Prostaglandins were first discovered by von Euler in the late 1940s to be active principles in semen that induced uterine contractility. Since then, interest in the field has waxed and waned. Indeed, through much of the 50s and 60s, prostaglandin research remained the arcane preserve of the dedicated few. It maintained a continuing emphasis on reproductive biology but was eclipsed in the popular vision by those other stepchildren of von Euler’s creativity, the catecholamines.
Because fashion is as ephemeral in science as it is elsewhere, disciplines may gain or lose broad appeal for reasons that have little to do with their significance. G.A. FitzGerald was drawn to the field by an article that nicely captured the rising wave of popularity that prostaglandin research was to enjoy by the end of the 70s. This article (1) by John Vane and his colleagues in London chronicled the elegant application of superfusion bioassay to the discovery and characterization of the bioactivity of prostaglandins and, most importantly, to elucidating the role of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) as cyclooxygenase (COX) inhibitors (2). This work synergized nicely with the characterization of prostanoid structures and the discovery of the lipoxygenase pathways of arachidonic acid (AA) metabolism, which were to deliver the Nobel Prize to Bergstrom, Samuelsson, and Vane in 1982 (3).
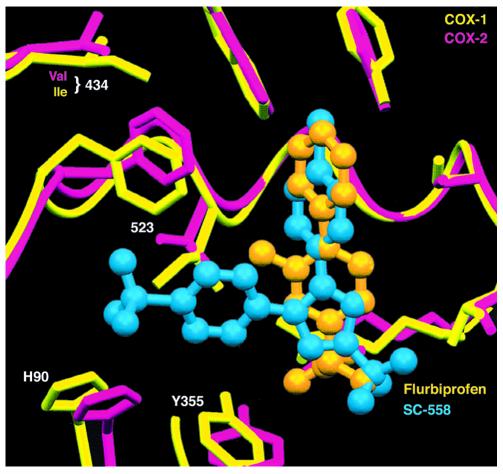
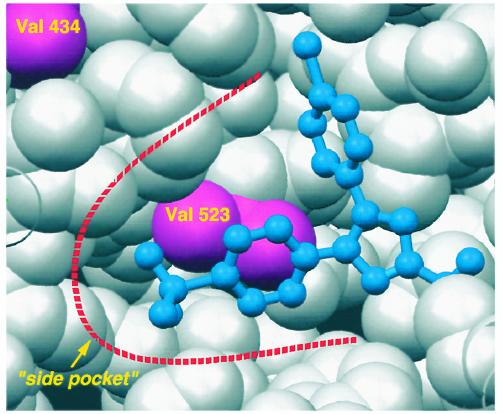
Since then, interest in the field has waned. However, the persistent focus of the few has yielded dividends. The advent of molecular methodology permitted the cloning and characterization of COX by DeWitt and Smith (4) and the discovery of a second form of the enzyme (5–7) more relevant to prostanoid formation in inflammation and cancer. Crystallization of both COXs revealed the molecular basis of aspirin action, initially described by Roth, Stanford, and Majerus (8), at the atomic level (9, 10). Both COXs are membrane-anchored proteins that exist as dimers and have remarkable structural similarity (Figure 1). The substrate AA gains access to the active site via a hydrophobic channel and access is blocked by interpolation of an acetyl residue on Ser 530 (Ser 516 in COX-2). The irreversibility of this interaction and the unique expression of COX-1 in the anucleate platelet underlies the clinical efficacy of low-dose aspirin (11). NSAIDs, by contrast, interact competitively with the active site (Figure 2) and, indeed, predosing with NSAIDs may interfere with the sustained antiplatelet effects of aspirin (12). While the tertiary structures of both COX isozymes are remarkably similar, COX-2 is characterized by a side pocket extension to the hydrophobic channel. Although the initial selective COX-2 inhibitors were discovered with the tools of classical biochemical pharmacology, structural studies reveal their localization in the side pocket (Figure 3), where they interact with slow, tight-binding kinetics (13).
Figure 1.
The COX-1 and COX-2 backbones, overlaid. COX-1 is shown in yellow, and COX-2 in pink. Note how the two structures are almost perfectly superimposable. The amphipathic helices that form the site of monotopic membrane attachment are indicated. The peroxidase (POX) active site lies on the opposite side of the molecule from the entrance to the COX active site channel. The actual position of the COX active center is marked by the asterisk, found near the center of the molecule.
Figure 2.
Isoform-selective inhibitor binding. The COX-1 and COX-2 active sites are shown superimposed (COX-1, yellow; COX-2, pink). Two inhibitors are seen: flurbiprofen (orange), a nonselective inhibitor, and SC-558 (blue), a COX-2–selective inhibitor. NSAIDs achieve COX inhibition by occupying the upper portion of the active site channel, preventing the fatty acid substrate from gaining access to the active site tyrosine seen at the upper right. Note how the COX-2–selective inhibitor projects leftward into a side pocket that is not exploited by the nonselective inhibitor.
Figure 3.
A view of the “side pocket” found at the side of the COX-2 active site. The COX-2–selective inhibitor SC-558 is shown in blue, bound in the active site. Protein residues are shown as van der Waals spheres; valine 434 and valine 523 are shown in pink. In COX-1, both these residues are isoleucines; the additional bulk contributed by the two extra methyl groups is sufficient to close down this small alcove so that no side pocket is found in COX-1.
The advent of COX-2 inhibitors and their introduction into clinical practice (14, 15) has rendered prostaglandin research fashionable (not to mention profitable) again. Before this shifting spotlight moves on, a review of contemporary prostanoid biology seems apposite. However, this series attempts to move beyond a simple recitation of developed expertise. In some cases, it challenges authors to address largely uncharted territory — the role and mechanism of action of AA itself rather than its products, for example. In others, it juxtaposes the perspectives of investigators with distinct areas of expertise; for example, proficiency in the structural biology of COXs with experience in COX gene inactivation. Finally, authors were encouraged to address themselves critically to outstanding issues; the potential role of prostanoids as nuclear receptor ligands, the biology and biochemistry of unorthodox products of the pathway, contemporary concepts of prostanoid disposition and the emerging pharmacology of COX inhibitors. It is hoped that this approach will serve both to update the general reader, but also to whet the jaded appetite of the COX-2 fashionista and prostanoid road warriors alike as they contemplate the challenges of this field in the years to come.
References
- 1.Gryglewski R, Vane JR. The release of prostaglandins and rabbit aorta contracting substance (RCS) from rabbit spleen and its antagonism by anti-inflammatory drugs. Br J Pharmacol. 1972;45:37–47. doi: 10.1111/j.1476-5381.1972.tb09574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moncada S, Ferreira SH, Vane JR. Prostaglandins, aspirin-like drugs and the oedema of inflammation. Nature. 1973;246:217–219. doi: 10.1038/246217a0. [DOI] [PubMed] [Google Scholar]
- 3.Oates JA. The 1982 Nobel prize in physiology or medicine. Science. 1982;218:765–768. doi: 10.1126/science.6753151. [DOI] [PubMed] [Google Scholar]
- 4.DeWitt DL, Smith WL. Primary structure of prostaglandin G/H synthase from sheep vesicular gland determined from the complementary DNA sequence. Proc Natl Acad Sci USA. 1988;85:1412–1416. doi: 10.1073/pnas.85.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie W, Chipman JG, Robertson DL, Erikson RL, Simmons DL. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci USA. 1991;88:2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kujubu DA, Herschman HR. Dexamethasone inhibits mitogen induction of the TIS10 prostaglandin synthase/cyclooxygenase gene. J Biol Chem. 1992;267:7991–7994. [PubMed] [Google Scholar]
- 7.O’Banion MK, Winn VD, Young DA. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc Natl Acad Sci USA. 1992;89:4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth GJ, Stanford N, Majerus PW. Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci USA. 1975;72:3073–3076. doi: 10.1073/pnas.72.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loll PJ, Picot D, Garavito RM. The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nat Struct Biol. 1995;2:637–643. doi: 10.1038/nsb0895-637. [DOI] [PubMed] [Google Scholar]
- 10.Kurumbail RG, Stevens AM, Gierse JK. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384:644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 11.Patrono C. Aspirin as an antiplatelet drug. N Engl J Med. 1994;330:1287–1294. doi: 10.1056/NEJM199405053301808. [DOI] [PubMed] [Google Scholar]
- 12.Catella-Lawson, F., et al. 2000. Non-steroidal anti-inflammatory drugs antagonize the irreversible anti-platelet effect of aspirin. Suppl. Br. J. Clin. Pharmacol. p. 26, no. 95.
- 13.Kalgutkar AS, et al. Biochemically based design of cyclooxygenase-2 (COX-2) inhibitors: facile conversion of nonsteroidal antiinflammatory drugs to potent and highly selective COX-2 inhibitors. Proc Natl Acad Sci USA. 2000;97:925–930. doi: 10.1073/pnas.97.2.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverstein FE, Faich G, Goldstein JL. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: The CLASS Study. A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 15.Bombardier C, et al. A double-blind comparison of rofecoxib and naproxen on the incidence of clinically important upper gastrointestinal events: The VIGOR Trial. N Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]