Abstract
HIV-1–specific cytotoxic T-lymphocyte (CTL) responses have been detected at a low frequency in many HIV-1–exposed, persistently seronegative (HEPS) subjects. However, it is unclear how CTLs could protect against HIV acquisition in HEPS subjects, when high levels of circulating CTL fail to prevent disease progression in most seropositive subjects. To address this issue we studied CD8+ lymphocyte responses to a panel of HIV-1 CTL epitopes in 91 HEPS and 87 HIV-1–infected Nairobi sex workers. HIV-specific responses in seropositive women focused strongly on epitopes rarely or never recognized in HEPS subjects, who targeted epitopes that were subdominant or unrecognized in infected women. These differences in epitope specificity were restricted by only those HLA class I alleles that are associated with a reduced risk of HIV-1 infection in this cohort. Late seroconversion in HEPS donors was associated with a switch in epitope specificity and/or immunodominance to those epitopes preferentially recognized by HIV-1–infected women. The likelihood of detecting HIV-1–specific responses in HEPS women increased with the duration of viral exposure, suggesting that HIV-1–specific CD8+ responses are acquired over time. The association between differential recognition of distinct CTL epitopes and protection from HIV-1 infection may have significant implications for vaccine design.
Introduction
There is substantial variation in susceptibility to HIV-1 infection, with some subjects remaining HIV-1 seronegative despite repeated exposure to virus (1–3). HIV-1–specific cytotoxic T-lymphocytes (CTLs) have been described in several HIV-1–exposed, persistently seronegative (HEPS) populations, and the generation of HIV-1–specific CTLs has become a key goal in the development of a protective HIV-1 vaccine (4). HIV-1–specific CTLs are also found in seropositive donors, where they are important in suppressing viremia and preventing disease progression (5–7), but are ultimately unable to prevent eventual immunosuppression and death (8). It remains unclear how HIV-1–specific CTL could play a role in protection against HIV-1 infection in HEPS subjects, while failing to eliminate the virus in the majority of those who are infected.
The epidemiology and immunology of HIV-1 has been studied for over 15 years in an observational cohort of sex workers based in the Pumwani slum area of Nairobi, Kenya (9). It is estimated that these women have over 60 unprotected sexual exposures to HIV-1 per year, resulting in intense HIV-1 infection pressure on women who are seronegative when joining the cohort. Rates of HIV-1 seroconversion are highest during the first year of cohort enrollment and reach a plateau after 3 years of seronegative follow-up, although seroconversion may occur as late as 10 years after enrollment (10). The subgroup of women who remain both seronegative and negative as determined by PCR for at least 3 years while continuing sex work are operationally defined as HIV-1 resistant (11). HIV-1–specific CTL have been described previously in both the both the blood and genital tract of this HIV-1–resistant subgroup, using IFN-γ enzyme-linked immunospot (ELISPOT) responses to HIV-1 CTL epitopes (10, 12, 13), lysis of autologous vaccinia HIV-1 env– and gag–infected targets (14), and peptide-stimulated bulk CTL assays (13).
Protection in HEPS subjects is unlikely to be due to a stronger HIV-specific CD8+ T-cell response, at least directed against epitopes defined in infected subjects, since the magnitude of this response to predefined epitopes is generally lower in both blood and genital tract than in those with persistent infection (12, 13). Reduced HIV-1 susceptibility is associated with specific class I HLA molecules (15); these alleles might restrict particularly efficient HIV-specific CTLs or present specific “protective” epitopes (13). Differential epitope recognition has been seen in primary HIV-1 infection, with dramatic differences between the specificity of HIV-1–specific CD8+ responses seen during primary and late HIV infection (16, 17). It is also possible that protective CTL responses are qualitatively different in these HEPS subjects from those in persistently infected women, since HIV-specific CTLs in infected subjects demonstrate impaired maturation and reduced perforin expression when compared with cytomegalovirus-specific (CMV-specific) CTLs (18).
To address these questions, we examined the magnitude and specificity of CD8+ T-cell responses to a panel of predefined CTL epitopes in the Kenyan sex worker cohort, using the IFN-γ ELISPOT assay. We confirm that the magnitude of responses in HEPS women is approximately tenfold lower than in infected subjects, and thus protection is not due to a stronger HIV-specific CD8+ response. The proportion of HEPS women demonstrating HIV-specific responses increases with increasing duration of HIV exposure, consistent with the development of acquired immunity to HIV. Finally, we demonstrate that class I–restricted CD8+ responses in chronically infected sex workers often focus on certain dominant epitopes. However, these epitopes are rarely recognized by HEPS sex workers, who often target different epitopes, particularly restricted through those HLA alleles prospectively shown to be associated with HIV resistance in this cohort (15). These findings suggest that qualitative, rather than quantitative, differences discriminate HEPS CD8+ responses from those of chronically infected subjects.
Methods
Study population and sampling.
Sex workers were enrolled from a dedicated sex worker clinic in Nairobi, Kenya (9), between 1995 and 1999. Seronegative sex workers were subdivided into three groups, based on the duration of previous HIV-1–uninfected follow-up in the cohort: those enrolled for over 3 years (thereby meeting criteria for HIV-1 resistance; group 1) (11), those enrolled for 1–3 years (group 2), and sex workers followed for less than 1 year (group 3). All HEPS women were confirmed to be HIV-1 uninfected using a PCR system that uses primers for env, nef, and vif HIV-1 provirus genes that have been specifically adapted to detect African clades and that is sensitive to below five viral copies per 2 × 105 PBMCs (19). Lower-risk HIV-1–uninfected control women with no history of sex work were enrolled from a mother-child health care clinic in the Pumwani district of Nairobi and from an infertility clinic in Nairobi’s Kenyatta National Hospital. Women with clinical or laboratory evidence of cervicitis were excluded. The Scientific and Ethical Review Committees of the University of Nairobi and the University of Manitoba approved the study, and informed consent was obtained from all study participants.
General laboratory methods.
Molecular HLA typing was performed on all study subjects using amplification refractory mutation system PCR (ARMS-PCR) with sequence specific primers, as described previously (20). HIV-1 serological testing employed a synthetic peptide enzyme immunoassay (Detect HIV; Biochem ImmunoSystems Inc., Montreal, Quebec, Canada), and positive tests were confirmed using a recombinant antigen enzyme immunoassay (Recombigen HIV-1/2 EIA; Cambridge Biotech Corp., Galway, Ireland). PBMCs were isolated from blood in acid citrate dextran, using Ficoll-Hypaque (Sigma Chemical Co., St. Louis, Missouri, USA) density-gradient centrifugation. All cell-culture assays were performed in RPMI-1640 supplemented with 10 mM HEPES and L-glutamine, pH 7.2 (Sigma Chemical Co.), 50 mM β-mercaptoethanol (Sigma Chemical Co.), 10 U/ml penicillin, 0.1 mg/ml streptomycin, 0.25 mg/ml amphotericin B, and 10% FCS (Sigma Chemical Co.), which had been heat inactivated at 56°C for 40 minutes.
HIV-1 epitope selection and peptide synthesis.
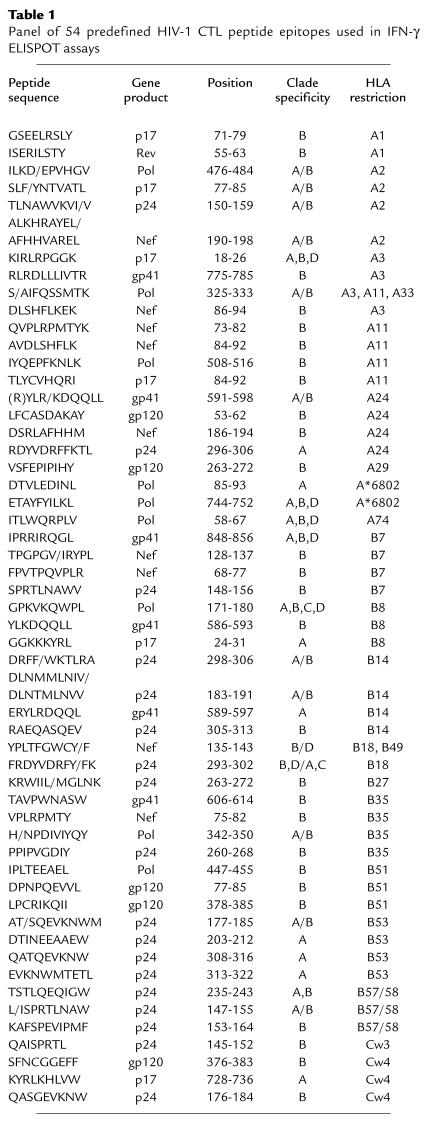
HIV-1 peptides were selected from a panel of 54 previously defined (21) A, B, and D clade CTL epitopes based on the class I HLA haplotype of the donor. Peptides were synthesized by F-moc chemistry using a Zinnser analytical synthesizer (Advanced Chemtech Inc., Louisville, Kentucky, USA), and purity was established by HPLC.
Peptide-based IFN-γ ELISPOT assays.
A modified ELISPOT assay was used to detect epitope-specific IFN-γ release from PBMCs, as described previously (12). Ninety-six-well nitrocellulose plates were precoated with a first-layer IFN-γ mAb, 1-DIK (MABTECH AB, Nacka, Sweden). At least 105 PBMCs were added to duplicate wells, either with predefined HIV-1 class I–restricted peptide epitopes at a concentration of 20 μM, with media alone (negative control), or with 1:100 phytohemagglutinin (PHA) (Murex Biotech Limited, Dartford, United Kingdom) as positive control). All peptides were run separately, without pooling of peptide epitopes. Where cell numbers permitted, assays were run with 2 × 105 PBMCs/well for HEPS subjects, and 1 × 105 for seropositive subjects. Plates were incubated overnight at 37°C in 5% CO2, cells discarded, and the plate incubated at room temperature for 3 hours with a second biotinylated anti–IFN-γ mAb (7-B6-1 biotin; MABTECH AB), followed by streptavidin-conjugated alkaline phosphatase (MABTECH AB) for 2 hours. Individual IFN-γ–producing cells were detected as dark blue spots using an alkaline phosphatase-conjugate substrate kit (Bio-Rad Laboratories, Hercules, California, USA). Spots were counted with a dissecting microscope (×40) prior to 1998, and from 1998 onward by using an automated ELISPOT reader (Autoimmun Diagnostika GmbH, Strassberg, Germany).
Criteria for a positive HIV–specific ELISPOT assay.
HIV-1–specific IFN-γ responses were reported as number of spot-forming units (SFUs) per 106 mononuclear cells, after subtraction of background IFN-γ secretion. To control for varying levels of background IFN-γ secretion, SFU in the HIV-1 peptide wells needed to be at least double that seen with media alone to be considered positive. An HIV-1–specific ELISPOT response was defined as described previously (10, 12, 22): (a) IFN-γ release seen in response to 1:100 PHA; (b) greater than or equal to 20 HIV-1–specific SFU/106 mononuclear cells; and (c) SFU in HIV-1 peptide wells exceeded background by a factor of at least 2.
An immunodominant epitope was defined as that HIV-1 epitope generating the highest-frequency IFN-γ response, provided that this response fulfilled criteria for a positive ELISPOT assay. Likewise, an allele-specific immunodominant epitope was defined as the epitope generating the highest frequency IFN-γ response from the panel of epitopes restricted by a particular HLA allele.
CD8+ lymphocyte depletion assays.
CD8+ lymphocyte depletion was performed using anti-CD8+ Ab–coated immunomagnetic beads (Dynabeads HLA cell prep I; Dynal Inc., Lake Success, New York, USA), according to the manufacturer’s instructions. Significant reduction of an ELISPOT response was defined as 50% or greater reduction in HIV-1–specific IFN-γ release after CD8+ lymphocyte depletion.
Data analysis.
Statistical analysis used the SPSS for Windows Rel. 9.0.0, 1998 package (SPSS Inc., Chicago, Illinois, USA). Comparison of means between study groups was performed by one-way ANOVA. Mantel-Haenszel χ-square test with calculation of likelihood ratios and confidence intervals was used to compare dichotomous variables between study groups. If ELISPOT assays had been performed on any individual at several time points, only the earliest result was included in the general analysis of response associations. However, an epitope-specific response detected in an individual at any time point was included in the analysis of epitope specificity. In comparing patterns of epitope recognition, the specificity of ELISPOT responses was examined within class I HLA alleles where at least two different HIV-1 epitopes had been studied (since two or more epitopes are needed to examine differences in specificity) in a minimum of eight subjects (to avoid basing conclusions on overly small subject numbers). To control for multiple comparisons at each class I allele, the P value calculated for epitope recognition at any given class I allele was corrected for the total number of epitopes tested for that allele.
Results
Frequency and magnitude of HIV-specific responses in Kenyan sex workers.
CD8+ T-cell responses were tested against a panel of 54 predefined CTL epitopes (listed in Table 1) in 178 sex workers (91 HEPS and 87 HIV-1–infected women, respectively), including all those seronegative women sampled during the 1995–1999 period for whom CTL epitopes appropriate for their HLA class I type were available. HIV-1 epitope–specific responses were more common in HIV-1–infected than HEPS sex workers (79 of 87, 91%, vs. 43 of 91, 47%; likelihood ratio (LR) = 42.4; P < 0.001), and dominant responses were approximately tenfold stronger in seropositive women (mean 983.4 vs. 85.8 SFU/million PBMCs; P < 0.001). On average, responses in HEPS sex workers exceeded background by a factor of 3.3 (range 1–26.7) and in HIV-1–infected sex workers by a factor of 36.1 (range 1–272).
Table 1.
Panel of 54 predefined HIV-1 CTL peptide epitopes used in IFN-γ ELISPOT assays
Responses were directed against a mean of 2.7 CTL epitopes overall and were broader in infected sex workers, directed against a mean of 3.1 epitopes versus 2.0 in HEPS (P = 0.007), although there was no significant difference in the number of gene products recognized (1.6 in infected vs. 1.4 in HEPS; P = 0.15). There was no difference between HIV-1–infected and HEPS subjects in the number of HLA alleles restricting ELISPOT responses (overall mean = 1.5 class I HLA alleles; 1.6 vs. 1.4 respectively; P = 0.2). Responders and nonresponders were similar in the number of epitopes screened and restricting HLA class I alleles tested. CD8+ depletion assays performed for 14 HEPS and 7 seropositive women confirmed that ELISPOT responses were mediated by CD8+ lymphocytes (data not shown). Thus CD8+ T-cell responses to predefined HIV-1 epitopes in HEPS women were neither stronger nor more broadly directed than in HIV-1–infected women.
Lack of HIV-specific responses in lower-risk controls.
ELISPOT assays were performed in 18 lower-risk Kenyan women with no history of commercial sex work. No HIV-1–specific responses were detected against a total of 130 CTL epitope peptides, using predefined criteria for a positive ELISPOT assay (12) (see Methods). The mean HIV-specific response in controls was 5.4 SFU/106 PBMCs, with an SD of 7.66, so that the mean + 2 SD was equal to 20.7 SFU/106. This correlated closely with our predefined cut-off for a positive assay.
Association of HEPS CD8+ responses with duration of HIV exposure.
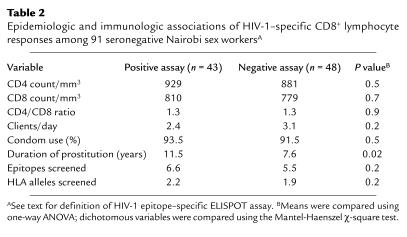
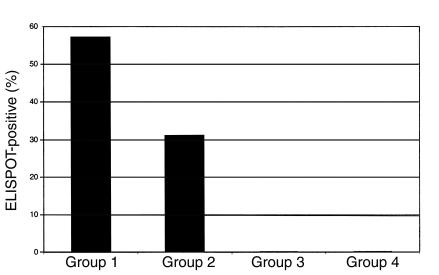
HEPS women with CD8+ responses (n = 43) were compared with those without CD8+ responses (n = 48) (Table 2). Responses were detected intermittently, and the proportion of time points with a positive assay was 0.62 (mean number of total assays 3.3, range 2–8; mean number with positive response 2.1, range 1–7). Responses were not associated with self-reported levels of condom use, the number of daily clients, absolute CD4/CD8 lymphocyte counts, CD4/CD8 ratio, or any single class I allele. However, women with a positive response had been engaged in commercial sex work for a longer time (11.5 vs. 7.6 years; P = 0.02), and there was a stepwise increase in the frequency of ELISPOT responses with increasing duration of seronegative clinical follow-up (Figure 1; LR = 14.3; P = 0.001). In addition, there was a positive correlation between the duration of uninfected clinical follow-up and the magnitude of HIV-1–specific responses (Pearson correlation = 0.31; P < 0.001). The association between the duration of uninfected HIV exposure and both the frequency and magnitude of HIV epitope-specific responses strongly suggests that immunity to HIV is acquired after cohort enrollment.
Table 2.
Epidemiologic and immunologic associations of HIV-1–specific CD8+ lymphocyte responses among 91 seronegative Nairobi sex workersA
Figure 1.
Association between duration of previous HIV-1 exposure and HIV-1–specific IFN-γ ELISPOT responses in HEPS sex workers. HIV-1–seronegative sex workers (n = 91) were divided into three groups, according to the duration of enrollment in the sex worker cohort: ≥3 years (group 1; n = 67); 1–3 years (group 2; n = 16); <1 year (group 3; n = 8). Group 4 consisted of 18 HIV-1–seronegative Kenyan women with no history of commercial sex work. The vertical bars represent the percentage of each group demonstrating HIV-1 CTL epitope-specific ELISPOT responses (see text for definition).
These data were generated using the predefined cut-off for a positive assay of 20 SFU/106 PBMCs above background (12). In the absence of universally established criteria for a positive ELISPOT assay, the robustness of this association was tested by using positive cut-offs of both 50 SFU and 100 SFU/106 PBMCs. In each case, a similar association was found between duration of follow-up and a positive ELISPOT assay (LR = 7.0, P = 0.03; and LR = 5.4, P = 0.07, respectively), although the proportion of HEPS women with a positive assay decreased as the cut-off was increased, reducing the statistical power of any comparisons.
Epitope specificity of responses in HEPS and HIV-infected donors.
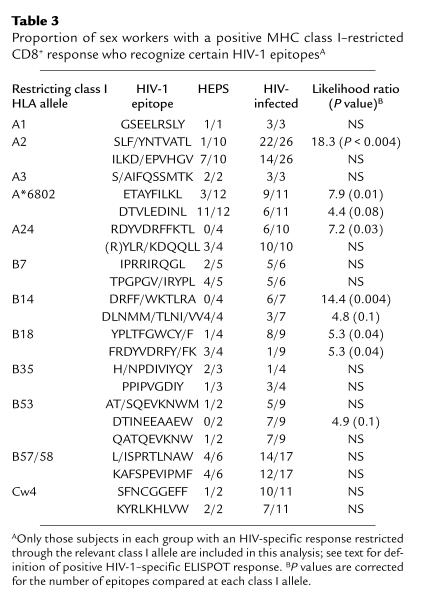
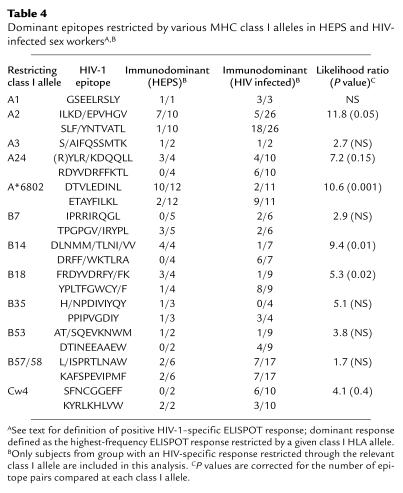
Results meeting selection criteria (see Methods) were available for 168 women (n = 83 HEPS, n = 85 HIV infected). When HEPS and HIV-infected subjects with CD8+ responses restricted through a particular class I allele were compared, HIV-infected sex workers often recognized epitopes rarely or never recognized by HEPS subjects. For instance, p17 epitope SLF/YNTVATL was recognized by 22 of 26 (85%) infected women who had a CD8+ response restricted through HLA class I allele A2, compared with only one of ten (10%) of HEPS (odds ratio [OR] 17.7; P < 0.004) (Table 3). Likewise, HIV-infected sex workers with responses restricted through A24 were more likely to respond to the p24 epitope RDYVDRFFKTL, through A*6802 to the pol epitope ETAYFILKL, through B14 to the nef epitope DRFF/WKTLRA, and through B18 to the nef epitope YPLTFGWCY/F (Table 3). In some cases, HEPS sex workers were also seen to preferentially recognize a different peptide epitope, in particular A*6802 pol epitope DTVLEDINL and the B18 p24 epitope FRDYVDRFY/FK. Almost all A2-restricted responses in HEPS were directed toward the pol epitope ILKD/EPVHGV (seven of ten responders), but responses to this epitope were also relatively common in HIV-infected subjects (14 of 25 responders). Strikingly, these differences in epitope specificity were only seen for responses restricted by class I HLA alleles A2, A24, A*6802, B14, and B18, previously shown to be associated with resistance to HIV-1 in this cohort (15). In addition, HEPS sex workers with at least one of the class I HLA alleles associated with protection from HIV-1 infection (15) were more likely to have an HIV-1–specific CD8+ response than those without any of these alleles (33 of 60 vs. 10 of 31; LR = 4.3, P = 0.04).
Table 3.
Proportion of sex workers with a positive MHC class I–restricted CD8+ response who recognize certain HIV-1 epitopesA
There were no differences between HEPS and HIV-1–infected sex workers in A2 allele subtypes, and no differences were apparent in A2-restricted epitope recognition based on A2 allele subtype (data not shown). There were no differences in the epitope specificity of CD8+ responses restricted by seven other class I alleles, with similar patterns of epitope recognition seen in HEPS and infected subjects (A1, A3, B7, B35, B53, B57/58, Cw4; Table 3). Insufficient subjects and/or epitopes were studied to examine differences in specificity at the remaining nine class I alleles.
Taken together, these data suggest that the protective effect of these HLA alleles in resistance to HIV infection could be related to a greater likelihood of generating a CTL response to a repertoire of “protective” HIV-1 epitopes.
Epitope immunodominance in HEPS and infected sex workers.
As noted, HIV–infected sex workers tended to focus CD8+ responses on epitopes infrequently recognized by HEPS sex workers, while epitopes recognized by HEPS women were not recognized, or were recognized at a lower frequency, by seropositive women. We therefore compared patterns of epitope immunodominance between HEPS and infected sex workers at specific class I alleles, correcting P values for the number of epitope pairs compared at each allele. Significant differences or strong trends in epitope immunodominance were seen for responses restricted by all those class I alleles associated with protection in the Nairobi sex worker cohort (A2, A24, A*6802, B14, and B18), but not for the other seven alleles analyzed (Table 4).
Table 4.
Dominant epitopes restricted by various MHC class I alleles in HEPS and HIV-infected sex workersA,B
Effect of late seroconversion on specificity of HIV-specific CD8+ responses.
Over the course of this study, HIV-1 seroconversion was observed in eight sex workers for whom preseroconversion ELISPOT assays had been performed (10). Seven of these women had met criteria for HIV-1 resistance, and one had not. Epidemiological correlates of late seroconversion have been described for six of these formerly HIV-resistant donors, which was strongly linked to reduced HIV-1 exposure (10). Prospective studies in other cohort members taking a break from sex work showed an associated fall in HIV-1–specific responses below the levels of detection, suggesting that repeated HIV-1 exposure was necessary to maintain protective effector T-cell responses (10).
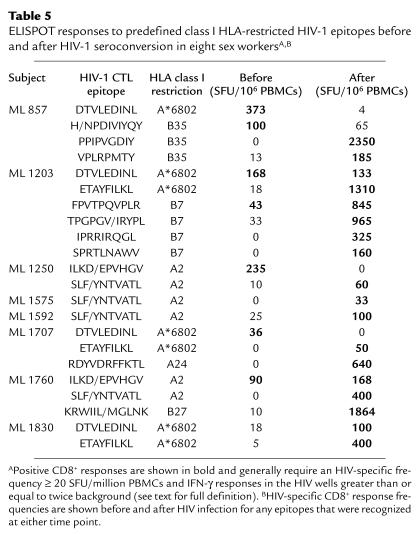
HIV-1–specific ELISPOT responses had been present before seroconversion in five of seven of women who had met criteria for HIV-1 resistance (10) and had not been detected in the one woman who had not. In these five women, CD8+ responses before seroconversion were compared with responses seen within 1 year after HIV infection. More epitopes and more gene products were recognized after infection (mean of 3.0 vs. 1.4 epitopes and 2.4 vs. 1.2 gene products; P = 0.08 and P = 0.004, respectively), and dominant CD8+ responses were stronger (1244.8 vs. 180.4 SFU/million PBMCs; P = 0.05). HIV-1 infection was therefore associated with broadening and strengthening of CD8+ responses.
In addition, CD8+ responses before and after HIV-1 infection showed the same pattern of differential epitope recognition as was seen in our larger cross-sectional analysis of the cohort (Table 5). An A*6802-restricted response to DTVLEDINL was seen in three subjects before seroconversion (ML 857, ML 1203, ML 1707), but after seroconversion was either lost (ML 857, ML 1707) or was superceded by responses to A*6802 ETAYFILKL (ML 1203). Likewise, A2-restricted responses to ILKD/EPVHGV in two HEPS sex workers were either lost completely (ML 1250) or became subdominant (ML 1760). In both these cases, the dominant A2-restricted epitope after HIV infection was SLF/YNTVATL. In the remaining three sex workers, no HIV-1–specific responses had been seen before infection, but dominant responses after infection recognized epitope A2 SLF/YNTVATL in two cases (ML 1575, ML 1592), and A*6802 ETAYFILKL in one case (ML 1830). It therefore seems that CD8+ epitope specificity is related to the HIV-1–infection status of an individual donor and is unlikely to reflect selection through polymorphic antigen-processing or other immune-response genes.
Table 5.
ELISPOT responses to predefined class I HLA-restricted HIV-1 epitopes before and after HIV-1 seroconversion in eight sex workersA,B
Discussion
The extraordinarily high risk of HIV-1 infection in the Nairobi sex worker cohort plateaus after 3 years of HIV-1–uninfected follow-up, an observation best explained by the presence of biologic factors mediating HIV-1 resistance in a minority of women (11). Both classical CTL responses (10, 13, 14) and IFN-γ responses directed against HIV-1 CTL epitopes (10, 12, 13) have been demonstrated previously in these HIV-1–resistant women, despite a lack of detectable HIV-1 infection or plasma IgG Ab responses. The current study constitutes the most complete examination of CD8+ lymphocyte responses performed in this cohort, examining 91 seronegative sex workers who had been followed previously for a mean of 5.4 years (range, 0–14 years).
The finding that HIV-1–specific CD8+ lymphocyte responses become more frequent and stronger in the seronegative women with the longest duration of HIV-1 exposure is the clearest association to date between CD8+ responses and resistance to HIV-1 infection and strongly suggests that HIV-1–specific immunity is acquired over time. However, because an observational study cannot prove causality, it remains possible that HIV-1–specific CD8+ lymphocyte responses are not protective, but constitute an epiphenomenon related to exposure of sex workers protected from HIV-1 infection by an alternate mechanism (which we know is not likely to be intrinsic resistance of CD4 cells, increased production of CC chemokines, or HIV-1 coreceptor abnormalities) (23). In addition, since HIV proviral DNA can been detected in the PBMCs of some HEPS subjects in other cohorts (24), it is also possible that HIV-“resistant” sex workers have been infected by HIV, but have been able to control viral replication to a level that is undetectable by conventional means.
Although HIV-1 CTL epitope-specific responses were common in both HEPS and seropositive women, we demonstrated significant differences between these groups in the epitope specificity of responses restricted by certain class I HLA alleles. This differential epitope recognition was seen only for responses restricted by class I alleles associated previously with a reduced risk of HIV-1 seroconversion in this cohort (15), namely HLA A2, A*6802, A24, B14, and B18 (allele B14 was associated with reduced seroconversion in this HLA study, although this did not reach statistical significance due to low subject numbers: OR = 0.36; 95% confidence interval [CI] 0.1–1.4; P = 0.1). The same phenomenon of differential recognition applied to eight HEPS sex workers who seroconverted. Either a switch in immunodominance was seen from a “resistant” to an “infected” epitope (three of eight: ML 1203, 1707, 1760), or a response to a “resistant” epitope was lost completely (two of seven: ML 857, 1250), or a lack of response was superceded by a response to an “infected” epitope (three of seven: ML 1575, 1592, 1830). This was not due to mutational escape within the resistant epitopes due to CTL-mediated immune pressure, since no viral sequence variation was seen in these epitopes from subjects ML 857, 1203, 1707, or 1760 (10). Although it is disappointing that seroconversion occurred despite preexisting HIV-1–specific CD8+ responses in these women, it is possible that late seroconversion in these women represents infection after waning of a protective CTL response in the temporary absence of antigen exposure (10).
The phenomenon of differential epitope recognition has been described recently in the context of primary versus chronic HIV-1 infection (16, 17). A2-restricted responses to the epitope SLYNTVATL were immunodominant during chronic infection (as they were within our cohort of HIV-1–infected sex workers), but were not seen in any of eleven subjects with primary infection. A SLYNTVATL-specific response did develop in two subjects 5–20 months after primary infection, but was not important in the initial control of viremia. We would likewise conclude that responses to the A2-restricted epitope SLYNTVATL, as well as to several other epitopes commonly recognized in the context of chronic HIV-1 infection, are not important in protection against HIV-1 infection. This is despite the fact that SLYNTVATL-specific CTL have been shown to be important in controlling viremia and disease progression in chronic HIV infection (6, 7). However, the timing of our sampling does not allow a detailed analysis of changes in epitope specificity during primary infection.
Several hypotheses could explain the association between protection from HIV-1 infection and differential epitope recognition. HEPS responses might be directed against more highly conserved regions of the virus, minimizing the possibility of viral escape, or against gene products critical for viral replication. However, although three of the differential epitopes overlap within the highly conserved major homology region (MHR) of HIV-1 gag, this region does not appear to be recognized preferentially by HEPS women: two of those epitopes (A24 RDYVDRFFKTL and B14 DRFF/WKTLRA) are more commonly recognized by HIV-1–infected women and one (B18 FRDYVDRFF/YK) by HEPS women. Furthermore, the heterogeneity of CTL epitopes preferentially recognized by HEPS subjects argues against a focus of these responses on particular viral gene products. Of the four resistant epitopes, two are found within HIV-1 pol (A2 ILKD/EPVHGV in reverse transcriptase and A*6802 DTVLEDINL in protease) and two within HIV-1 p24 (B14 DLNM/TLNI/VV and B18 FRDYVDRFY/FK).
Low-cell surface density of naturally processed CTL epitope peptides may limit the effectiveness of HIV-specific CTL. However, Tsomides and colleagues (25) have demonstrated that the A2-restricted epitope SLYNTVATL (preferentially recognized by HIV-1–infected women in our study) is displayed on the cell surface at approximately 30 times the level of ILKEPVHGV (preferentially recognized by HEPS women). Variations in cell-surface expression of CTL epitopes are therefore unlikely to explain the phenomenon of differential epitope recognition. This observation has parallels with studies in a murine model of lymphocytic choriomeningitis virus (LCMV) infection, which showed that the relative abundance of different CTL epitopes on infected cells closely correlated with the magnitude of the epitope-specific CTL response. However, the quantitative hierarchy of CTL activity was not reflected in the ability of these CTLs to protect against LCMV infection following adoptive transfer: the best protection in this study was mediated by CTLs specific for a subdominant epitope (26).
Finally, it is possible that there are functional differences between CTLs specific for different epitopes, particularly since HIV-1–specific CTLs in chronically infected donors demonstrate relatively low levels of perforin expression and cytolytic function (18). It is feasible that this impaired CTL function could be related in some way to the epitope specificity of CTL in seropositive donors, but given the low frequency of PBMC responses in HEPS subjects, detailed examination of CTL function may require the cloning and subsequent expansion of these low-frequency CTL, since they are generally below the detection limit of the ex vivo tetramer assay (27).
In our study, HIV-1–specific responses were not detected in 42% of women meeting criteria for HIV-1 resistance. This could be due to the protection of some HEPS subjects in this cohort by other immune responses, including HIV-1–specific IgA (28–30), CD4-mediated T-helper responses (14, 30), and noncytolytic CD8+–mediated inhibition of HIV-1 (31). However, it is also possible that CTL epitope responses were present, but fell below the threshold of detection of our assays. HIV-specific CTL are detected intermittently in HEPS individuals, possibly due to varying levels of antigen exposure or assay limitations (10, 32). More importantly, the panel of 54 HIV-1 CTL epitopes used in the ELISPOT assays did not include every CTL epitope described to date, so certain epitope responses might have been missed and the estimated breadth of response underestimated. Finally, the finding of differential epitope recognition raises the possibility that HEPS women target epitopes completely distinct from those mapped in HIV-1–infected subjects. Since almost all HIV-1 CTL epitopes to date have been mapped in infected donors, responses to unique HEPS epitopes could be missed. CTL-based preventive HIV-1 vaccine studies may benefit from a focus on previously defined CTL epitopes that are preferentially recognized in HEPS individuals, as well as from a search for novel protective epitopes within HEPS cohorts.
Acknowledgments
We would like to thank Jane Kamene for assistance in subject recruitment; Kati di Gleria for peptide synthesis; and the women of the Pumwani cohort for many years of cooperation and enthusiasm. This work was supported by grants from the UK Medical Research Council (S.L. Rowland-Jones, T. Dong); the Elizabeth Glaser Pediatric AIDS Foundation (S.L. Rowland-Jones); and the Medical Research Council of Canada (R. Kaul, F.A. Plummer). F.A. Plummer is a Senior Scientist of the Medical Research Council of Canada and a Canada Research Chair, K.S. MacDonald is a Career Scientist of the Ontario HIV Treatment Network, and S.L. Rowland-Jones is an Elizabeth Glaser Scientist of the Pediatric AIDS Foundation.
Footnotes
Rupert Kaul and Tao Dong contributed equally to this work.
References
- 1.Rowland-Jones SL, McMichael A. Immune responses in HIV-exposed seronegatives: have they repelled the virus? Curr Opin Immunol. 1995;7:448–455. doi: 10.1016/0952-7915(95)80087-5. [DOI] [PubMed] [Google Scholar]
- 2.Haynes BF, Pantaleo G, Fauci AS. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 3.Clerici M, Shearer GM. Correlates of protection in HIV infection and the progression of HIV infection to AIDS. Immunol Lett. 1996;51:69–73. doi: 10.1016/0165-2478(96)02557-6. [DOI] [PubMed] [Google Scholar]
- 4.Letvin NL. Progress in the development of an HIV-1 vaccine. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 5.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogg GS, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 7.Ogg GS, et al. Longitudinal phenotypic analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes: correlation with disease progression. J Virol. 1999;73:9153–9160. doi: 10.1128/jvi.73.11.9153-9160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wodarz D, Nowak MA. Evolutionary dynamics of HIV-induced subversion of the immune response. Immunol Rev. 1999;168:75–89. doi: 10.1111/j.1600-065x.1999.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 9.Simonsen JN, et al. HIV infection among lower socioeconomic strata prostitutes in Nairobi. AIDS. 1990;4:139–144. doi: 10.1097/00002030-199002000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Kaul R, et al. Late seroconversion in HIV-resistant Nairobi prostitutes despite pre-existing HIV-specific CD8+ responses. J Clin Invest. 2001;107:341–349. doi: 10.1172/JCI10714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowke KR, et al. Resistance to HIV-1 infection among persistently seronegative prostitutes in Nairobi, Kenya. Lancet. 1996;348:1347–1351. doi: 10.1016/S0140-6736(95)12269-2. [DOI] [PubMed] [Google Scholar]
- 12.Kaul R, et al. HIV-1 specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1 resistant prostitutes in Nairobi. J Immunol. 2000;164:1602–1611. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- 13.Rowland-Jones SL, et al. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J Clin Invest. 1998;102:1758–1765. doi: 10.1172/JCI4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowke KR, et al. HIV-1-specific cellular immune responses among HIV-1-resistant sex workers. Immunol Cell Biol. 2000;78:586–595. doi: 10.1046/j.1440-1711.2000.00944.x. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald KS, et al. Influence of HLA supertypes on susceptibility and resistance to human immunodeficiency virus type 1 infection. J Infect Dis. 2000;181:1581–1589. doi: 10.1086/315472. [DOI] [PubMed] [Google Scholar]
- 16.Altfeld M, et al. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001;193:169–180. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goulder PJ, et al. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J Exp Med. 2001;193:181–194. doi: 10.1084/jem.193.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appay V, et al. HIV-specific CD8+ cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawood MR, Allan R, Fowke K, Embree J, Hammond GW. Development of oligonucleotide primers and probes against structural and regulatory genes of human immunodeficiency virus type 1 (HIV-1) and their use for amplification of HIV-1 provirus by using polymerase chain reaction. J Clin Microbiol. 1992;30:2279–2283. doi: 10.1128/jcm.30.9.2279-2283.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bunce M, et al. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355–367. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 21.Brander, C., and Walker, B.D. 1999. List of epitopes by HLA presenting molecule. HIV Molecular Immunology Database: Los Alamos National Laboratory: Theoretical Biology and Biophysics, Los Alamos, New Mexico, USA. IB1–IB24.
- 22.Biasin M, et al. Mucosal and systemic immune activation is present in human immunodeficiency virus-exposed seronegative women. J Infect Dis. 2000;182:1365–1374. doi: 10.1086/315873. [DOI] [PubMed] [Google Scholar]
- 23.Fowke KR, et al. HIV type 1 resistance in Kenyan sex workers is not associated with altered cellular susceptibility to HIV type 1 infection or enhanced beta-chemokine production. AIDS Res Hum Retroviruses. 1998;14:1521–1530. doi: 10.1089/aid.1998.14.1521. [DOI] [PubMed] [Google Scholar]
- 24.Zhu, T., et al. 1999. Evidence for HIV-1 latent infection in exposed seronegative individuals (abstract #8). 6th Conference on Retroviruses and Opportunistic Infections. Chicago, Illinois, USA.
- 25.Tsomides TJ, et al. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J Exp Med Sci. 1994;180:1283–1293. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallimore A, et al. A protective cytotoxic T cell response to a subdominant epitope is influenced by the stability of the MHC class I/peptide complex and the overall spectrum of viral peptides generated within infected cells. Eur J Immunol. 1998;28:3301–3311. doi: 10.1002/(SICI)1521-4141(199810)28:10<3301::AID-IMMU3301>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 27.Kaul, R., and Rowland-Jones, S.L. 1999. Methods of detection of HIV-specific CTL and their role in protection against HIV infection. HIV Molecular Immunology Database: Los Alamos National Laboratory: Theoretical Biology and Biophysics, Los Alamos, New Mexico, USA. IV27–IV36.
- 28.Devito C, et al. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J Immunol. 2000;165:5170–5176. doi: 10.4049/jimmunol.165.9.5170. [DOI] [PubMed] [Google Scholar]
- 29.Devito C, et al. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS. 2000;14:1917–1920. doi: 10.1097/00002030-200009080-00006. [DOI] [PubMed] [Google Scholar]
- 30.Kaul R, et al. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS. 1999;13:23–29. doi: 10.1097/00002030-199901140-00004. [DOI] [PubMed] [Google Scholar]
- 31.Stranford SA, et al. Lack of infection in HIV-exposed individuals is associated with a strong CD8(+) cell noncytotoxic anti-HIV response. Proc Natl Acad Sci USA. 1999;96:1030–1035. doi: 10.1073/pnas.96.3.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goh WC, et al. Protection against human immunodeficiency virus type 1 infection in persons with repeated exposure: evidence for T cell immunity in the absence of inherited CCR5 coreceptor defects. J Infect Dis. 1999;179:548–557. doi: 10.1086/314632. [DOI] [PubMed] [Google Scholar]