinrich J. Mechanical loading stimulates differentiation of periodontal osteoblasts in a mouse osteoinduction model: effect on type I collagen and alkaline phosphatase genes. Calcif Tissue Int. 2000;67:163–172. doi: 10.1007/s00223001105. [DOI] [PubMed] [Google Scholar]
- 37.Pavlin, D., Zadro, R., and Gluhak-Heinrich, J. 2001. Temporal pattern of stimulation of osteoblast-associated genes during mechanically-induced osteogenesis in vivo: early responses of osteocalcin and type I collagen. Connect. Tissue Res. In press. [DOI] [PubMed]
- 38.Gluhak-Heinrich J, Villarreal A, Pavlin D. Reciprocal regulation of osteopontin gene during mechanically induced bone formation and resorption. J Bone Miner Res. 2000;15(Suppl. 1):M086. (Abstr.) [Google Scholar]
- 39.Terai K, et al. Role of osteopontin in bone remodeling caused by mechanical stress. J Bone Miner Res. 1999;14:839–849. doi: 10.1359/jbmr.1999.14.6.839. [DOI] [PubMed] [Google Scholar]
- 40.Dodds RA, et al. Human osteoclasts, not osteoblasts, deposit osteopontin onto resorption surfaces: an in vitro and ex vivo study of remodeling bone. J Bone Miner Res. 1995;10:1666–1680. doi: 10.1002/jbmr.5650101109. [DOI] [PubMed] [Google Scholar]