Abstract
ADP is an important platelet agonist causing shape change and aggregation required for physiological hemostasis. We recently demonstrated the existence of two distinct G protein-coupled ADP receptors on platelets, one coupled to phospholipase C, P2Y1, and the other to inhibition of adenylyl cyclase, P2TAC. In this study, using specific antagonists for these two receptors, we demonstrated that concomitant intracellular signaling from both the P2TAC and P2Y1 receptors is essential for ADP-induced platelet aggregation. Inhibition of signaling through either receptor, by specific antagonists, is sufficient to block ADP-induced platelet aggregation. Furthermore, signaling through the P2TAC receptor could be replaced by activation of α2A-adrenergic receptors. On the other hand, activation of serotonin receptors supplements signaling through the P2Y1 receptor. Moreover, this mechanism of ADP-induced platelet aggregation could be mimicked by coactivation of two non-ADP receptors coupled to Gi and Gq, neither of which can cause platelet aggregation by itself. We propose that platelet aggregation results from concomitant signaling from both the Gi and Gq, a mechanism by which G protein-coupled receptors elicit a physiological response.
ADP is the first small molecular weight platelet agonist to be identified (1). When stimulated with ADP, platelets undergo shape change, release granule contents, and produce thromboxane A2 (2, 3). In addition, ADP activates the fibrinogen receptor, causing platelets to bind fibrinogen and aggregate (2, 3). The receptors through which extracellular nucleotides elicit physiological responses have been classified as P2 receptors and are divided into P2X ligand-gated ion channels and P2Y G protein-coupled receptors (4). These receptor subtypes are numbered in the order of cloning, and to date 7 subtypes of P2X receptors and 10 subtypes of P2Y receptors have been cloned (5, 6). Sage and coworkers (7) recently demonstrated a P2X receptor in platelets and proposed that this receptor is the P2X1 receptor subtype mediating rapid calcium influx. We have provided evidence for two distinct G protein-coupled ADP receptors, one coupled to the inhibition of adenylyl cyclase, the P2TAC receptor, and the other coupled to the activation of phospholipase C, the P2Y1 receptor, in human platelets (8, 9). Several compounds, including ARL 66096, ticlopidine, and clopidogrel, have been utilized to block ADP-induced inhibition of adenylyl cyclase and subsequent platelet aggregation both in vitro and in vivo (3, 8), suggesting that the P2TAC receptor mediates ADP-induced platelet aggregation.
Here, we delineate the role of the three ADP receptors, the P2TAC, P2Y1, and P2X1 receptors, in ADP-induced human platelet aggregation and demonstrate that some agonist-induced physiological responses may require simultaneous activation of multiple receptor subtypes by the same agonist, resulting in converging signal transduction pathways leading to a physiological response.
EXPERIMENTAL PROCEDURES
Materials.
Adenosine 3′-phosphate 5′-phosphosulfate (A3P5PS), adenosine 3′-phosphate 5′-phosphate (A3P5P), adenosine 2′-phosphate 5′-phosphate (A2P5P), serotonin, and epinephrine were obtained from Sigma. ARL 66096 was a gift from Astra Research Laboratories (Loughborough, U.K.; formerly Fisons). All other materials and methods used have been described (8, 10, 11).
Isolation of Platelets.
Blood was collected from informed healthy human volunteers in acid/citrate/dextrose after the nature and possible consequences of the studies were explained. Platelet-rich plasma was obtained by centrifugation at 180 × g for 15 min at ambient temperature, incubated with 1 mM aspirin for 1 h at 37°C, and recentrifuged at 800 × g for 15 min at ambient temperature. Platelets were isolated from plasma by centrifugation at 800 × g for 15 min and resuspended in the final buffer consisting of 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 3.0 mM NaH2PO4, 5 mM glucose, 10 mM Hepes (pH 7.4), 0.2% BSA, and 20 μg/ml apyrase. The platelet count was adjusted to 2 × 108 cells per ml. All experiments were carried out in buffers with no added Ca2+ unless otherwise noted.
Platelet Aggregation.
Platelet shape change and aggregation (0.5-ml samples) were measured in a lumi-aggregometer (Chrono-Log, Havertown, PA) with stirring at 37°C. Fibrinogen (1 mg/ml) was added to all samples. The base line was set by using the platelet suspension diluted 1:1 with platelet suspension buffer to increase the gain of the aggregometer output.
Measurement of cAMP.
Platelet-rich plasma was incubated with 2 μCi/ml [3H]adenine and aspirin (1 mM) for 1 h at 37°C. Platelets were isolated from plasma by centrifugation at 800 × g for 15 min and resuspended in the same buffer as was used for aggregation. Reactions were stopped with 1 M HCl, and 4,000 dpm of [14C]cAMP was added as the recovery standard. cAMP was determined as detailed earlier (8) and expressed as a percentage of total [3H]adenine nucleotides.
RESULTS AND DISCUSSION
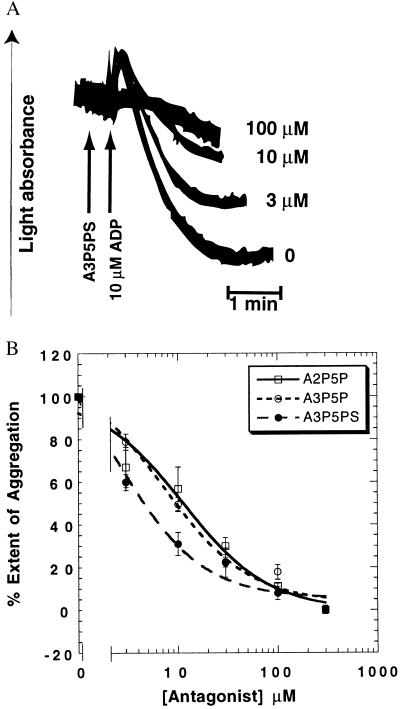
We recently demonstrated that the P2Y1 receptor is coupled to phospholipase C activation in platelets (9). In the present study we investigated the role of the P2Y1 receptor in ADP-induced platelet aggregation by using aspirin treated and washed human platelets in the presence of exogenous fibrinogen (1 mg/ml). We eliminated the contribution of thromboxane A2, produced by ADP-induced activation of phospholipase A2, by using aspirin to inactivate cyclooxygenase. The P2Y1 receptor-selective antagonist A3P5PS (12) inhibited both the rate and extent of ADP-induced platelet aggregation in a concentration-dependent manner (Fig. 1). These results were confirmed with two other P2Y1 receptor-selective antagonists (12), A3P5P and A2P5P (Fig. 1B). The potency of the antagonists (A3P5PS > A3P5P ≈ A2P5P) at the extent of ADP-induced platelet aggregation is similar to that at the cloned P2Y1 receptor (12). Similar results were obtained with 2-methylthio-ADP (2-MeSADP) as an agonist (not shown). These data indicate that activation of the P2Y1 receptor is required for ADP-induced platelet aggregation.
Figure 1.
Effect of P2Y1 receptor-selective antagonists on 10 μM ADP-induced platelet aggregation. Human platelets were washed and resuspended in Tyrode’s buffer, and aggregation was measured in the absence of added extracellular calcium, as described in Experimental Procedures. (A) Representative traces of various concentrations of A3P5PS on ADP-induced aggregation of aspirin-treated and washed platelets (8). Additions are shown with an arrow. Concentrations of A3P5PS are shown on the right. (B) Effect of P2Y1 receptor-selective antagonists on the extent of ADP-induced aggregation. The extent of aggregation in the presence of antagonists was normalized to that in the absence of the antagonist (taken as 100%).
Several lines of evidence have implicated the ADP receptor coupled to the inhibition of adenylyl cyclase, P2TAC, in platelet aggregation induced by ADP. Synthetic agonists of the P2TAC receptor, generally 2-substituted derivatives of ADP, are also inducers of platelet aggregation (13, 14). Antagonists of this receptor, such as ATP and ARL 66096, have been shown to block both ADP-induced adenylyl cyclase inhibition (8, 15) and platelet aggregation (15, 16). Furthermore, a significant correlation was found between affinity constant values for eight different nucleotide analogs as blockers of both ADP-induced adenylyl cyclase inhibition and aggregation (17). The thienopyridines, ticlopidine and clopidogrel, when administered in vivo, abrogate ADP-induced inhibition of adenylyl cyclase and platelet aggregation (18–20). Finally, two patients with congenital defects in ADP-induced inhibition of adenylyl cyclase in platelets are also deficient in ADP-induced platelet aggregation (21, 22). Hence, it is believed that P2TAC receptor activation by ADP leads to platelet aggregation.
In light of our interesting observation that inhibition of the P2Y1 receptor could also block ADP-induced platelet aggregation, we further investigated the nature of inhibition of ADP-induced platelet aggregation by ARL 66096, a P2TAC receptor-selective antagonist, and the P2Y1 antagonists.
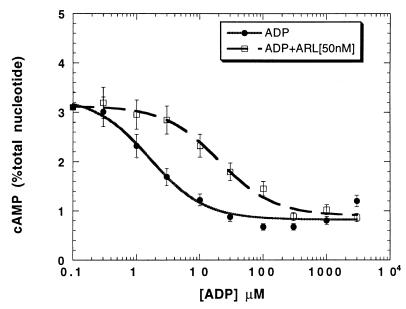
Platelet aggregation can be inhibited by increased cAMP levels, and physiological agonists, such as prostacyclin, inhibit platelet aggregation through this mechanism (23). Hence, we investigated whether the P2TAC or P2Y1 receptor-selective antagonists inhibit platelet aggregation by stimulation of adenylyl cyclase. None of these P2 receptor antagonists increased intracellular cAMP levels, whereas iloprost (a stable analog of prostacyclin) did (not shown). The P2Y1 receptor-selective antagonists A3P5PS, A3P5P, and A2P5P have been shown to be competitive inhibitors of agonist-stimulated inositol phosphate formation (12) and do not block ADP-induced adenylyl cyclase inhibition in platelets (9). We have shown that ARL 66096 abrogates ADP-induced inhibition of adenylyl cyclase in platelets (9). Investigations of the mechanism of action of ARL 66096 reveal that this compound caused a rightward shift of the ADP dose–response curve (Fig. 2), confirming that ARL 66096 acts by simple competition at the P2TAC receptor. We previously demonstrated that ARL 66096 has no effect on ADP-induced inositol phosphate formation in platelets (8). These results conclusively demonstrate the specificity of ARL 66096 for the P2TAC receptor and A3P5PS, A3P5P, and A2P5P for the P2Y1 receptor.
Figure 2.
Effect of ARL 66096 on ADP-induced inhibition of platelet adenylyl cyclase. Effect of ADP on platelet adenylyl cyclase was determined at various concentrations in the presence and absence of a single dose of 50 nM ARL 66096. The data are normalized to response to the maximal concentration of ADP (taken as 100%).
The human P2Y1 receptor is known to couple to mobilization of calcium from intracellular stores through activation of phospholipase C and formation of inositol trisphosphate, possibly by coupling to the heterotrimeric G protein, Gq (11, 24). Thus, signaling through Gq must be essential for ADP-induced platelet aggregation. Our conclusion is supported by at least two other studies (25, 26). A patient with a congenital defect with decreased amounts of Gαq was reported to have abnormal ADP-induced platelet aggregation (25). Furthermore, ADP-induced platelet aggregation and shape change were abolished in Gαq-deficient mice, confirming the role of Gq in ADP-induced platelet aggregation (26). Our studies reveal that the signaling events through both Gq and Gi are essential for platelet aggregation. In the case of ADP, signaling via Gq and Gi is achieved by simultaneous activation of two different receptors. The thrombin receptor has been shown to couple to both the activation of phospholipase C and inhibition of adenylyl cyclase, by coupling to different G proteins, Gq and Gi, (27), and hence thrombin causes platelet aggregation by activation of Gq and Gi pathways with the same receptor (28). Similarly, thromboxane A2 receptors also can couple to Gi and Gq, although it is not clear whether this is achieved by different receptor subtypes (or splice variants) (29, 30). Interestingly, platelet aggregation induced by thrombin or U46619, a thromboxane mimetic, was also abrogated in mice lacking Gαq (26), corroborating the essential role of the Gq-mediated signaling pathway in platelet aggregation. Of the other platelet-aggregating agents, lysophosphatidic acid can couple to both Gi and Gq (31), whereas the phorbol ester phorbol 12-myristate 13-acetate and the calcium ionophore A23187 probably activate a downstream signaling molecule in both of the pathways. Whether the Gi-mediated signaling is essential for all aggregating agents still remains to be evaluated.
Platelets also contain several other G proteins, including, Gz, Gs, G12, G13, and G16 (32, 33). Although increase in cAMP levels through activation of Gs inhibits agonist-induced platelet aggregation (23), the functional role of the other G proteins in this response needs to be determined. Platelet shape change induced by ADP, but not by thrombin and thromboxane, was abolished in mouse platelets lacking Gq, and it was proposed that thrombin and thromboxane might cause platelet shape change through coupling to other G proteins (26).
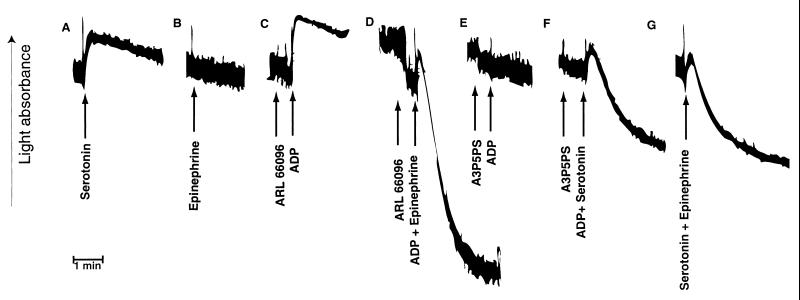
We tested the paradigm of coactivation of ADP receptors by mimicking the signaling with two independent G protein-coupled receptors on platelets. Serotonin, a weak platelet agonist, activates the 5-hydroxytryptamine receptor subtype 2A on platelets, which is coupled to activation of phospholipase C but not to inhibition of adenylyl cyclase (34). Thus, activation of platelets by serotonin is similar to the activation of the P2Y1 receptor by ADP. On the other hand, analogous to the P2TAC receptor activation, epinephrine causes inhibition of adenylyl cyclase, through activation of the α2A-adrenergic receptors on platelets. Epinephrine, which promotes only inhibition of adenylyl cyclase, by coupling to Gi, does not cause platelet aggregation, although it potentiates platelet aggregation induced by other agonists, which activate Gq (35, 36). As shown in Fig. 3, activation of either the α2A-adrenergic receptor or the serotonin receptor alone failed to cause platelet aggregation. Similar to selective activation of the P2Y1 receptor (8), activation by serotonin alone caused platelet shape change. When signaling through the P2TAC receptor was blocked with ARL 66096 (Fig. 3C), it could be replaced by activation of α2A-adrenergic receptors to result in platelet aggregation (Fig. 3D). On the other hand, P2Y1 receptor-mediated signaling (Fig. 3E) could be replaced by serotonin receptor activation (Fig. 3F). Furthermore, coactivation of the α2A-adrenergic and serotonin receptors, mimicking the activation of the P2TAC and the P2Y1 receptors, respectively, also resulted in platelet aggregation (Fig. 3G). The simultaneous addition of agonists is important for coactivation of signaling pathways. Delayed addition of the second agonist (30 s apart) resulted in no aggregation, whereas shorter intervals resulted in partial aggregation. The overall extent and rate of aggregation appear to be a reflection of the combined strength of the Gq- and Gi-coupled signaling pathways. These data clearly demonstrate that concomitant signaling by Gq-coupled and the Gi-coupled pathways is essential for platelet aggregation.
Figure 3.
Mimicking ADP-induced platelet aggregation by activation of two different G protein-coupled receptors on platelets. Aggregation was measured in the absence of added extracellular calcium. Representative aggregation traces are shown with additions as indicated by arrows. The following were used: 5 μM serotonin, 1 μM epinephrine, 10 μM ADP, 300 nM ARL 66096, and 200 μM A3P5PS.
The requirement of converging signaling pathways from two different G proteins has been previously demonstrated in agonist-induced DNA synthesis and cell proliferation (31, 37). Signaling through both pertussis toxin-sensitive and -insensitive G proteins is essential for the mitogenic action of thrombin and serotonin in Chinese hamster lung cells (37, 38) and for lysophosphatidic acid in rat 1 cells (39). Microinjection of inhibitory antibodies to different α-subunits of G proteins and subsequent activation by thrombin confirmed that the dual signaling from Gq and Gi or Go is required for thrombin-induced DNA synthesis (40, 41). Thus, it is conceivable that a similar mechanism is responsible for the activation of fibrinogen receptor in platelets.
2-Substituted derivatives of ADP have been found to induce platelet aggregation that correlates with their ability to inhibit platelet adenylyl cyclase, indicating that these compounds are potent agonists at the P2TAC receptor. We (8) and others (42) have shown that 2-MeSADP is a potent agonist at the platelet P2Y1 receptor, mediating inositol trisphosphate formation and mobilization of calcium from intracellular stores. Hence, we believe that the 2-substituted ADP analogs are also agonists at the P2Y1 receptor, and these synthetic agonists cause platelet aggregation through dual activation of the P2TAC and P2Y1 receptors.
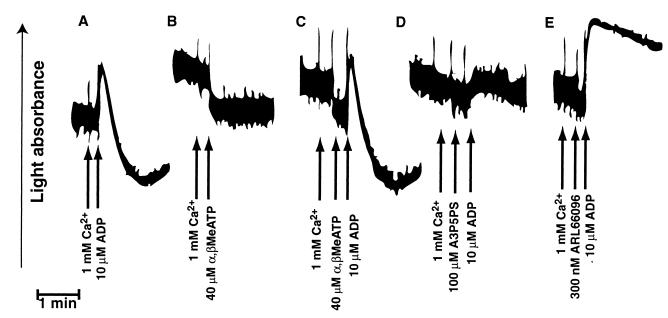
MacKenzie et al. (7) demonstrated an ADP-gated channel on platelets, suggested to be the P2X1 receptor, that could cause rapid calcium influx. We investigated whether coactivation of the P2X1 receptor and the P2TAC or P2Y1 receptor could cause platelet aggregation. A P2X receptor-selective agonist [α,β-methylene]ATP (α,β-MeATP), has previously been shown to have no effect on the P2Y1 or P2TAC receptors. In the presence of extracellular calcium, α,β-MeATP neither caused nor potentiated platelet aggregation induced by ADP (Fig. 4A–C). In addition, α,β-MeATP did not inhibit ADP-induced platelet aggregation, whereas the P2TAC antagonist ARL 66096 or the P2Y1 antagonist A3P5PS did (Fig. 4C–E). As observed earlier (8), unlike A3P5PS, ARL 66096 did not abrogate ADP-induced platelet shape change. These data indicate that the P2X1 receptor does not play a significant role in ADP-induced platelet aggregation. Furthermore, coactivation of the P2X1 receptor and either the P2Y1 or P2TAC receptor is also not sufficient to cause platelet aggregation. Thus, rapid calcium influx mediated by the P2X1 receptor is not sufficient to elicit either shape change (9) or platelet aggregation.
Figure 4.
Effect of P2X receptor-selective agonist on platelet aggregation. Representative aggregation traces are shown with additions indicated by arrows. Aggregation was measured as described for Fig. 1.
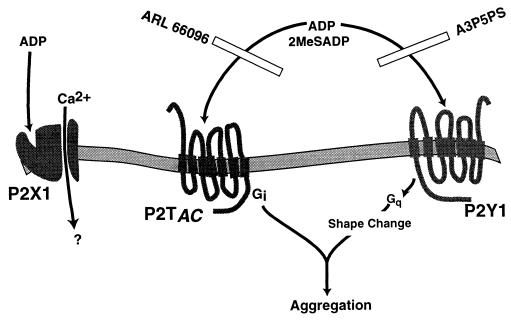
The coactivation mechanism of two different subtypes of P2 receptors in ADP-induced platelet activation is depicted in Fig. 5. Although the P2Y1 receptor alone can mediate ADP-induced platelet shape change (9), signaling events from both the P2Y1 and P2TAC receptors are essential for ADP-induced platelet aggregation. This model also explains the inability of epinephrine to cause platelet aggregation in the absence of positive feedback from thromboxane A2 or ADP, although it can potentiate other platelet-aggregating agents (35, 36). We propose that concomitant signaling through Gi and Gq is the general mechanism by which fibrinogen receptor activation and subsequent platelet aggregation occurs.
Figure 5.
Model depicting the signal transduction and physiological events mediated by the three ADP receptors on platelets.
Acknowledgments
We thank Drs. James L. Daniel, Barrie Ashby, Lee-Yuan Liu-Chen, and J. Bryan Smith (Department of Pharmacology, Temple University Medical School), Dr. Jeffrey L. Benovic (Kimmel Cancer Institute, Thomas Jefferson University, Philadelphia), and Dr. Ian Kirk and colleagues (Astra-Charnwood, Loughborough, U.K.) for critically reviewing the manuscript. This work was supported in part by a grant in aid from the American Heart Association, Southeastern Pennsylvania Affiliate (to S.P.K.). This work was performed during the tenure of an Established Investigator Award in Thrombosis from the American Heart Association and Genentech to S.P.K.
Note Added in Proof
Subsequent to the original submission of this manuscript, Savi and coworkers (43) reported that A3P5P blocks 2MeSADP-induced rabbit platelet aggregation, which was restored by activation with serotonin.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: α,β-MeATP, [α, β-methylene]ATP; A3P5PS, adenosine 3′-phosphate 5′-phosphosulfate; A3P5P, adenosine 3′-phosphate 5′-phosphate; A2P5P, adenosine 2′-phosphate 5′-phosphate; ARL 66096, 2-propylthio-d-β,γ-difluoromethyleneadenosine 5′-triphosphate; 2-MeSADP, 2-methylthio-ADP, P2TAC, platelet ADP receptor coupled to inhibition of adenylyl cyclase.
References
- 1.Gaarder A, Jonsen A, Laland S, Hellem A J, Owren P. Nature (London) 1961;192:531–532. doi: 10.1038/192531a0. [DOI] [PubMed] [Google Scholar]
- 2.Gachet C, Hechler B, Leon C, Vial C, Leray C, Ohlmann P, Cazenave J P. Thromb Haemost. 1997;77:271–275. [PubMed] [Google Scholar]
- 3.Mills D C B. Thromb Haemostasis. 1996;76:835–856. [PubMed] [Google Scholar]
- 4.Fredholm B B, Abbracchio M P, Burnstock G, Daly J W, Harden T K, Jacobson K A, Leff P, Williams M. Pharmacol Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- 5.Fredholm B B, Abbracchio M P, Burnstock G, Dubyak G R, Harden T K, Jacobson K A, Schwabe U, Williams M. Trends Pharmacol Sci. 1997;18:79–82. doi: 10.1016/s0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Communi D, Govaerts C, Parmentier M, Boeynaems J-M. J Biol Chem. 1997;272:31969–31973. doi: 10.1074/jbc.272.51.31969. [DOI] [PubMed] [Google Scholar]
- 7.MacKenzie A B, Mahaut-Smith M P, Sage S O. J Biol Chem. 1996;271:2879–2881. doi: 10.1074/jbc.271.6.2879. [DOI] [PubMed] [Google Scholar]
- 8.Daniel J L, Dangelmaier C, Jin J, Ashby B, Smith J B, Kunapuli S P. J Biol Chem. 1998;273:2024–2029. doi: 10.1074/jbc.273.4.2024. [DOI] [PubMed] [Google Scholar]
- 9.Jin J, Daniel J L, Kunapuli S P. J Biol Chem. 1998;273:2030–2034. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- 10.Akbar G K M, Dasari V R, Webb T E, Ayyanathan K, Pillarisetti K, Sandhu A K, Athwal R S, Daniel J L, Ashby B, Barnard E A, Kunapuli S P. J Biol Chem. 1996;271:18363–18367. doi: 10.1074/jbc.271.31.18363. [DOI] [PubMed] [Google Scholar]
- 11.Schachter J B, Li Q, Boyer J L, Nicholas R A, Harden T K. Br J Pharmacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyer J L, Romeroavila T, Schachter J B, Harden T K. Mol Pharmacol. 1996;50:1323–1329. [PubMed] [Google Scholar]
- 13.Cusack N J, Hourani S M. Br J Pharmacol. 1982;77:329–333. doi: 10.1111/j.1476-5381.1982.tb09302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hourani S M, Hall D A. Trends Pharmacol Sci. 1994;15:103–108. doi: 10.1016/0165-6147(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 15.Macfarlane D E, Mills D C B. Blood. 1975;46:309–320. [PubMed] [Google Scholar]
- 16.Humphries R G, Robertson M J, Leff P. Trends Pharmacol Sci. 1995;16:179–181. doi: 10.1016/s0165-6147(00)89018-5. [DOI] [PubMed] [Google Scholar]
- 17.Cusack N J, Hourani S M O. Br J Pharmacol. 1982;76:221–227. doi: 10.1111/j.1476-5381.1982.tb09210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mills D C B, Puri R N, Hu C-J, Minnitti C, Grana G, Freedman M, Colman R F, Colman R W. Atheroscler Thromb. 1992;12:430–436. doi: 10.1161/01.atv.12.4.430. [DOI] [PubMed] [Google Scholar]
- 19.Gachet C, Cazenave J-P, Ohlmann P, Bouloux C, Defreyn G, Driot F, Maffrand J-P. Biochem Pharmacol. 1990;40:2683–2687. doi: 10.1016/0006-2952(90)90587-b. [DOI] [PubMed] [Google Scholar]
- 20.Defreyn G, Gachet G, Savi P, Driot F, Cazenave J-P, Maffrand J-P. Thromb Haemostasis. 1991;65:186–190. [PubMed] [Google Scholar]
- 21.Cattaneo M, Lecchi A, Randi A M, McGregor J L, Mannucci P M. Blood. 1992;80:2787–2796. [PubMed] [Google Scholar]
- 22.Nurden P, Savi P, Heilmann E, Bihour C, Herbert J-M, Maffrand J-P, Nurden A. J Clin Invest. 1995;95:1612–1622. doi: 10.1172/JCI117835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salzman E W. N Engl J Med. 1972;286:358–363. doi: 10.1056/NEJM197202172860708. [DOI] [PubMed] [Google Scholar]
- 24.Leon C, Hechler B, Vial C, Leray C, Cazenave J P, Gachet C. FEBS Lett. 1997;403:26–30. doi: 10.1016/s0014-5793(97)00022-7. [DOI] [PubMed] [Google Scholar]
- 25.Gabbeta J, Yang X, Kowalska M A, Sun L, Dhanasekaran D, Rao A K. Proc Natl Acad Sci USA. 1997;94:8750–8755. doi: 10.1073/pnas.94.16.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Offermanns S, Toombs C F, Hu Y-H, Simon M I. Nature (London) 1997;389:183–186. doi: 10.1038/38284. [DOI] [PubMed] [Google Scholar]
- 27.Hung D T, Wong Y H, Vu T K H, Coughlin S R. J Biol Chem. 1992;267:20831–20834. [PubMed] [Google Scholar]
- 28.Siess W. News Physiol Sci. 1991;6:51–56. [Google Scholar]
- 29.Hirata T, Kakizuka A, Ushikubi F, Fuse I, Okuma M, Narumiya S. J Clin Invest. 1994;94:1662–1667. doi: 10.1172/JCI117510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirata T, Ushikubi F, Kakizuka A, Okuma M, Narumiya S. J Clin Invest. 1996;97:949–956. doi: 10.1172/JCI118518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moolenaar W H. J Biol Chem. 1995;270:12949–12952. doi: 10.1074/jbc.270.22.12949. [DOI] [PubMed] [Google Scholar]
- 32.van Willigen G, Donath J, Lapetina E G, Akkerman J W. Biochem Biophys Res Commun. 1995;214:254–262. doi: 10.1006/bbrc.1995.2282. [DOI] [PubMed] [Google Scholar]
- 33.Offermanns S, Hu Y H, Simon M I. J Biol Chem. 1996;271:26044–26048. doi: 10.1074/jbc.271.42.26044. [DOI] [PubMed] [Google Scholar]
- 34.Hourani S M O, Cusack N J. Pharmacol Rev. 1991;43:243–298. [PubMed] [Google Scholar]
- 35.Lanza F, Beretz A, Stierle A, Hanau D, Kubina M, Cazenave J P. Am J Physiol. 1988;255:H1276–1288. doi: 10.1152/ajpheart.1988.255.6.H1276. [DOI] [PubMed] [Google Scholar]
- 36.Steen V M, Holmsen H, Aarbakke G. Thromb Haemostasis. 1993;70:506–513. [PubMed] [Google Scholar]
- 37.Pouyssegur J, Seuwen K. Annu Rev Physiol. 1992;54:195–210. doi: 10.1146/annurev.ph.54.030192.001211. [DOI] [PubMed] [Google Scholar]
- 38.Seuwen K, Magnaldo I, Pouyssegur J. Nature (London) 1988;335:254–256. doi: 10.1038/335254a0. [DOI] [PubMed] [Google Scholar]
- 39.van Corven E J, Groenink A, Jalink K, Eichholtz T, Moolenaar W H. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- 40.LaMorte V J, Harootunian A T, Spiegel A M, Tsien R Y, Feramisco J R. J Cell Biol. 1993;121:91–99. doi: 10.1083/jcb.121.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baffy G, Yang L, Raj S, Manning D R, Williamson J R. J Biol Chem. 1994;269:8483–8487. [PubMed] [Google Scholar]
- 42.Hall D A, Hourani S M O. Br J Pharmacol. 1993;108:728–733. doi: 10.1111/j.1476-5381.1993.tb12869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savi P, Beauverger P, Labouret C, Delfaud M, Salel V, Kaghad M, Herbert J M. FEBS Lett. 1998;422:291–295. doi: 10.1016/s0014-5793(98)00025-8. [DOI] [PubMed] [Google Scholar]