Abstract
Autoantigen-specific T cells have tissue-specific homing properties, suggesting that these cells may be ideal vehicles for the local delivery of immunoregulatory molecules. We tested this hypothesis by using type II collagen–specific (CII-specific) CD4+ T hybridomas or primary CD4+ T cells after gene transfer, as vehicles to deliver an immunoregulatory protein for the treatment of collagen-induced arthritis (CIA), a mouse model of rheumatoid arthritis (RA). CII-specific T cells or hybridomas were transduced using retroviral vectors to constitutively express the IL-12 antagonist, IL-12 p40. Transfer of engineered CD4+ T cells after immunization significantly inhibited the development of CIA, while cells transduced with vector control had no effect. The beneficial effect on CIA of IL-12 p40-transduced T cells required TCR specificity against CII, since transfer of T cells specific for another antigen producing equivalent amounts of IL-12 p40 had no effect. In vivo cell detection using bioluminescent labels and RT-PCR showed that transferred CII-reactive T-cell hybridomas accumulated in inflamed joints in mice with CIA. These results indicate that the local delivery of IL-12 p40 by T cells inhibited CIA by suppressing autoimmune responses at the site of inflammation. Modifying antigen-specific T cells by retroviral transduction for local expression of immunoregulatory proteins thus offers a promising strategy for treating RA.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammatory synovitis and subsequent progressive destruction of articular tissue. The etiologic cause of RA has not been clearly delineated, but cumulative evidence suggests that CD4+ T cell–mediated autoimmune responses play a critical role in the pathogenesis of RA (1). IFN-γ–producing Th1 cells appear to be pivotal in the development of autoimmune arthritis in both humans and animal models, whereas Th2 cells that secrete IL-4 or IL-10 are protective (2). Thus, recent therapeutic strategies have focused on modulating the response of CD4+ T cells. Depletion of CD4+ T cells was effective in treating a mouse model of RA, type II collagen–induced (CII-induced) arthritis (3). Unfortunately, the same treatment seemed less effective in RA (4).
To increase the specificity of therapies for RA, emphasis has shifted to targeting cytokines and their receptors. Neutralization of proinflammatory cytokines by mAb’s or soluble receptors can efficiently control RA, revealing the potential of modulating the cytokine balance as a therapeutic strategy for controlling RA (5). However, systemic administration of anti-inflammatory cytokines or neutralizing mAb’s against proinflammatory cytokines is antigen nonspecific and often results in systemic immune suppression.
The local delivery of “regulatory proteins” that could modulate an autoimmune response would be a desirable new approach to the treatment of RA. Autoantigen-specific CD4+ T cells can transfer organ-specific autoimmune disease in mice, and CD4+ T cells can be found in target organs in both human and mouse models of autoimmunity; thus, autoantigen-specific CD4+ T cells have tissue-specific homing properties. These findings suggest that CII-reactive CD4+ T cells, retrovirally transduced to express regulatory proteins, may be ideal candidates for the local delivery of gene therapy. We and others have demonstrated that expression of “immunoregulatory proteins” by autoantigen-specific CD4+ T cells could ameliorate the clinical signs of experimental autoimmune encephalomyelitis (EAE) after adoptive transfer (6, 7). These previous reports also provided indirect evidence that homing to the site of inflammation was necessary for the therapeutic effect.
Development of the Th1 subset during an immune response is influenced by the cytokines present during the initial phase of the immune response, where a bioactive cytokine, IL-12, plays a major role. IL-12 is a heterodimeric protein composed of 35-kDa (p35) and 40-kDa (p40) subunits; the latter is responsible for receptor binding (8). It has been demonstrated that the expression of p35 and p40 is differentially regulated and that IL-12 p40 can be produced as a homodimer or a monomer in the absence of p35 and act as an IL-12 antagonist in vitro and in vivo (9–11). It is thus possible that the development of Th1-mediated autoimmune arthritis might be inhibited by IL-12 p40 (12). In the study described below, we demonstrate that the constitutive delivery of IL-12 p40 by retroviral-transduced CII-specific T lymphocytes ameliorated collagen-induced arthritis (CIA). By using bioluminescence real-time imaging (13–16), we demonstrated that antigen-specific therapeutic T cells migrated into and persisted in the inflamed joints. These data support our hypothesis that the therapeutic effect was the result of expression of the regulatory protein in the inflamed joints, not in regional lymph nodes. These data further suggest that local delivery of therapeutic proteins via antigen-specific T cells will be a promising strategy for controlling RA locally, at the site of inflammation.
Methods
Mice.
Male DBA/1 LacJ (H-2q) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA) and were used at 7–10 weeks of age. CII-specific TCR transgenic (Tg) mice on the DBA/1 LacL background, were kindly provided by Warren C. Ladiges (University of Washington, Seattle, Washington, USA; see ref. 17). Myelin basic protein–specific (MBP-specific) TCR Tg mice have been described elsewhere (18).
Generation of T-cell hybridomas.
Spleen cells from either CII-specific or MBP-specific TCR Tg mice were stimulated with specific peptide antigens (40 μg/ml for CII and 30 μg/ml for MBP). Forty-eight hours after stimulation, CD4+ T cells were purified by magnetic activated cell sorting using anti-CD4 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany). These cells (106) were then fused with the BW5147 TCR-αβ–negative T-cell line (106) using 40% polyethylene glycol. Twenty-four hours after the fusion, the cells were selected by sodium hypoxanthine, aminopterin, and thymidine–containing media. CII- or MBP-specific cells were further selected by staining with anti-Tg TCR Vβ8.2 Ab by FACScan (Becton Dickinson Immunocytometry Systems, San Jose, California, USA), with purity above 98%.
Retrovirus construct, transfection, and infection.
The pGCIRES retroviral plasmid was constructed as described previously (19). The gene for green fluorescent protein (GFP; ref. 20) was PCR-amplified using Pfu-polymerase (Stratagene, La Jolla, California, USA) and primers MB.GFP5′: 5′ actagctgacgcggccgcccATGGTGAGCAAGGGCGAGGAGCTGTTCA-3′, with uppercase letters corresponding to the GFP sequence; and MB.GFP3′: 5′-ttcagagatcatgagctcggatccacctccacctgatccaccgcctccggatccaccaccgccggccccCTTGTACAGCTCGTCCATGCCGTGAGT-3′, the latter encoding for a poly-glycine-serine, [G4S]3, linker (21). The PCR product was cloned into the vector pGL3 upstream of the modified firefly luciferase gene (Promega Corp., Madison, Wisconsin, USA). The resulting GFP-Luc fusion gene was then inserted into the pGCIRES plasmid that was termed pGC-GFP-Luc. A pGCy retroviral plasmid (6,700 bp) was constructed as described (19), except a yellow fluorescent protein (YFP; CLONTECH Laboratories, Palo Alto, California, USA) was used as the reporter protein (22). Murine IL-12 p40 cDNA was obtained from Riken Gene Bank (Tsukuba, Japan) with the approval of H. Hamada (10). The IL-12 p40 fragment (1.0 kb) was obtained and subcloned into pGCy (G.L. Costa et al., manuscript submitted for publication) and termed pGCy-mIL-12 p40. Transfection of a producer line to make viral supernatants and infection was performed as described previously (19). At 48 hours after infection, cells were analyzed for transduction and sorted by flow cytometric analysis of either the GFP or YFP protein. Primary CD4+ T cells were purified and transduced using the same protocols as described (19).
Immunofluorescence and flow cytometry.
The cells were first preincubated with anti-FcγR mAb (2.4G2; PharMingen, San Diego, California, USA) to block nonspecific binding of mAb to FcγR and then incubated with phycoerythrin-conjugated and/or FITC-conjugated mAb for 30 minutes at 4°C. The mAb’s against CD4 (GK1.5) and TCR Vβ8.2 (MR5-2) were obtained from PharMingen. The stained cells were analyzed on a FACScan (Becton Dickinson Immunocytometry Systems). The FACS data were analyzed using FlowJo (Tree Star Software, San Carlos, California, USA).
Induction of CIA, treatment, and clinical assessment of arthritis.
CIA was induced as described previously (23). Briefly, DBA/1 LacJ mice were immunized intradermally at the tail base with 200 μg of bovine CII (University of Utah, Salt Lake City, Utah, USA) in 0.05 M acetic acid, emulsified with an equal volume of CFA containing 100 μg of H37Ra Mycobacterium tuberculosis (Difco Laboratories, Detroit, Michigan, USA). On day 21, the mice were boosted by intradermal injection with 200 μg of bovine CII emulsified with incomplete Freund’s adjuvant (IFA) at the base of the tail.
Starting 1 day before the booster immunization (day 20), groups of DBA/1 LacJ mice received intradermal or intravenous injection of 106 cells of pGCy-mIL-12 p40–transduced CII-specific T-cell hybridomas or 5 × 106 cells of CII-specific primary T cells. pGCy-transduced CII-specific T-cell hybridomas (CII-YFP) and pGCy-mIL-12 p40–transduced MBP-specific T-cell hybridomas were used as controls. For imaging experiments, prearthritic mice (day 21) or mice that had severe arthritis (day 35) were transferred with the same number of CII-specific T-cell hybridomas that were transduced with the GFP-luciferase fusion gene with or without the mIL-12 p40 gene. The clinical development of CIA was scored by daily observation where the inflammation of all four paws was graded from 0 to 4 as described (23). Each paw was graded, and the four scores were totaled so that the maximal possible score per mouse was 16.
T-cell stimulation assay.
Draining lymph node (DLN) cells were isolated from three to four mice in each group at either 3 or 7 days after the booster immunization. DLN cells (5 × 105) were stimulated with bovine CII, and proliferative responses were determined as described (23). For assessment of cytokine production, culture supernatants were collected after 48 hours and stored at –80°C until the ELISA assay was performed.
ELISA for serum anti-CII Ab’s and quantification of cytokines.
Sera from each group of mice was individually collected at 7 days after booster immunization. CII-specific IgG1 and IgG2a were determined as described previously (23). Cell-free culture supernatants were assayed for IL-4, IL-10, IFN-γ, and IL-12 p40 concentration by using specific ELISAs according to the protocol recommended by the manufacturer. All anticytokine mAb’s and cytokine standards were obtained from PharMingen (10, 23).
Imaging of T-cell trafficking.
Before imaging, mice were anesthetized with Avertin (250 mg/kg; Sigma-Aldrich, Milwaukee, Wisconsin, USA). An aqueous solution of the substrate luciferin (126 mg/kg; Xenogen Corp., Alameda, California, USA) was injected into the peritoneal cavity 5 minutes before imaging. The animals were then placed in the light-tight chamber of a low-light imaging system equipped with a cooled charge coupled device (CCD) camera and a Navitar f 0.95 lens (IVIS; Xenogen Corp.). Then a gray-scale body-surface reference image was collected under weak illumination. Photons emitted from luciferase within the animal and then transmitted through the tissue were collected using the IVIS system with 5-minute integration times. A pseudocolor image representing light intensity (blue is least intense and red is most intense) was generated using LivingImage Software (Xenogen Corp.) as an overlay on the IGOR image analysis package (WaveMetrics, Lake Oswego, Oregon, USA). Gray-scale reference images and pseudocolor images were superimposed by the LivingImage software, and annotations were added.
RT-PCR.
Total RNA was isolated from either DLN cells or cells from collagenase-digested ankle joints using RNeasy Mini Kit (QIAGEN Inc., Valencia, California, USA). First-strand cDNA was synthesized using oligo-dT primers and SuperScript reverse transcriptase (Life Technologies Inc., Rockville, Maryland, USA) from 5 μg of each RNA sample. For PCR, cDNA products were amplified in a reaction mixture containing 2 μM each of 5′ and 3′ primers (sense 5′-TCGCCACCATGGTGAGCAAGGGCG-3′ and antisense 5′-TCCTCCGGATCATTACTTGTACAGCTCGTCCAT-3′ for YFP, and sense 5′-GTGGGCCGCTCTAGGCACCA-3′ and antisense 5′-CGGTTGGCCTTAGGGTTCAGGGGGG-3′ for β-actin; Operon Technologies Inc., Alameda, California, USA), 225 μM each dNTP, 1 U of Taq DNA polymerase (Promega Corp.), and 1× PCR buffer. PCR was performed on a PTC-100 thermal controller (MJ Research Inc., Waltham, Massachusetts, USA) for 40 cycles (95°C for 1 minute, 62°C for 90 seconds, and 72°C for 1 minute) followed by a 5-minute extension at 72°C. The PCR products were electrophoresed in 1.5% agarose gel and visualized by ultraviolet light.
Statistical analysis.
Mann Whitney’s rank sum test was performed to determine the statistical significance. A P value less than 0.05 was considered to be significant.
Results
Characterization of T-cell hybridomas.
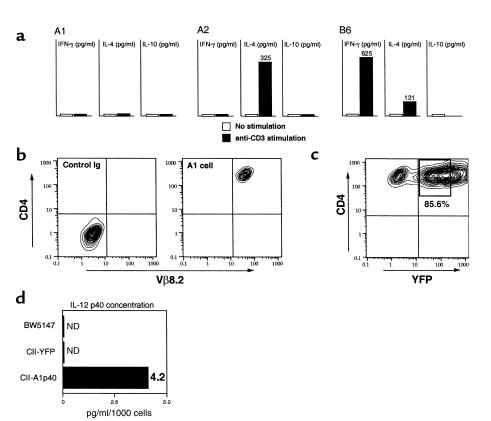
We generated several CII-specific CD4+ T-cell hybridomas from CII-specific TCR Tg mice to use in attempts to deliver immunoregulatory molecules locally. This Tg TCR recognized CII in an MHC-restricted, antigen-specific manner (ref. 17 and our unpublished data). We used three different lines in this study (termed A1, A2, and B6) that displayed different profiles of endogenous cytokine expression (Figure 1a). However, each of these T-cell lines expressed high levels of CD4 and Tg TCR Vβ8.2 chain as determined by FACS analysis (Figure 1b). To generate regulatory protein expressing CD4+ T cells, CII-specific CD4+ T-cell hybridomas from all three lines were transduced with the pGCy-mIL-12 p40 retroviral vector. Greater than 80% of the CII-specific CD4+ T-cell hybridomas were transduced and expressed the mIL-12 p40 gene (Figure 1c). We have demonstrated previously a direct correlation between expression of the fluorescent reporter gene and the gene upstream of the IRES sequence in these vectors (19), which allows control of the production of IL-12 p40 by the expression level of the reporter, YFP. YFP-positive cells were sorted and termed A1p40, A2p40, and B6p40, corresponding to the A1, A2, and B6 parental lines. Significant levels of IL-12 p40 were detected in the YFP-positive but not in the pGCy vector–transduced cells (CII-YFP) or the parental cells (Figure 1d). Bioactive IL-12 was not detected in any cells, and production of IL-12 p40 was confirmed using Western blot analysis (data not shown). No significant differences in the growth rates in vitro nor alteration of cytokine profiles was observed following transduction (data not shown).
Figure 1.
Characterization of CII-specific CD4+ T-cell hybridomas. (a) Cytokine production from CII-specific CD4+ T-cell hybridomas. Three different CII-specific CD4+ T-cell hybridomas were (or were not) stimulated with immobilized anti-CD3 mAb (5 μg/ml). Forty-eight hours later, cell-free supernatants were collected and assayed for IFN-γ, IL-4, and IL-10 levels using ELISA. Data are shown as the mean value from triplicate wells. (b) Expression of CD4 and TCR Vβ8.2 on hybridoma cells. T-cell hybridomas were stained with FITC-conjugated anti-TCR Vβ8.2 mAb and phycoerythrin-conjugated (PE-conjugated) anti-CD4 mAb. Immunofluorescent staining was analyzed by using two-color flow cytometry. Data are displayed as contour plots (four-decade scale), and quadrant markers were positioned to include greater than 98% of control IgG–stained cells in the lower left, as shown. A FACS profile of A1 cells is shown, and similar data were obtained from the other two hybridomas. (c) Transduction of the mIL-12 p40 gene into hybridoma cells. CII-specific CD4+ T-cell hybridomas (A1 cells) were transduced with the pGCy-mIL-12 p40 retroviral vector as described in Methods. At 48 hours after infection, the cells were analyzed for transduction efficiency by flow-cytometric analysis of YFP protein. Similar transduction efficiency was obtained with the other two hybridomas. The percentage represents transduction efficiency and the boxed region (bold outlines) represents the sorted population. (d) Production of IL-12 p40. Supernatants were collected after culturing the cells (106/ml) for 24 hours, after which the IL-12 p40 concentration was measured using ELISA. Data represent the mean of triplicate wells. ND, not detected.
Transfer of mIL-12 p40–transduced T-cell hybridomas prevents the development of CIA.
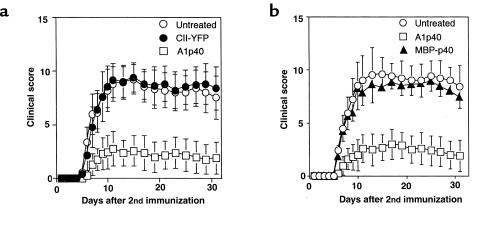
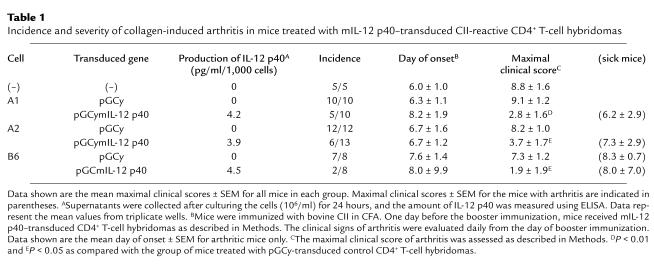
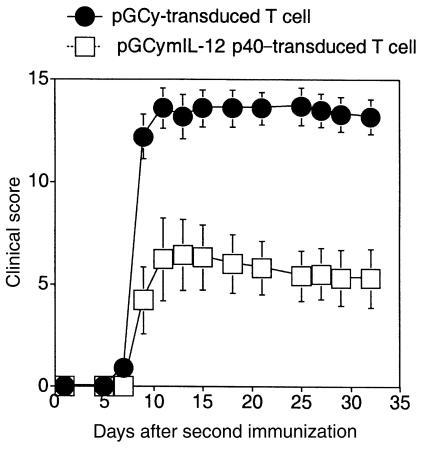
To examine if local delivery of an immunoregulatory protein is sufficient to suppress autoimmune arthritis, CII-specific T-cell hybridomas, either transduced with a retrovirus encoding the murine IL-12 p40 subunit and the marker protein YFP, or only the marker protein, were transferred into CII-immunized mice. In the first set of experiments, groups of mice received injections intraperitoneally with cells from one of the CII-specific T-cell hybridomas (A1) that either expressed YFP or YFP and IL-12 p40. These cells were administered at day 20 after primary immunization, 1 day before the booster immunization. As shown by data presented in Figure 2a, the mice given control vector-transduced cells (CII-YFP) developed severe arthritis, as did the untreated mice. In contrast, the transfer of IL-12 p40–producing cells (A1p40) efficiently inhibited the development of CIA (Figure 2a and Table 1). Injection either intraperitoneally or intravenously with CII-specific IL-12 p40–producing T cells inhibited CIA development (data not shown). In another set of experiments, CII-immunized mice were treated with transduced hybridomas (B6p40 and A2p40) that produced IL-12 p40 and either IL-4 or IFN-γ. Regardless of the level of endogenous cytokine production, transfer of vector-transduced B6 or A2 cells did not significantly exacerbate or ameliorate arthritis, and transfer of either B6p40 or A2p40 inhibited the development of CIA in a manner similar to that seen with A1p40 (Table 1). These results indicated that transfer of T cells transduced to express the IL-12 p40 gene was sufficient to prevent the development of CIA.
Figure 2.
Adoptive transfer of mIL-12 p40–transduced CII-specific CD4+ T-cell hybridomas prevents the development of CIA. DBA/1 LacJ mice were immunized with bovine CII (200 μg/mouse) in CFA. On day 21, the mice were boosted by intradermal injection with CII in IFA. Starting 1 day before the booster injection, the mice were given either pGCy-mIL-12 p40– or pGCy-transduced CII-specific CD4+ T-cell hybridomas (106/mice) intraperitoneally. Untreated CII-immunized DBA/1 LacJ mice were used as controls. The development of CIA was evaluated as described in Methods. Data are shown as the mean ± SEM of clinical scores at the indicated time points after the booster immunization. (b) Amelioration of CIA by mIL-12 p40 gene therapy requires TCR specificity. DBA/1 LacJ mice (n = 7 for each group) were immunized, and the development of CIA was evaluated as in a. These mice received either CII-specific or MBP-specific mIL-12 p40–transduced CD4+ T-cell hybridomas (106/mouse) intraperitoneally 1 day before the booster immunization. Similar results were obtained from two other experiments.
Table 1.
Incidence and severity of collagen-induced arthritis in mice treated with mIL-12 p40–transduced CII-reactive CD4+ T-cell hybridomas
To examine whether TCR specificity was required for the amelioration of CIA, we transferred either disease-relevant CII-specific or disease-irrelevant MBP-specific CD4+ T-cell hybridomas (IL-12 p40–transduced MBP-reactive T-cell hybridomas that had been shown to inhibit the induction of EAE; G.L. Costa et al., manuscript submitted for publication) that made similar amounts of IL-12 p40 (4.2 pg/ml/1000 cells for CII-specific cells and 3.9 pg/ml/1,000 cells for MBP-specific cells) into CII-immunized mice. As shown by data presented in Figure 2b, transfer of MBP-specific IL-12 p40–transduced T cells had no effect on the clinical course of CIA despite their demonstrable therapeutic effect in EAE (G.L. Costa et al., manuscript submitted for publication). This result suggested that the beneficial effect on CIA of IL-12 p40–transduced T cells required TCR specificity against CII.
Injection of mIL-12 p40–transduced T-cell hybridomas did not inhibit T-cell proliferation or systemic Ab production, but modulated cytokine balance in DLN.
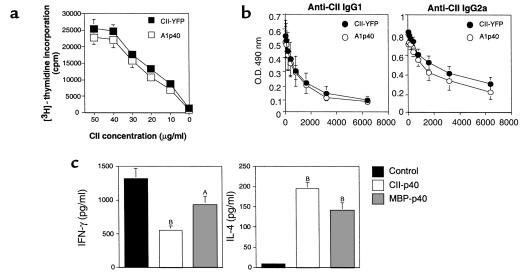
To determine if IL-12 p40 gene therapy might affect T-cell and B-cell responses, lymphocytes were isolated from the DLNs of A1p40-transferred CII-immunized mice or control mice 7 days after the second immunization and restimulated with CII in vitro. As shown by data presented in Figure 3a, DLN cells from the mice treated with A1p40 proliferated in response to antigen as well as those from the control mice. Next, we measured CII-specific IgG1 and IgG2a Ab’s in the serum of treated mice 7 days after the second immunization. The treatment with IL-12 p40–transduced T cells had no significant effect on the serum levels of anti-CII IgG1 or IgG2a Ab’s (Figure 3b). These results suggested that treatment with IL-12 p40–transduced T cells did not affect the antigen-specific T-cell activation or systemic Ab responses.
Figure 3.
T-cell and B-cell responses in mice treated with mIL-12 p40–transduced T-cell hybridomas. (a) DBA/1 LacJ mice were immunized with CII and treated as described in Figure 2. DLN (inguinal and popliteal) cells were isolated 7 days after the booster immunization and cultured in the presence of indicated amount of CII for 96 hours. Cultures were pulsed with [3H]-thymidine for the last 16 hours. Data are shown as the mean nuclide incorporation ± SEM of triplicate cultures and are representative of three separate experiments with similar results. (b) Sera from individual mice were collected 7 days after the booster immunization. CII-specific Ab levels were measured by subclass-specific ELISA. Data are representative of at least seven mice in each group. Similar results were obtained in two other experiments. (c) DLN cells were prepared from the mice treated with YFP mIL-12 p40 gene–transduced CII-specific or MBP-specific CD4+ T-cell hybridomas 4 days after the booster immunization and cultured in the presence or absence of CII (40 μg/ml) for 48 hours. Culture supernatants were assayed for cytokine levels of IFN-γ (left) and IL-4 (right). Data are shown as the mean cytokine level ± SEM of triplicate cultures. Similar results were obtained in three independent experiments. AP < 0.05 and BP < 0.01 as compared with the control group.
Since this treatment might affect the balance of cytokine production, we examined cytokine production in the DLN lymphocytes of mice treated with various transduced T cells. DLN cells from the CII-immunized mice treated with either A1p40 or MBP-p40 were restimulated with CII in vitro, and IFN-γ and IL-4 levels in the culture supernatants were measured using ELISA. Interestingly, transfer of MBP-p40 cells, which had no disease-ameliorating effects in CIA (Figure 2b), modulated cytokine production as efficiently as did A1p40: CII-specific IFN-γ production was suppressed in mice treated with A1p40 or MBP-p40, while IL-4 production was augmented (Figure 3c). Therefore, IL-12 p40 produced by either antigen-specific or antigen-nonspecific T cells suppressed Th1-type immune responses and augmented Th2 responses in the DLN. Since the transfer of MBP-p40 cells did not affect the clinical signs of CIA (Figure 2b), these results indicated that the modulation of cytokine production in the draining lymph node did not play a major role in amelioration of CIA by the CII-specific IL-12 p40 gene therapy. These results suggested that the transfer of CII-specific IL-12 p40–producing T-cell hybridomas preferentially inhibited inflammation locally in the joints, raising the possibility that a key feature was the homing of the transduced cells to the inflamed tissue.
Antigen-specific T-cell hybridomas accumulate in the site of inflammation.
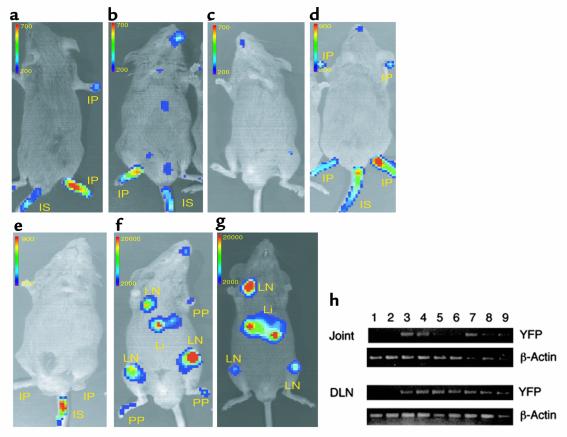
To examine directly whether CII-specific T-cell hybridomas home to the site of inflammation, we transduced CII-specific T-cell hybridomas with a gene encoding a GFP-luciferase fusion protein, CII-GFP-Luc, and tested the patterns of cell trafficking using whole-body bioluminescence imaging of the labeled cells in living animals (13–16). This technique has been used to monitor tumor-cell growth in vivo; it has demonstrated excellent sensitivity (15, 16) and should allow visualization of the trafficking of antigen-specific CD4+ T cells in vivo. Initially, we injected CII-GFP-Luc (derived from the A1 cell line) into mice that had severe arthritis and followed the mice with serial images for 2 weeks. Three days after the cell transfer, photons emitted from the cells were detected in arthritic joints from all mice tested (Figure 4a). Interestingly, GFP-Luc–transduced MBP-specific T-cell hybridomas (MBP-GFP-Luc) initially homed to inflamed joints as efficiently as the CII-GFP-Luc cells (Figure 4b). However, accumulation of CII-GFP-Luc cells into normal, noninflamed joints was not observed in naive mice (n = 6), suggesting that the MBP-GFP-Luc cells homed nonspecifically to the inflammation (Figure 4c). The trafficking of MBP-GFP-Luc cells to the inflamed joints was transient, whereas the CII-GFP-Luc cells were detected in the arthritic joints for more than 7 days after the injection (Figure 4, d and e). The other CII-reactive IL-12 p40–producing T-cell hybridomas, A2 and B6, migrated into and remained in the inflamed joints in a manner similar to the A1 cells (data not shown). Asymptomatic immunized mice were then given CII-specific or MBP-specific mIL-12 p40 and GFP-Luc double-transduced T-cell hybridomas (termed CII-p40-Luc or MBP-p40-Luc). Again, the CII-p40-Luc cells preferentially and rapidly homed to joints (five of six mice) that were not overtly inflamed and to DLN (six of six mice) within 3 days and remained for more than 7 days, whereas MBP-p40-Luc cells preferentially accumulated in the DLN and liver 7 days after the injection, but not in the noninflamed joints (Figure 4, f and g). These results indicate that both CII- and MBP-specific transduced T cells efficiently trafficked into the inflamed joints, but that TCR specificity seemed to be required for detection in preinflamed joints and, ultimately, for effective therapy in the inflamed joints. Taken together, these data suggest that CII-specific IL-12 p40–producing hybridomas inhibited CIA inflammation locally by homing to and remaining in the inflamed joints. We performed RT-PCR analyses, which confirmed the imaging data: we detected the YFP gene by RT-PCR in DLN and joints from CII-immunized mice treated with CII-YFP or A1p40 within 4 days after the cell transfer, but not in nontreated mice (Figure 4h).
Figure 4.
Trafficking capacity of CII-specific CD4+ T-cell hybridomas. CII-immunized DBA/1 LacJ mice with severe arthritis (a–e) or without arthritis (prearthritic; f and g) received either CII-specific (a, c, and d) or MBP-specific (b and e) CD4+ T-cell hybridomas expressing a GFP-luciferase reporter gene (106/mouse) intravenously. pGC-Luc and pGCy-mIL-12 p40 double-transduced CII-specific (f) or MBP-specific (g) T-cell hybridomas were also administrated. Unimmunized naive mice were used as a control (c). The images were obtained on day 3 (a–c and f), on day 5 (d and e), and on day 7 (g). Images are representative of at least six mice in each group. The color scale represents luminescent signal intensity, with blue indicating the least intense and red the most intense light originating from the transduced cells in the animals. (h) Total RNA was isolated from either DLN cells or cells from ankle joints of untreated CII-immunized mice (lane 1 and 2), CII-immunized mice treated with pGCy-transduced CII-reactive T-cell hybridomas (lane 3 and 4), or pGCy-mIL-12–transduced CII-reactive T-cell hybridomas (lane 5–8). mRNA from pGCy-transduced CII-specific T-cell hybridomas was used as positive control (lane 9). RT-PCR was performed as described in Methods. PP, prearthritic paw; IP, inflamed paw; LN, lymph node; Li, liver; IS, injection site.
CIA treatment using CII-specific IL-12 p40–transduced primary CD4+ T cells.
We demonstrated that the transfer of IL-12 p40–transduced CD4+ T-cell hybridomas effectively inhibited the development of CIA. However, the use of CD4+ T-cell hybridomas may be impractical in human trials. We therefore tested whether CII-specific IL-12 p40–transduced primary CD4+ T cells without tumorigenic potential had the same therapeutic effects as IL-12 p40–expressing hybridoma cells. Nontransformed CD4+ T cells were isolated from CII-specific TCR Tg mice, activated with CII and transduced to express the IL-12 p40 gene. More than 40% of the primary CD4+ T cells were transduced (data not shown) and sorted by FACS. As shown by data presented in Figure 5, transfer of the CII-specific IL-12 p40–producing primary CD4+ T cells ameliorated CIA clinical signs. These data indicate that the delivery of IL-12 p40 by retrovirus-transduced primary T cells can effectively prevent the development of CIA.
Figure 5.
The development of CIA is inhibited by the transfer of CII-specific IL-12 p40–transduced primary CD4+ T cells. DBA/1 LacJ mice (n = 10 for each group) were immunized as in Figure 2. These mice received YFP with or without IL-12 p40–transduced CII-specific primary CD4+ T cells (5 × 106 cells/mice) intravenously 1 day before the booster immunization. The development of CIA was evaluated as described in Figure 2. Data are shown as the mean ± SEM of clinical scores at the indicated time points after the booster immunization.
Discussion
There has been an increased understanding of the phenomenon of immune dysregulation in several autoimmune diseases during the past several years, and it is now possible to treat several autoimmune diseases based on this knowledge. An example is the dramatic clinical benefit of blocking TNF in human RA (24, 25). However, most current therapeutic strategies for treatment of autoimmune disease (including TNF blockade) include potent and nonspecific immune suppression that may result in systemic toxicity and increased risk for infections and malignancies. Thus, local gene therapy using adoptive transfer of transduced antigen-specific CD4+ T cells expressing regulatory proteins may serve as a better long-term option for treating autoimmune diseases (26, 27). Furthermore, localized delivery of a preselected amount of a regulatory protein to a specific site should ensure maximum therapeutic effect in the area of inflammation while minimizing the exposure of nontargeted organs to the gene products and markedly reducing the risk of undesirable systemic side effects. In the present study, it was demonstrated that local delivery of an immunoregulatory protein (IL-12 p40) in the CIA model could be achieved using antigen-specific T cells as a gene-delivery vehicle. Amelioration of CIA by CII-specific IL-12 p40–transduced CD4+ T cells was due to local delivery and retention of IL-12 p40 expression in the inflamed joint. This was demonstrated by the following: First, antigen recognition was required by the transduced T cells to ameliorate CIA, since MBP-specific IL-12 p40–expressing T cells that produced amounts of IL-12 p40 equivalent to CII-specific IL-12 p40-expressing T cells and had proven efficacy in EAE (G.L. Costa et al., manuscript submitted for publication) had no therapeutic effect in this model of CIA despite transient migration to and localization in the inflamed paws. The possibility that MBP-specific IL-12 p40–transduced T cells were not therapeutic due to their B10.PL (H-2u) origin is unlikely, because both T-cell hybridomas had allogeneic properties similar to the host derived from the BW5147 cell-fusion partner. Furthermore, both MBP-specific and CII-specific T cells could be detected at least 2 weeks after transfer by bioluminescence real-time imaging (data not shown). These data strongly suggest that CII-specific IL-12 p40–expressing T cells have a site-specific effect mediated not only by homing to, but retention in, the site of inflammation. Second, we demonstrated that CII-specific CD4+ T cells preferentially accumulated in the joints by bioluminescence real-time imaging (Figure 4). RT-PCR analysis confirmed the imaging data (Figure 4h). Interestingly, MBP-specific T cells were also found to migrate into the inflamed joints, but this phenomenon was transient. It is probable that antigen nonspecific T cells migrate nonspecifically into the site of inflammation because of chemokines and adhesion molecules expressed at the site of inflammation. This “nonspecific” migration was transient, the MBP-specific T cells were not retained in the inflamed joints, and no therapeutic benefit was observed. The fact that MBP-specific T-cell hybridomas did not migrate into the prearthritic joints supports our hypothesis. Taken together, these results strongly suggest that T cell–mediated adoptive gene therapy is site and antigen specific due to local delivery and retention.
Additional support for the therapeutic benefit being derived from the local effects of this form of adoptive gene therapy is that transfer of either CII-specific or MBP-specific IL-12 p40–transduced T cells did not inhibit T-cell proliferation, and both transiently suppressed Th1-type cytokine production and augmented Th2-type cytokine production in the DLN cells. Despite these similarities, only CII-reactive T cells were therapeutic in CIA. Thus, development of CIA seemed to be inhibited by local suppression of Th1-type autoimmune responses in the joint.
The concept of local production of IL-12 p40 inhibiting CIA is supported by several reports that CIA is inhibited by systemic administration of anti–IL-12 mAb or IL-12 p40 (28, 29). From these published studies, however, it was not clear whether anti–IL-12 mAb or IL-12 p40 inhibited inflammatory responses locally, in the joints, or systemically. Because the transfer of MBP-specific IL-12 p40–producing T-cell hybridomas modulated cytokine production in DLN when examined 4 days after the cell transfer, but did not suppress CIA development, modulation of cytokine production in DLN did not correlate with the therapeutic effects. In contrast, CII-specific IL-12 p40 producing T cells homed to and remained in the joints, which correlated with suppressed joint inflammation. Modulation of cytokine production was IL-12 p40 specific, since transfer of pGCy-transduced CII-specific T-cell hybridomas did not affect cytokine production compared with untreated CII-immunized mice (data not shown). Therefore, both CII-specific and MBP-specific T-cell hybridomas migrated nonspecifically to DLN and modulated cytokine production in the DLN of the immunized mice. To exclude the possibility that allogenicity of the CII-specific IL-12 p40–producing T-cell hybridomas affected immune response leading to amelioration of CIA, we also used syngeneic CII-specific IL-12 p40–producing primary T cells from CII-specific TCR Tg DBA/1 mice. As shown by data presented in Figure 5, transfer of these transduced primary cells also efficiently suppressed the development of CIA. Taken together, antigen recognition by CII-specific TCR is necessary for the selective retention of T cells in the inflamed joints, and expression of IL-12 p40 is necessary for the suppression of joint inflammation, and both are required for the local gene therapy. Consistent with this observation are recent reports suggesting that synovial cells from RA patients produce IL-12 and other proinflammatory cytokines in response to CD40 ligation (30). IL-12 has been shown to induce IFN-γ production and control the production of other proinflammatory cytokines (8). Thus, local production of IL-12 p40 by these transduced T cells likely suppressed joint inflammation locally by inhibiting the effect of bioactive IL-12.
In most autoimmune diseases, T cells play a critical role in the disease pathogenesis. The ability to transduce CD4+ T cells using retroviruses that carry genes encoding therapeutic proteins has lead to the possibility of using the homing of antigen-specific cells to deliver the immunomodulatory cytokines locally (19). We demonstrated evidence for trafficking and retention of autoantigen-specific CD4+ T cells in sites of autoimmune inflammation. Previous barriers of retroviral transduction for the application of gene therapy have included low proviral integration frequency in immune cells, proviral promoter shutdown, and inadequate isolation and expansion of transduced cells. In fact, only 3–5% transduction efficiency was obtained in previous studies using retrovirus-mediated TGF-β or IL-10 gene transduction into T cells (31, 32). However, as we have reported, the system used in these studies has resulted in high transduction efficiency that has made retroviral-mediated adoptive T cell–mediated gene therapy easier in murine models of autoimmune disease (19). Adoptive T cell–mediated gene therapy may have great advantages over other gene delivery tools for treating autoimmunity because autoantigen reactive T cells can target the inflamed autoantigen-expressing tissues.
In conclusion, we demonstrated directly that autoantigen-specific CD4+ T cells trafficked into inflamed autoantigen-expressing tissues. Using retroviral-transduced CD4+ T cells as a vehicle for delivery of IL-12 p40, we demonstrated that autoimmune arthritis could be efficiently controlled by CII-reactive T cells transduced to express IL-12 p40 without systemic immune suppression. Therefore, targeting antigen-specific T cells by retroviral transduction is a promising strategy for controlling autoimmune disease at the site of inflammation.
Acknowledgments
We thank Hirofumi Hamada for providing the mIL-12 p40 cDNA and Robyn Kizer for assistance in the preparation of this manuscript. This work was supported by NIH grants AI-39646 and AI-36535, the NIH contract NO1-AR-6-2227 (C.G. Fathman), unrestricted gifts from the Mary L. Johnson and Hess Research Funds (C.H. Contag), and an Arthritis Foundation Physician Scientist Award (C.M. Seroogy). A. Nakajima was partly supported by the Japan Rheumatism Foundation.
References
- 1.Panayi GS. T cell-dependent pathways in rheumatoid arthritis. Curr Opin Rheumatol. 1977;9:236–240. doi: 10.1097/00002281-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 2.O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 3.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 4.Epstein WV. Expectation bias in rheumatoid arthritis clinical trials. The anti-CD4 monoclonal antibody experience. Arthritis Rheum. 1996;39:1773–1780. doi: 10.1002/art.1780391102. [DOI] [PubMed] [Google Scholar]
- 5.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 6.Shaw MK, et al. Local delivery of interleukin 4 by retrovirus-transduced T lymphocytes ameliorates experimental autoimmune encephalomyelitis. J Exp Med. 1997;185:1711–1714. doi: 10.1084/jem.185.9.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathisen PM, Yu M, Johnson JM, Drazba JA, Touhy VK. Treatment of experimental autoimmune encephalomyelitis with genetically modified memory T cells. J Exp Med. 1997;186:159–164. doi: 10.1084/jem.186.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gately MK, et al. The interleukin-12/interleukin-12-receptor system: role in normal and pathogenic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 9.Mattner F, et al. The interleukin-12 subunit p40 specifically inhibits effects of the interleukin-12 heterodimer. Eur J Immunol. 1993;23:2202–2208. doi: 10.1002/eji.1830230923. [DOI] [PubMed] [Google Scholar]
- 10.Kato K, et al. Local production of the p40 subunit of interleukin 12 suppresses T-helper 1-mediated immune responses and prevents allogeneic myoblast rejection. Proc Natl Acad Sci USA. 1996;93:9085–9089. doi: 10.1073/pnas.93.17.9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuda H, et al. Local expression of immunoregulatory IL-12p40 gene prolonged syngeneic islet graft survival in diabetic NOD mice. J Clin Invest. 1998;102:1807–1814. doi: 10.1172/JCI2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trembleau S, Germann T, Gately MK, Adorini L. The role of IL-12 in the induction of organ-specific autoimmune diseases. Immunol Today. 1995;16:383–386. doi: 10.1016/0167-5699(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 13.Contag CH, et al. Photonic detection of bacterial pathogens in living hosts. Mol Microbiol. 1995;18:593–603. doi: 10.1111/j.1365-2958.1995.mmi_18040593.x. [DOI] [PubMed] [Google Scholar]
- 14.Contag PR, Olomu IN, Stevenson DK, Contag CH. Bioluminescent indicator in living mammals. Nat Med. 1999;4:245–247. doi: 10.1038/nm0298-245. [DOI] [PubMed] [Google Scholar]
- 15.Edinger M, et al. Noninvasive assessment of tumor cell proliferation in animal models. Neoplasia. 1999;1:303–310. doi: 10.1038/sj.neo.7900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sweeney TJ, et al. Visualizing the kinetics of tumor-cell clearance in living animals. Proc Natl Acad Sci USA. 1999;96:12044–12049. doi: 10.1073/pnas.96.21.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osman GE, et al. Expression of a type II collagen-specific TCR transgene accelerates the onset of arthritis in mice. Int Immunol. 1998;10:1613–1622. doi: 10.1093/intimm/10.11.1613. [DOI] [PubMed] [Google Scholar]
- 18.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 19.Costa GL, et al. Targeting rare populations of murine antigen-specific T lymphocytes by retroviral transduction for potential application in gene therapy for autoimmune disease. J Immunol. 2000;164:3581–3590. doi: 10.4049/jimmunol.164.7.3581. [DOI] [PubMed] [Google Scholar]
- 20.Cormack BP, Valdivia RH, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 21.McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990;348:552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- 22.Heim R, Tsien RY. Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Curr Biol. 1996;6:178–182. doi: 10.1016/s0960-9822(02)00450-5. [DOI] [PubMed] [Google Scholar]
- 23.Yoshioka T, Nakajima A, et al. Contribution of OX40/OX40 ligand interaction to the pathogenesis of rheumatoid arthritis. Eur J Immunol. 2000;30:2815–2823. doi: 10.1002/1521-4141(200010)30:10<2815::AID-IMMU2815>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 24.Feldmann M, Eliott MJ, Woody JN, Maini RN. Anti-tumor necrosis factor-α therapy of rheumatoid arthritis. Adv Immunol. 1997;64:283–350. doi: 10.1016/s0065-2776(08)60891-3. [DOI] [PubMed] [Google Scholar]
- 25.Moreland LW, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 26.Seroogy CM, Fathman CG. The application of gene therapy in autoimmune diseases. Gene Ther. 2000;7:9–13. doi: 10.1038/sj.gt.3301111. [DOI] [PubMed] [Google Scholar]
- 27.Tsokos GC, Nepom GT. Gene therapy in the treatment of autoimmune diseases. J Clin Invest. 2000;106:181–183. doi: 10.1172/JCI10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malfait AM, et al. Blockade of IL-12 during the induction of collagen-induced arthritis (CIA) markedly attenuates the severity of arthritis. Clin Exp Immunol. 1998;111:377–383. doi: 10.1046/j.1365-2249.1998.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Germann T, Hess H, Szeliga J, Rude E. Characterization of the adjuvant effect of IL-12 inhibitors in type II collagen-induced arthritis. Ann NY Acad Sci. 1996;795:227–240. doi: 10.1111/j.1749-6632.1996.tb52672.x. [DOI] [PubMed] [Google Scholar]
- 30.Kitagawa M, et al. Differential regulation of rheumatoid synovial cell interleukin-12 production by tumor necrosis factor alpha and CD40 signals. Arthritis Rheum. 1999;42:1917–1926. doi: 10.1002/1529-0131(199909)42:9<1917::AID-ANR18>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 31.Chen LZ, et al. Gene therapy in allergic encephalomyelitis using myelin basic protein-specific T cells engineered to express latent transforming growth factor-β1. Proc Natl Acad Sci USA. 1998;95:12516–12521. doi: 10.1073/pnas.95.21.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setoguchi K, et al. Antigen-specific T cells transduced with IL-10 ameliorate experimentally induced arthritis without impairing the systemic immune response to the antigen. J Immunol. 2000;165:5980–5986. doi: 10.4049/jimmunol.165.10.5980. [DOI] [PubMed] [Google Scholar]