Bone is a highly hospitable environment for colonization and growth of metastatic tumors, and some of the most common human malignancies, notably breast cancer and prostate cancer, have a strong propensity to produce skeletal metastases (1). Tumor cells, in turn, can produce a spectrum of skeletal manifestations which spans diffuse osteopenia, focal osteolysis, focal osteogenesis, and osteomalacia (2).
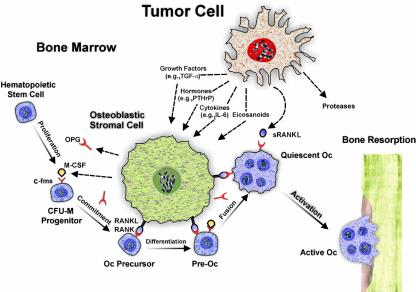
The most common skeletal manifestation of malignancy is focal osteolysis in association with metastases. In order for tumor cells to grow and invade mineralized bone, osteolysis must occur. Osteoclasts appear uniquely adapted to produce the microenvironment and the biochemical milieu that are needed to resorb bone. Although previous reports have indicated that some tumor cells appear capable of assuming an osteoclast phenotype and directly resorbing bone (3), the bulk of the evidence suggests that most tumor cells act indirectly by co-opting the physiologic mechanisms that normally favor bone resorption. Thus, they release agents such as hormones, eicosanoids, growth factors, and cytokines into the bone microenvironment, which act on osteoblastic stromal cells to enhance the production of osteoclast activating factors. Most notable of these is the cell membrane–associated protein termed receptor activator of NF-κB ligand (RANKL), which is a member of the TNF family of cytokines. RANKL can then bind to its cognate receptor (RANK) on osteoclast precursors and, in the presence of M-CSF, enhance the differentiation and fusion of these cells to produce functioning multinucleated osteoclasts (4) (Figure 1). Concomitantly, production of a soluble decoy receptor for RANKL, termed osteoprotegerin (OPG) (5), may be downregulated (6), thus eliminating one means by which the ensuing osteolysis could be repressed.
Figure 1.
Schematic representation of tumor-cell induced osteolysis. A tumor cell may release soluble mediators such as hormones (e.g., PTHrP), eicosanoids, cytokines (e.g., IL-6), or growth factors (e.g., TGF-α) that act on an osteoblastic stromal cell. The stromal cell produces RANKL, which binds to its cognate receptor, RANK, expressed on osteoclast (Oc) precursors. In the presence of M-CSF, which acts on its receptor, c-fms, RANKL can enhance the formation of active osteoclasts that carry out bone resorption. Tumor cells have also been occasionally reported to directly release sRANKL, a soluble form of RANKL. Additionally, proteases can be produced by tumor cells and facilitate their invasion of nonmineralized tissue.
Mineralized bone matrix is a rich source of stored growth factors such as TGF-β. Such growth factors, once released from degraded bone matrix, may further accelerate growth of the tumor, which can now expand within the lysed area. Such growth factors also appear capable of further increasing the release from tumor cells of osteolytic mediators, such as parathyroid hormone–related peptide (PTHrP) (7, 8). A cycle may therefore be initiated that consists of release of osteolytic mediators by tumor cells, bone degradation, release of growth factors from degraded bone, enhanced tumor cell growth, and further release of osteolytic mediators (9).
Therapeutic implications for osteolytic cancers
The osteoclast offers a critical target for therapies designed to break this pathological cycle and help manage malignancy-induced osteolysis. By binding and neutralizing RANKL, OPG can diminish the production of functioning osteoclasts, and, indeed, this factor has been reported to block bone resorption in animal models of hypercalcemia of malignancy (10). Nevertheless, OPG appears to have little direct effect on tumor cell growth or survival. Bisphosphonates are potent bone resorption inhibitors that have a high affinity for mineralized matrix and are taken up by osteoclasts, in which they induce apoptosis (11). They too have been reported to diminish bone resorption in malignancy. Furthermore, some evidence indicates that bisphosphonates reduce adhesion (12) and induce apoptosis (13) in tumor cells metastatic to bone. Consequently the actions of OPG and bisphosphonates may be complementary.
Osteolysis and prostate cancer
Although less common than focal osteolysis, focal osteogenesis (which lead to the so called “osteoblastic” lesions) may occur in association with skeletal metastases of certain tumors, notably prostate cancer. Since such tumors must occupy space within the bone matrix, they too are invariably associated with osteoclastic osteolysis. There has been long-standing histological evidence for this, which has been confirmed by histomorphometric analyses as well as studies of biochemical markers of bone resorption. The osteogenic component of the skeletal reaction to prostate cancer, however, remains the most characteristic, the most intriguing, and the most enigmatic.
Because bone formation generally follows bone resorption during bone turnover, lesions of osteolytic tumor cells are generally associated with some evidence of bone repair. Occasionally, however, these events become uncoupled, as in tumors producing very high concentrations of PTHrP (14), or in association with multiple myeloma (15), where osteolysis seems to proceed virtually independently of new bone formation. In prostate cancer, the degree of osteogenesis appears in excess of that generally observed as part of the coupling process, tilting the balance toward new bone formation. The osteoblastic growth factors in prostate cancer cells (2) that drive this process are still not well understood, despite considerable efforts to unravel their effects on cancer-induced focal osteogenesis.
In this issue of the JCI, Zhang et al. (16) use a xenograft model to show that OPG inhibits osteoclastic osteolysis and tumor survival when administered to SCID mice injected within the tibia with a human prostate cancer cell line. Tumor cells directly produce a soluble form of RANKL (sRANKL), which appears to mediate tumor-induced osteoclastogenesis. This study provides good evidence for the critical role played by osteolysis in facilitating the establishment of tumor cells in bone and points to the use of OPG or other resorption inhibitors as early adjuvant therapy to prevent the spread of prostate tumor to bone. Clinical trials of bisphosphonates to reduce the incidence and sequelae of skeletal metastases in advanced breast cancer have to date met with only moderate success (17), but earlier introduction of bone resorption inhibitors could prove more helpful.
The observation by Zhang et al. (16) that their tumor cells themselves produce sRANKL is of interest, but whether human prostate cancer cells do so in vivo will require further study. In general, RANKL expression has not been reported in osteolytic tumor cells (18, 19), although one recent report identifies a soluble form of RANKL in a squamous cell carcinoma derived from a malignancy associated with hypercalcemia (20). The frequency with which this RANKL variant is produced remains to be determined.
Interestingly, Zhang et al. (16) observe that OPG treatment prevents osteoblastic as well as osteolytic lesions in their model. Although the pathogenesis of the osteoblastic lesions remains unknown, reducing the tumor burden in bone would certainly be beneficial no matter what the final mechanism of the tumor-induced osteogenesis. Overall, therefore, this study emphasize the critical role played by osteolysis for tumor cell colonization, as well as the importance of the RANKL system in this process. Inhibitors of bone resorption thus appear all the more promising as tools to manage skeletal metastases, especially if they can be introduced early in the course of cancer therapy.
References
- 1.Goltzman D. Mechanisms of the development of osteoblastic metastases. Cancer. 1997;80:1581–1587. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1581::aid-cncr8>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Goltzman D, Karaplis AC, Kremer R, Rabbani SA. Molecular basis of the spectrum of skeletal complications of neoplasia. Cancer. 2000;88:2903–2908. doi: 10.1002/1097-0142(20000615)88:12+<2903::aid-cncr4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Eilon G, Mundy GR. Direct resorption of bone by human breast cancer cells in vitro. Nature. 1978;276:726–728. doi: 10.1038/276726a0. [DOI] [PubMed] [Google Scholar]
- 4.Suda T, Kobayashi K, Jimi E, Udagawa N, Takahashi N. The molecular basis of osteoclast differentiation and activation. Novartis Found Symp. 2001;232:235–247; discussion 247–250. doi: 10.1002/0470846658.ch16. [DOI] [PubMed] [Google Scholar]
- 5.Kong YY, Boyle WJ, Penninger JM. Osteoprotegerin ligand: a regulator of immune responses and bone physiology. Immunol Today. 2000;21:495–502. doi: 10.1016/s0167-5699(00)01718-7. [DOI] [PubMed] [Google Scholar]
- 6.Lee SK, Lorenzo JA. Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclast-like cell formation. Endocrinology. 1999;140:3552–3561. doi: 10.1210/endo.140.8.6887. [DOI] [PubMed] [Google Scholar]
- 7.Guise TA. Molecular mechanisms of osteolytic bone metastases. Cancer. 2000;88:2892–2898. doi: 10.1002/1097-0142(20000615)88:12+<2892::aid-cncr2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Rabbani SA, Gladu J, Harakidas P, Jamison B, Goltzman D. Over-production of parathyroid hormone-related peptide results in increased osteolytic skeletal metastasis by prostate cancer cells in vivo. Int J Cancer. 1999;80:257–264. doi: 10.1002/(sici)1097-0215(19990118)80:2<257::aid-ijc15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Chirgwin JM, Guise TA. Molecular mechanisms of tumor-bone interactions in osteolytic metastases. Crit Rev Eukaryot Gene Expr. 2000;10:159–178. [PubMed] [Google Scholar]
- 10.Capparelli C, et al. Osteoprotegerin prevents and reverses hypercalcemia in a murine model of humoral hypercalcemia of malignancy. Cancer Res. 2000;60:783–787. [PubMed] [Google Scholar]
- 11.Rogers MJ, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–2978. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 12.Van der Pluijim G, et al. Bisphosphonates inhibit the adhesion of breast cancer cells to bone matrices in vitro. J Clin Invest. 1996;98:698–704. doi: 10.1172/JCI118841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senaratne SG, Pirianov G, Mansi JL, Arnett TR, Colston KW. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer. 2000;82:1459–1468. doi: 10.1054/bjoc.1999.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart AF, et al. Quantitative bone histomorphometry in humoral hypercalcemia of malignancy: uncoupling of bone cell activity. J Clin Endocrinol Metab. 1982;55:219–227. doi: 10.1210/jcem-55-2-219. [DOI] [PubMed] [Google Scholar]
- 15.Woitge HW, et al. Biochemical markers of bone formation in patients with plasma cell dyscrasias and benign osteoporosis. Clin Chem. 2001;47:686–693. [PubMed] [Google Scholar]
- 16.Zhang J, et al. Osteoprotegerin inhibits prostate cancer–induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest. 2001;107:1235–1244. doi: 10.1172/JCI11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berenson JR, Lipton A. Bisphosphonates in the treatment of malignant bone disease. Annu Rev Med. 1999;50:237–248. doi: 10.1146/annurev.med.50.1.237. [DOI] [PubMed] [Google Scholar]
- 18.Thomas RJ, et al. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology. 1999;140:4451–4458. doi: 10.1210/endo.140.10.7037. [DOI] [PubMed] [Google Scholar]
- 19.Chikatsu N, et al. Interactions between cancer and bone marrow cells induce osteoclast differentiation factor expression and osteoclast-like cell formation in vitro. Biochem Biophys Res Commun. 2000;267:632–637. doi: 10.1006/bbrc.1999.2008. [DOI] [PubMed] [Google Scholar]
- 20.Nagai M, Kyakumoto S, Sato N. Cancer cells responsible for humoral hypercalcemia express mRNA encoding a secreted form of ODF-TRANCE that induces osteoclast formation. Biochem Biophys Res Commun. 2000;269:532–536. doi: 10.1006/bbrc.2000.2314. [DOI] [PubMed] [Google Scholar]