Between 1965 and the mid-1980s, investigators laid a durable foundation for understanding the regulated formation and metabolic disposition of eicosanoids. First, they sought, and found in arachidonic acid, the biosynthetic precursor for the prostaglandins. Second, they identified phospholipids as the cellular compartment that harbored arachidonic acid, and they identified phospholipases as the enzymes essential for its liberation and for the ensuing biosynthesis of prostaglandins (1). Third, they deduced the existence of transient intermediates in the prostanoid biosynthetic pathway, leading to the eventual discovery of the prostaglandin endoperoxides, PGG2 and PGH2 (2, 3). Fourth, they established that the enzyme PGH synthase, or cyclooxygenase (COX), was a molecular target of great medical significance in reproductive, cardiovascular, and inflammatory disorders (4). Fifth, they established that rapid, comprehensive pulmonary metabolism limited the steady-state levels and duration of action of prostanoids, implying that they acted as autocoid lipid mediators, not hormones. Finally, they identified new prostanoids (5, 6) and their biosynthetic enzymes, as well as new lipoxygenase enzymatic pathways and their leukotriene products (7, 8), as medically significant molecules.
Over this period, the most prominent themes in eicosanoid research were the identification of novel eicosanoid mediators, the determination of their molecular structures, and the establishment of their pharmacological activities. In a typical study of the time, investigators exposed tissues or cells to 50–100 μM of exogenous arachidonic acid, a concentration five- to tenfold greater than the Km for COX or lipoxygenases (Km ≈ 10 μM) (see Brash, this Perspective series, ref. 9). The contemporary model for eicosanoid biosynthesis, circa 1985, asserted that liberation of free arachidonic acid by phospholipase was the rate-limiting step in biosynthesis. Accordingly, providing saturating amounts of exogenous arachidonic acid ought to reveal all biosynthetic products and pathways.
By 1985 or thereabouts, the quest for novel eicosanoid mediators of medical significance had reached the point of diminishing returns. Simultaneously, more and more investigators sought to understand the role of eicosanoid biosynthesis in disease processes. Accordingly, investigators shifted from exogenous arachidonic acid and Ca2+ ionophore as preferred tools and embraced natural ligands, relevant to disease, to initiate receptor-coupled activation of eicosanoid formation.
By the late 1980s, two lines of experimental inquiry began to provoke a reconsideration and refinement of the accepted model of eicosanoid biosynthesis. First, kinetic and quantitative aspects of eicosanoid biosynthesis initiated by growth factors or cytokines on mitotically competent cells suggested that it was an oversimplification to regard availability of arachidonic acid as the sole, rate-limiting step in cellular eicosanoid biosynthesis. Second, the discovery of the 5-lipoxygenase activating protein (FLAP) in 1990 proved unambiguously that some eicosanoids (leukotrienes) originated under conditions that were not rate-limited by arachidonic acid availability. The discovery of novel regulatory processes, particularly Ca2++-dependent redistribution of 5-lipoxygenase (5-LO) and the interaction between 5-LO and FLAP (10), offered a new framework on which to reconstruct the earlier model of eicosanoid biosynthesis.
Here, we comment on several mechanisms through which cells attain more subtle control of eicosanoid biosynthesis than would be possible by simply limiting the availability of arachidonic acid. We first discuss the coordinated action of specific phospholipases, the enzymes that generate this substrate, with specific COXs and PGH isomerase enzymes. We then consider the restricted expression of eicosanoid biosynthetic enzymes and, finally, turn to the “suicide” inactivation of these biosynthetic enzymes, a general mechanism that may help terminate their proinflammatory function.
Functional coupling of phospholipases, COXs, and PGH isomerases
In 1989 Lin et al. (11) provided an accurate glimpse of two regulatory processes that have assumed great importance during the past decade. First, they showed that PDGF, a typical growth factor, caused a sustained increase in PGE2 biosynthesis by NIH 3T3 cells, commencing within 10 minutes and reaching a maximum after 2 hours. Second, they showed that cycloheximide inhibited more than 90% of the PGE2 synthesis initiated by PDGF, suggesting that de novo protein synthesis, presumably de novo synthesis of the COX enzyme, contributed to the process. However, additional experiments in which NIH 3T3 cells were exposed to exogenous arachidonic acid, instead of PDGF, showed that these cells had ample basal capacity for COX-catalyzed formation of PGE2. Furthermore, the steady-state level of COX mRNA in NIH 3T3 cells rose, but this rise followed, rather than preceded, the increase in PGE2 synthesis. Finally, COX protein levels did not rise appreciably following PDGF stimulation, even when the corresponding mRNA levels were elevated.
These data prompted two questions. First, why would cells rely on de novo synthesis of COX enzyme if they already had sufficient COX enzymatic capacity to convert any available arachidonic acid into PGE2? Second, if de novo synthesis of COX enzyme did account for increased PGE2 formation in cells incubated with PGDF, why were the temporal and stoichiometric relationships between mRNA accumulation, protein accumulation, and PGE2 formation so distorted? Herschman and colleagues’ discovery (12) of the COX-2 isoenzyme (see Smith and Langenbach, this Perspective series, ref. 13) would eventually clarify the quantitative and temporal distortions observed by Lin et al. (11). However, absent this discovery, Lin et al. proposed that coupling of the PDGF receptor to phospholipase and ultimately to COX relied on de novo synthesis of an unidentified protein. In other words, the PDGF-mediated increase in cellular PGE2 synthesis depends on de novo synthesis of proteins that coordinate the availability of arachidonic acid to its metabolism by COX during growth factor receptor occupancy. This hypothesis remains attractive and active today. For instance, Murakami et al. (14) have proposed the existence of a hypothetical accessory protein that integrates phospholipase–COX-2 interactions to explain how COX-2 expression enables cPLA2 activation. Given the renewed interest in functional coupling among the enzymes of the arachidonic acid cascade (15), it will be interesting to see if as-yet unidentified coupling proteins promote interactions among component receptors and enzymes.
During the past year the concept of coupling among the phospholipase-COX-PGH isomerase enzymes has gained particular experimental support and clarity from the investigations by Kudo and colleagues (14, 16, 17). By expressing any of several forms of phospholipase A2 (the Ca2+-dependent cytosolic phospholipase cPLA2, the secretory phospholipase sPLA2; or the Ca2+-independent phospholipase iPLA2) in combination with ectopically expressed COX-1 or COX-2, these authors have established a hierarchy of functional interactions among the enzymes.
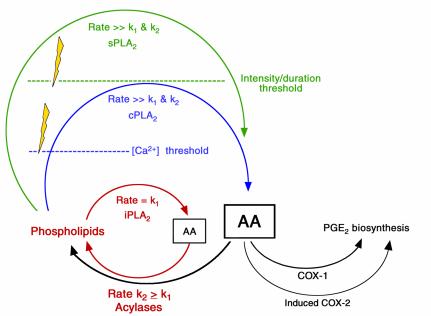
First, under what might be termed basal conditions (Figure 1, red text and arrows), the Ca2+-independent iPLA2 is the dominant phospholipase involved in the liberation of arachidonic acid and related polyunsaturated fatty acids from membrane phospholipids. iPLA2 serves primarily in cell membrane remodeling and does not induce eicosanoid biosynthesis because, under basal conditions, the rate of arachidonic release via iPLA2 is less than or equal to the rate of its reincorporation into cell membranes; thus there is negligible accumulation of free arachidonic acid substrate for conversion by either COX isoenzyme. Effectively, the acylase enzymes competitively inhibit the COX isoenzymes.
Figure 1.
Stages of eicosanoid biosynthesis. Under basal conditions, release and metabolism of arachidonic acid (AA) are balanced, leaving little AA available as a substrate for COXs, so little prostaglandin E2 is formed. With the breaching (lightning bolts) of two successive metabolic thresholds, two additional phospholipases are activated, permitting the accumulation of AA and the formation of its downstream metabolites. Prior to stimulation, the cycle shown in red predominates, wherein membrane phospholipids are hydrolyzed by the phospholipase A2 isoform iPLA2, yielding low quantities of AA that are recycled by the action of acylases to reform membrane phospholipid. However, following growth factor receptor activation, Ca2+ levels rise and the inducible phospholipase cPLA2 (shown in blue) begins to generate sufficient AA that COX-1 and, later, COX-2 become active. COX-1 activity is coupled to a cytosolic form of the PGH-PGE isomerase, whereas COX-2 is coupled to a membrane-bound form of the enzyme. Following prolonged receptor occupancy and activation, yet another phospholipase, sPLA2 (shown in green), is induced. This enzyme is secreted and can therefore activate prostaglandin synthesis in neighboring cells by a paracrine mechanism.
Second, following receptor activation, intracellular Ca2+ levels rise abruptly, exceeding a Ca2+ concentration threshold. At this point, the Ca2+-dependent cPLA2 becomes the dominant phospholipase involved in liberation of arachidonic acid from membrane phospholipids (Figure 1, blue text and arrows). During receptor activation, the rate of arachidonic acid release by cPLA2 exceeds the rate of its reincorporation into cell membranes; thus there is sufficient accumulation of free arachidonic acid substrate for metabolism by either of the COX isoenzymes or both together. The model implies that inaugural formation of PGE2 involves preferential coupling between cPLA2 and COX-1 and a cytosolic PGH-PGE isomerase, all of which are expressed constitutively. As time passes and conditions favor the induction of COX-2 and a membrane-bound PGH-PGE isomerase, terminal formation of PGE2 involves coupling between cPLA2 and these latter two enzymes.
Finally, when exposure to receptor ligands is enduring and intense, the inducible, secreted sPLA2 isoenzyme begins to participate, creating an amplification loop to align arachidonic acid availability with the sustained capacity for biosynthesis by inducible COX-2 and inducible PGH-PGE isomerase-2. This paracrine amplification loop helps release eicosanoid formation from its focal origins, allowing it to spread to surrounding cells (Figure 1, green text and arrows).
We draw attention to two further observations by Kudo and colleagues (14, 16, 17). First, COX-1 and COX-2 have rather similar Km for arachidonic acid, yet it is COX-2 that metabolizes the bulk of the available arachidonic acid when the substrate is present at low concentrations (see Smith and Langenbach, this Perspective series, ref. 13). Studies of COX enzyme kinetics by Kulmacz and colleagues (18) point to cellular levels of hydrogen peroxide, which may contribute to COX activation, as a basis for this phenomenon, but it is an unusual trait that warrants investigation. Second, the occurrence of the PGH-PGE isomerase enzymes within discrete cellular compartments, cytosol and membrane, corresponds with but does not necessarily explain their preferential coupling to COX-1 and COX-2. A compartmentalization mechanism seems flawed, since both COX isoenzymes are located on membranes. In seeking an explanation, one is drawn, again, to hypothetical proteins (11, 12) that might couple the separate steps depicted in Figure 1.
Restricted expression of oxygenase and isomerase enzymes
Restricted enzyme expression provides a second mechanism for regulating eicosanoid biosynthesis. For example, restricted expression of thromboxane (Tx) synthase in platelets and restricted expression of PGI2 synthase in endothelial cells account for the medical benefits of low-dose aspirin in thrombo-occlusive disorders (19). The distribution also accounts for the prothrombotic properties of platelets and the antithrombotic properties of the vascular endothelium (20, 21). Restricted expression of 5-LO is also critical for the so-called transcellular biosynthesis of leukotrienes, in which an eicosanoid precursor generated by granulocytes or other cells can be enzymatically activated by platelets or endothelial cells (22–24). Likewise, the inducible expression of COX-2 during inflammation provides the rationale for using COX-2–selective inhibitors in treating rheumatological disorders and helps explain the general observation that COX-1 participates in physiological processes whereas COX-2 acts in disease.
Emerging evidence suggests that restricted expression of COX-2 will be as important in oncology and developmental biology as it has been in understanding inflammation. For instance, nonsteroidal anti-inflammatory drugs (NSAIDs) that inhibit COX-1 and COX-2 with comparable efficacy have been associated with cleft palate during embryogenesis in experimental animals and in humans (25). FDA-approved COX-2–selective inhibitors presumably have no greater liability than do nonselective inhibitors. As their use grows it will be interesting to see whether they have reduced liability. In a different developmental context, recent experiments show an obligate role for COX isoenzymes in T cell maturation in the fetal thymus. Rocca et al. (26) propose a minimal model suggesting that PGE2 formation by COX-1 converts CD4–/CD8– thymocytes into CD4+/CD8+ and that PGE2 formation by COX-2 subsequently converts CD4+/CD8+ into the CD4+ lineage. This investigation provides a glimpse of the type of experimental design, involving both genetic and pharmacological experiments, that seems necessary to investigate the role of eicosanoid enzymes in developmental biology.
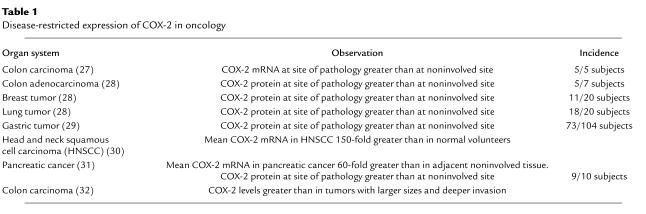
Disease-restricted expression of COX-2 is not limited to inflammation. Several types of neoplastic and preneoplastic tissue overexpress COX-2, compared with adjacent noninvolved tissue, with incidences ranging from about 50–90% in studies involving a minimum of ten subjects (27–32) (Table 1). Is this induction a cause or an effect of tumor progression? Investigations with the Min+/– mouse model of intestinal polyposis suggest that COX-2 expression favors progression from a preneoplastic to a neoplastic condition. Chulada et al. (33) report that homologous disruption of the COX-2 gene diminishes polyp formation in Min+/– mice by about 80%. Investigations with these mice also suggest that the COX-1 isoenzyme participates in tumorigenesis. Episodically, investigators have reported that stromal tissue adjacent to tumor harbors elevated levels of the COX-1 isoenzyme, not COX-2 (34). Such reports reinforce the notion that disease-restricted expression of COX-2 is associated directly with the disease at the cellular level, but they also indicate that altered eicosanoid biosynthesis occurring as an adaptation to the diseased state of neighboring cells can be mediated by COX-1.
Table 1.
Disease-restricted expression of COX-2 in oncology
The high expression of COX-2 in neoplastic tissue derives, in part, from the accumulation of somatic mutations that persistently activate the ras pathway or various receptor tyrosine kinase pathways. Most tumors should exhibit this trait, but to explain the minority of cases without COX-2 expression, Toyota et al. (35) examined CpG island methylation in COX-2 and found that methylation of CpG islands near exon 1 silenced COX-2 expression in 12 of 92 (13%) sporadic colorectal cancers and 7 of 50 (14%) colorectal adenomas. This observation has important implications. First, adenomas with a methylation-silenced COX-2 gene may have a diminished response to treatment with COX-2–specific inhibitors or a better prognosis, depending on what other genetic defects are harbored by the tumor. Second, and more speculatively, a similar mechanism may apply to other oxygenase genes that are induced downstream of tumorigenic signaling pathways. Consistent with a number of other reports, Gao et al. (36) have shown that 12-lipoxygenase is expressed in a disease-restricted fashion in prostate cancer. Among 122 subjects examined, 12-lipoxygenase expression levels were elevated in about 50%, and 12-lipoxygenase elevation correlated with stage. It would be interesting to determine whether CpG methylation silences transcription of 12-lipoxygenase or other lipoxygenase enzymes in tumors that fail to show this inductive response.
“Suicide inactivation” of eicosanoid biosynthetic enzymes
The regulated formation of eicosanoids, as depicted in Figure 1, implies that cells have appreciable ability to amplify the rate and amount of eicosanoid biosynthesis. Once these processes reach maximal velocity, how can the cell return to its basal state? No doubt, part of the answer involves silencing the expression of sPLA2, COX-2, and the other biosynthetic enzymes, but a phenomenon termed “suicide inactivation,” or mechanism-based autoinactivation, could also impose a vital, but overlooked, limit on eicosanoid biosynthesis. This process occurs for COX (37), Tx synthase (38), PGI synthase (39), leukotriene A4 hydrolase (40), and the 5-, 12-, and 15-lipoxygenases (41, 42).
Suicide inactivation is often regarded as a special tactic for developing irreversible pharmacological inhibitors. It is seldom regarded as a regulatory process. However, many substrate-product pairs in eicosanoid biosynthesis contain at least one electrophile, which might be expected to interact with and irreversibly modify the enzyme involved. In this context, it seems less surprising that these enzymes are subject to two alternative fates. One fate is a normal catalytic cycle, yielding product and regenerating enzyme. A second fate is a catalytic cycle leading to irreversible inactivation of the enzyme instead of product formation.
Little is known about the intracellular degradation of COX-2 protein, or other suicide-inactivated proteins. For instance, how is autoinactivated COX recognized and targeted for degradation? Is this different from degradation of active COX? Does cellular accumulation of suicide-inactivated enzyme account, in part, for any poor correlation between protein expression and product formation (e.g., COX-2 protein versus PGE2)? If protein degradation processes fail to degrade suicide-inactivated enzymes, are there any cellular consequences? The implications of suicide inactivation have not yet been fully appreciated, or fully integrated, into models of eicosanoid biosynthesis. Future efforts to correlate the amount of cellular enzymes with the amount of product will need to take into account the catalytic history of the enzyme.
Acknowledgments
F.A. Fitzpatrick is the Dee Glenn & Ida Smith Chair of Cancer Research and an investigator of the Huntsman Cancer Institute. This work was supported by RO1 AI26730 (to F.A. Fitzpatrick), R01 GM061823, and a gift from the Jewish Communal Fund (to R. Soberman).
References
- 1.Anggard E, Samuelsson B. Biosynthesis of prostaglandins from arachidonic acid in guinea pig lung. Prostaglandins and related factors. 38. J Biol Chem. 1965;240:3518–3521. [PubMed] [Google Scholar]
- 2.Lands WE, Samuelsson B. Phospholipid precursors of prostaglandins. Biochim Biophys Acta. 1968;164:426–429. doi: 10.1016/0005-2760(68)90168-9. [DOI] [PubMed] [Google Scholar]
- 3.Hamberg M, Svensson J, Samuelsson B. Prostaglandin endoperoxides. A new concept concerning the mode of action and release of prostaglandins. Proc Natl Acad Sci USA. 1974;71:3824–3828. doi: 10.1073/pnas.71.10.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nugteren DH, Hazelhof E. Isolation and properties of intermediates in prostaglandin biosynthesis. Biochim Biophys Acta. 1973;326:448–461. doi: 10.1016/0005-2760(73)90145-8. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira SH, Moncada S, Vane JR. Indomethacin and aspirin abolish prostaglandin release from the spleen. Nat New Biol. 1971;231:237–239. doi: 10.1038/newbio231237a0. [DOI] [PubMed] [Google Scholar]
- 6.Hamberg M, Svensson J, Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci USA. 1975;72:2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nugteren DH. Arachidonate lipoxygenase in blood platelets. Biochim Biophys Acta. 1975;380:299–307. doi: 10.1016/0005-2760(75)90016-8. [DOI] [PubMed] [Google Scholar]
- 8.Borgeat P, Hamberg M, Samuelsson B. Transformation of arachidonic acid and homo-gamma-linolenic acid by rabbit polymorphonuclear leukocytes. Monohydroxy acids from novel lipoxygenases. J Biol Chem. 1976;251:7816–7820. [PubMed] [Google Scholar]
- 9.Brash AR. Arachidonic acid as a bioactive molecule. J Clin Invest. 2001;107:1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon RA, et al. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990;343:282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- 11.Lin AH, Bienkowski MJ, Gorman RR. Regulation of prostaglandin H synthase mRNA levels and prostaglandin biosynthesis by platelet-derived growth factor. J Biol Chem. 1989;264:17379–17283. [PubMed] [Google Scholar]
- 12.Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991;266:12866–12872. [PubMed] [Google Scholar]
- 13.Smith, W.L., and Langenbach, R. 2001. Why there are two cyclooxygenase isozymes. J. Clin. Invest. In press. [DOI] [PMC free article] [PubMed]
- 14.Murakami M, Kambe T, Shimbara S, Kudo I. Functional coupling between various phospholipase A2s and cyclooxygenases in immediate and delayed prostanoid biosynthetic pathways. J Biol Chem. 1999;274:3103–3115. doi: 10.1074/jbc.274.5.3103. [DOI] [PubMed] [Google Scholar]
- 15.Reddy ST, Herschman HR. Prostaglandin synthase-1 and prostaglandin synthase-2 are coupled to distinct phospholipases for the generation of prostaglandin D2 in activated mast cells. J Biol Chem. 1997;272:3231–3237. doi: 10.1074/jbc.272.6.3231. [DOI] [PubMed] [Google Scholar]
- 16.Tanioka T, Nakatani Y, Semmyo N, Murakami M, Kudo I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J Biol Chem. 2000;275:32775–32782. doi: 10.1074/jbc.M003504200. [DOI] [PubMed] [Google Scholar]
- 17.Murakami M, et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem. 2000;275:32783–32792. doi: 10.1074/jbc.M003505200. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Pawelek TR, Kulmacz RJ. Hydroperoxide dependence and cooperative cyclooxygenase kinetics in prostaglandin H synthase-1 and -2. J Biol Chem. 1999;274:20301–20306. doi: 10.1074/jbc.274.29.20301. [DOI] [PubMed] [Google Scholar]
- 19.Clarke RJ, Mayo G, Price P, FitzGerald GA. Suppression of thromboxane A2 but not of systemic prostacyclin by controlled-release aspirin. N Engl J Med. 1991;325:1137–1141. doi: 10.1056/NEJM199110173251605. [DOI] [PubMed] [Google Scholar]
- 20.Marcus AJ, Weksler BB, Jaffe EA, Broekman MJ. Synthesis of prostacyclin from platelet-derived endoperoxides by cultured human endothelial cells. J Clin Invest. 1980;66:979–986. doi: 10.1172/JCI109967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowak J, FitzGerald GA. Redirection of prostaglandin endoperoxide metabolism at the platelet-vascular interface in man. J Clin Invest. 1989;83:380–385. doi: 10.1172/JCI113895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGee JE, Fitzpatrick FA. Erythrocyte-neutrophil interactions: formation of leukotriene B4 by transcellular biosynthesis. Proc Natl Acad Sci USA. 1986;83:1349–1353. doi: 10.1073/pnas.83.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maclouf JA, Murphy RC. Transcellular metabolism of neutrophil-derived leukotriene A4 by human platelets. A potential cellular source of leukotriene C4. J Biol Chem. 1988;263:174–181. [PubMed] [Google Scholar]
- 24.Feinmark SJ, Cannon PJ. Endothelial cell leukotriene C4 synthesis results from intercellular transfer of leukotriene A4 synthesized by polymorphonuclear leukocytes. J Biol Chem. 1986;261:16466–16472. [PubMed] [Google Scholar]
- 25.Saxen I. Associations between oral clefts and drugs taken during pregnancy. Int J Epidemiol. 1975;4:37–44. doi: 10.1093/ije/4.1.37. [DOI] [PubMed] [Google Scholar]
- 26.Rocca B, et al. Distinct roles of prostaglandin H synthases 1 and 2 in T-cell development. J Clin Invest. 1999;103:1469–1477. doi: 10.1172/JCI6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kutchera W, et al. Prostaglandin H synthase 2 is expressed abnormally in human colon cancer: evidence for a transcriptional effect. Proc Natl Acad Sci USA. 1996;93:4816–4820. doi: 10.1073/pnas.93.10.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soslow RA, et al. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–2645. doi: 10.1002/1097-0142(20001215)89:12<2637::aid-cncr17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 29.Lim HY, et al. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res. 2000;6:519–525. [PubMed] [Google Scholar]
- 30.Chan G, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- 31.Tucker ON, et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–990. [PubMed] [Google Scholar]
- 32.Fujita T, et al. Size- and invasion-dependent increase in cyclooxygenase 2 levels in human colorectal carcinomas. Cancer Res. 1998;58:4823–4826. [PubMed] [Google Scholar]
- 33.Chulada PC, et al. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res. 2000;60:4705–4708. [PubMed] [Google Scholar]
- 34.Hwang D, Scollard D, Byrne J, Levine E. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst. 1998;90:455–460. doi: 10.1093/jnci/90.6.455. [DOI] [PubMed] [Google Scholar]
- 35.Toyota M, et al. Aberrant methylation of the Cyclooxygenase-2 CpG island in colorectal tumors. Cancer Res. 2000;60:4044–4048. [PubMed] [Google Scholar]
- 36.Gao X, Porter AT, Honn KV. Involvement of the multiple tumor suppressor genes and 12-lipoxygenase in human prostate cancer. Therapeutic implications. Adv Exp Med Biol. 1997;407:41–53. doi: 10.1007/978-1-4899-1813-0_7. [DOI] [PubMed] [Google Scholar]
- 37.Smith WL, Lands WE. Oxygenation of polyunsaturated fatty acids during prostaglandin biosynthesis by sheep vesicular gland. Biochemistry. 1972;11:3276–3285. doi: 10.1021/bi00767a024. [DOI] [PubMed] [Google Scholar]
- 38.Jones DA, Fitzpatrick FA. “Suicide” inactivation of thromboxane A2 synthase. Characteristics of mechanism-based inactivation with isolated enzyme and intact platelets. J Biol Chem. 1990;265:20166–20171. [PubMed] [Google Scholar]
- 39.Wade ML, Voelkel NF, Fitzpatrick FA. "Suicide" inactivation of prostaglandin I2 synthase: characterization of mechanism-based inactivation with isolated enzyme and endothelial cells. Arch Biochem Biophys. 1995;321:453–458. doi: 10.1006/abbi.1995.1417. [DOI] [PubMed] [Google Scholar]
- 40.Orning L, Jones DA, Fitzpatrick FA. Mechanism-based inactivation of leukotriene A4 hydrolase during leukotriene B4 formation by human erythrocytes. J Biol Chem. 1990;265:14911–14916. [PubMed] [Google Scholar]
- 41.Smith WL, Lands WE. The self-catalyzed destruction of lipoxygenase. Biochem Biophys Res Commun. 1970;41:846–851. doi: 10.1016/0006-291x(70)90160-9. [DOI] [PubMed] [Google Scholar]
- 42.Lepley RA, Fitzpatrick FA. Irreversible inactivation of 5-lipoxygenase by leukotriene A4. Characterization of product inactivation with purified enzyme and intact leukocytes. J Biol Chem. 1994;269:2627–2631. [PubMed] [Google Scholar]