Abstract
Mitogen-activated protein kinase (MAPK) cascades are involved in inflammation and tissue destruction in rheumatoid arthritis (RA). In particular, c-Jun N-terminal kinase (JNK) is highly activated in RA fibroblast-like synoviocytes and synovium. However, defining the precise function of this kinase has been difficult because a selective JNK inhibitor has not been available. We now report the use of a novel selective JNK inhibitor and JNK knockout mice to determine the function of JNK in synoviocyte biology and inflammatory arthritis. The novel JNK inhibitor SP600125 (anthra[1,9-cd]pyrazol-6(2H)-one) completely blocked IL-1–induced accumulation of phospho-Jun and induction of c-Jun transcription in synoviocytes. Furthermore, AP-1 binding and collagenase mRNA accumulation were completely suppressed by SP600125. In contrast, complete inhibition of p38 had no effect, and ERK inhibition had only a modest effect. The essential role of JNK was confirmed in cultured synoviocytes from JNK1 knockout mice and JNK2 knockout mice, each of which had a partial defect in IL-1–induced AP-1 activation and collagenase-3 expression. Administration of SP600125 modestly decreased the rat paw swelling in rat adjuvant-induced arthritis. More striking was the near-complete inhibition of radiographic damage that was associated with decreased AP-1 activity and collagenase-3 gene expression. Therefore, JNK is a critical MAPK pathway for IL-1–induced collagenase gene expression in synoviocytes and in joint arthritis, indicating that JNK is an important therapeutic target for RA.
Introduction
Mitogen-activated protein kinase (MAPK) likely plays a critical role in the pathogenesis of rheumatoid arthritis (RA), which is a chronic inflammatory disease marked by cytokine production, synovial lining hyperplasia, and joint destruction. Three major MAPK families that differ in their substrate specificity and responses to stress have been identified in vertebrates and have been implicated in RA: c-Jun N-terminal kinase (JNK), extracellular regulating kinase (ERK), and p38 kinase (1). MAPKs phosphorylate selected intracellular proteins, including transcription factors, that subsequently regulate gene expression by transcriptional and posttranscriptional mechanisms (2, 3). MAPKs are, in turn, activated by phosphorylation at conserved threonine and tyrosine residues by upstream dual-specific MAPK kinases (MAPKKs), which themselves are activated by MAPKK kinases (4).
The role of cytokines in the pathogenesis of RA is increasingly appreciated (5), but the signal transduction pathways that determine matrix degradation are only partially understood. Overexpression of matrix metalloproteinases (MMPs), which play a critical role in rheumatoid joint destruction, is of particular interest (6). MMP production might be regulated, in part, by increased activation of c-Jun amino-terminal kinase (JNK) since this MAPK activates key transcription factors involved in MMP gene expression. Several JNK isoforms, encoded by three genes, phosphorylate specific sites (serine 63 and serine 73) on the amino-terminal transactivation domain of c-Jun after exposure to ultraviolet irradiation, growth factors, or cytokines (7, 8). By phosphorylating these sites, the JNKs enhance the transcriptional activity of AP-1, a key regulator of MMP production.
Our previous studies demonstrated that IL-1 is a potent inducer of JNK phosphorylation and collagenase gene expression in RA synoviocytes (9). However, evaluation of this pathway in arthritis has been hampered by the lack of selective compounds to block JNK function in vivo and in vitro. Using a novel selective JNK inhibitor (10), we now report that JNK blockade suppresses MMP and bone destruction in an animal model of arthritis. Furthermore, data from synoviocytes derived from JNK knockout mice confirmed the importance of JNK in metalloproteinase expression.
Methods
Patient selection and cell preparation.
Fibroblast-like synoviocytes (FLS) were isolated from RA synovial tissues obtained at joint replacement surgery as described previously (11). The diagnosis of RA conformed to the 1987 revised American College of Rheumatology criteria (12). Briefly, the tissues were minced and incubated with 1 mg/ml collagenase in serum-free DMEM (Life Technologies Inc., Grand Island, New York, USA) for 2 hours at 37°C, filtered through a nylon mesh, extensively washed, and cultured in DMEM supplemented with 10% FCS (endotoxin content less than 0.006 ng/ml; Life Technologies Inc.), penicillin, streptomycin, and L-glutamine in a humidified 5% CO2 atmosphere. After overnight culture, nonadherent cells were removed, and adherent cells were cultivated in DMEM plus 10% FCS. At confluence, cells were trypsinized, split at a 1:3 ratio, and recultured in medium. Synoviocytes were used from passages three through nine in these experiments, during which time they were a homogeneous population of FLSs (<1% CD11b, <1% phagocytic, and <1% Fc-gamma RII receptor positive).
Reagents.
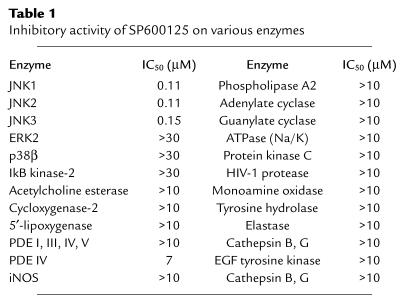
SP600125 (anthra[1,9-cd]pyrazol-6(2H)-one) (see Figure 1) is a novel JNK inhibitor synthesized by the Department of Chemistry at Signal Research Division of Celgene Inc., San Diego, California, USA. The IC50 for this compound on various kinases and other enzymes are shown in Table 1. These studies were performed on the recombinant enzymes (see below for methods). The chemistry and biochemical analysis will be reported elsewhere (10). SB203580 (p38 inhibitor, IC50; 10 nM) was purchased from Calbiochem-Novabiochem Corp. (San Diego, California, USA) and PD98059 (MEK1/2 inhibitor, IC50 10 μM) was obtained from New England Biolabs Inc., Beverly, Massachusetts, USA). The following reagents were also used: IL-1β (Boehringer Mannheim Biochemicals Inc., Indianapolis, Indiana, USA), glutathione-S-transferase-c-Jun (GST-c-Jun) and glutathione-S-transferase-activating transcription factor-2 (GST-ATF2) (Signal Pharmaceuticals Inc., San Diego, California, USA), complete protease inhibitor cocktail (Boehringer Mannheim Biochemicals Inc.), protein A-Sepharose 4B-CL (Promega Corp., Madison, Wisconsin, USA).
Figure 1.
Structure of SP600125, a selective JNK inhibitor.
Table 1.
Inhibitory activity of SP600125 on various enzymes
In vitro enzyme inhibition studies.
The IC50 for SP600125 on JNK was measured using recombinant JNK2 with GST-c-Jun (amino acids 1–79) as a substrate. All reactions were performed in duplicate for 60 minutes at room temperature and contained the following: 50 ng JNK2, 20 mM HEPES, pH 7.6, 10 mM MgCl2, 1.5 mM DTT, 0.5 μCi γ32P-ATP, 50 mM NaCl, 0.03% Triton X-100, 0.1 mM EDTA. JNK2 activity was measured at 2 μM ATP and 2 μM GST-cJun with increasing concentrations of SP600125 to determine the IC50. ERK1, IκB kinase (IKK) , and p38-2 assays were similar to the JNK assay except for the use of different recombinant enzymes and substrate. The ERK, p38-2 and IKK assays measured the phosphorylation of myelin basic protein, GST-ATF2 and GST-IκB (amino acids 1–54), respectively. Similar analyses were performed using increasing concentrations of SP600125 with the appropriate substrate by Cerep Inc. (Redmond, Washington, USA) to inhibit a panel of recombinant enzymes as shown in Table 1.
JNK1 and JNK2 knockout mouse synoviocytes.
Six-week-old JNK1 and JNK2 knockout mice were produced by C57BL/6 backcrossing with SV12a and then backcrossing with C57BL/6 for another five generations to obtain homozygous progeny, and mice homozygous for the targeted gene were analyzed for phenotype (13, 14). Synovial tissue was microdissected from the ankle joints of mice and subsequently minced and incubated with 1 mg/ml collagenase in serum-free DMEM (Life Technologies Inc.) for 2 hours at 37°C, filtered through a nylon mesh, extensively washed, and cultured in DMEM supplemented with 10% FCS (endotoxin content less than 0.006 ng/ml; Life Technologies Inc.), penicillin, streptomycin, and L-glutamine in a humidified 5% CO2 atmosphere. After overnight culture, nonadherent cells were removed and adherent cells were cultivated in DMEM plus 10% FCS. At confluence, cells were trypsinized, split at a 1:3 ratio, and recultured in medium.
In vitro kinase assays.
Confluent RA FLS in 100-mm-diameter dishes were pretreated with MAPK inhibitors for 30 minutes and further incubated with IL-1 (2 ng/ml) for 15 minutes, or cells were treated with IL-1 alone for 15 minutes. Kinase assays were performed using a modification of methods described previously (4). Cells were washed with cold PBS two times, and cells were scraped directly into lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 1% Triton X-100, 20 mM β-glycerophosphate, 50 mM sodium fluoride, 1 mM Na3VO4, 10 μg/ml aprotinin, 1 μM pepstatin A, 1 mM PMSF) and homogenized by ten passes through a 26-gauge needle fitted to a 1-ml syringe. The homogenate was centrifuged for 10 minutes at 14,000 g, and the supernatant was retained for immunoprecipitation. Samples containing 500 μg of protein and 5% FCS in a total volume of 100 μl were incubated with specific anti-p38 or anti-JNK MAPK Ab (at a dilution of 1:1,000) (New England BioLabs Inc.) and 3 μg of GST-c-Jun or GST-ATF2 for 3 hours at 4°C. These recombinant substrates serve as phosphorylation targets for the immunoprecipitates and are phosphorylated by either JNK (GST-c-Jun) or JNK/p38 (GST-ATF2), respectively. Then 30 μl of 50% slurry of protein A-Sepharose 4B-CL in PBS was added, and the mixture was incubated for 1 hour on a rotation wheel. After centrifugation for 5 minutes at 3,000 g, immunocomplexes were washed three times with a low-salt buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.2% Triton X-100, 2 mM EDTA, 2 mM EGTA, 0.1% SDS) and three times with a high-salt buffer (50 mM Tris-HCl, pH 7.4, 500 mM NaCl, 0.2% Triton X-100, 2 mM EDTA, 2 mM EGTA, 0.1% SDS) and once with 20 mM HEPES, pH 7.4, 20 mM MgCl2 before the kinase reaction was started by adding 30 μl of kinase buffer (25 mM HEPES, pH 7.4, 25 mM MgCl2, 20 mM β-glycerophosphate, 0.1 mM Na3VO4, 2 mM dithiothreitol, 20 μM ATP, 2 μCi γ[32P]-ATP) for 30 minutes at 37°C. Samples were heated for 5 minutes at 95°C, separated using 10% SDS-PAGE, and visualized by autoradiography. For studies in adjuvant arthritis, rats were immunized with complete Freund’s adjuvant as described above and treated with 30 mg/kg SP600125 subcutaneously, beginning on day 8. The animals were sacrificed on day 14, and the ankle joints were minced in 2 ml of the lysis buffer. The protein extracts were then processed as described for tissue culture cells.
Western blot analysis.
Synoviocytes were pretreated with medium or MAPK inhibitors for 30 minutes and further incubated with IL-1 (2 ng/ml) for 15 minutes. Protein samples (25 μg/lane) from FLS were run on a 10% SDS-PAGE and transferred onto a nitrocellulose membrane at 140 mA in 25 mM Tris-HCl, pH 8.3, 192 mM glycine, and 50% methanol. Western blot analysis was performed using SAPK/JNK assay kit, PhosphoPlus MAPK Ab kit, and PhosphoPlus p38 MAP kinase (Thr180/Tyr182) Ab kit (New England BioLabs Inc.), according to the manufacturer’s instructions. Briefly, filters were blocked with TBS plus 0.1% Tween-20 and 5% dry milk for 1–3 hours, followed by incubation with the appropriate Ab at 4°C overnight. The membrane was washed three times and incubated with HRP-conjugated secondary Ab for 1 hour at room temperature (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA). The proteins were visualized by chemiluminescence with hydrogen peroxide and luminol as a substrate using Kodak X-AR film (Eastman Kodak Co. Scientific Imaging Systems, Rochester, New York, USA).
Northern blot analysis.
FLSs were pretreated with medium or MAPK inhibitors for 30 minutes and further incubated with IL-1 (2 ng/ml) for 18 hours. Total RNA was isolated using 1 ml of RNA STAT-60 (Tel-Test Inc., Friendswood, Texas, USA) per 4 × 106 cells. Equal amounts of RNA (20 μg of total RNA) were fractionated in a 1.2% agarose gel containing 5.5% formaldehyde. RNA was then transferred to a nylon membrane using the turbo blotter system (Schleicher & Schuell Inc., Keene, New Hampshire, USA) and cross-linked at 80°C for 60 minutes. The blots were prehybridized in 50% formamide, 5× saline-sodium phosphate-EDTA (SSPE), 5× Denhardt’s solution, 1% SDS, 200 μg/ml ssDNA, and 50 μg/ml tRNA. The cDNA probes were denatured and labeled by random-primed incorporation of α[32P]-dATP (Ambion Inc., Austin, Texas, USA). The probes were denatured at 100°C, and the blots were hybridized overnight at 42°C. The membrane was washed in 2× SSPE and 0.1% SDS at 37°C and exposed to Kodak X-Omat AR film (Eastman Kodak Co. Scientific Imaging Systems) with an intensifying screen for 18–24 hours at –80°C. Image analysis was performed on digitized images using the NIH image software (Bethesda, Maryland, USA).
Preparation of nuclear extracts.
Confluent RA FLS in 100-mm-diameter dishes were pretreated with medium or MAPK inhibitors for 30 minutes and further incubated with IL-1 (2 ng/ml) for 60 minutes. The cells were then lysed with 1 ml buffer A (10 mM HEPES, pH 7.4, 1.5 mM MgCl2, 10 mM KCl, 1 mM DTT, 1 mM PMSF, and 0.1% Nonidet P-40 [NP-40]) as described previously (15), incubated on ice for 15 minutes, and centrifuged at 850 g at 4°C. The supernatants were discarded and the pellets resuspended in 4 ml of buffer A without NP-40. The samples were centrifuged again, and the supernatant was discarded. One hundred microliters of buffer C (25% glycerol, 20 mM HEPES, pH 7.9, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, pH 8.0, 1 mM DTT, and 1 mM PMSF) was added to the pellets and the samples were rocked at 4°C for 30 minutes. Particulate matter was pelleted for 30 minutes at 4°C in a microfuge, and the supernatant was aliquoted and stored at –80°C.
Electrophoretic mobility shift assay.
The Bandshift kit (Promega Corp.) was used according to the manufacturer’s instructions. Consensus and control oligonucleotides (Santa Cruz Biotechnology Inc.) were labeled by polynucleotide kinase incorporation of γ[32P]-dATP. Oligonucleotides sequences included the AP-1 consensus (5′ to 3′) (CGCTTGATGACTTGGCCGGAA) (CGCTTGATGACTTGGCCGGAA). After the oligonucleotide was radiolabeled, the nuclear extracts (4 μg of protein in 2 μl of nuclear extract) were mixed with 20 pmol of the appropriate γ[32P]-ATP–labeled consensus or mutant oligonucleotide in a total volume of 20 μl for 30 minutes at room temperature. The samples were then resolved on a 4% polyacrylamide gel. The gel was transferred to Whatman paper, dried, and visualized by autoradiography. Controls were performed in each case with mutant oligonucleotides or cold oligonucleotides to compete with labeled sequences.
Adjuvant arthritis model.
Male Lewis rats (150–200 g) were immunized with complete Freund’s adjuvant on day 0 (16). In this model, arthritis typically begins on day 10 and reaches a plateau from day 16 to 20. Treatment with subcutaneous SP600125 or vehicle (40% polyethylene glycol [PEG] 400 in PBS) was begun on day 8 and was continued daily. Paw swelling was determined by water displacement plethysmography. Roentgenograms were obtained of the right hind paw to assess bone changes using a semiquantitative scoring system: demineralization (0–2+); ankle and mid-foot erosions (0–2+); calcaneal erosion (0–1+); heterotopic bone formation (0–1+), with a maximum possible score of 6. A histologic scoring system was used to evaluate joint inflammation and damage: synovial inflammation (0–4+), cartilage integrity (0–4+), bone erosions (0–4+), marrow infiltration (0–4+), and extra-articular inflammation (0–4+), with a maximum score of 16.
Statistics.
Data were compared using Student’s t test, and a P value less than 0.05 was considered significant. Data are presented as mean plus or minus SEM.
Results
Effect of MAPK inhibitors on JNK activity in RA FLS.
Initial studies were performed to determine the effect of the MAPK inhibitors on phosphorylation of transcription factors c-Jun and ATF2 using in vitro kinase assays. To compare the activity of the different MAPK and MAPKK inhibitors, RA FLS were incubated with the novel JNK inhibitor SP600125 (see Figure 1 for structure and Table 1 for a profile of activity on various enzymes), the MEK inhibitor PD98059 (which blocks ERK activation), or the p38α/β inhibitor SB203580 for 30 minutes and then stimulated with IL-1. IL-1–stimulated JNK activity measured with a GST-Jun substrate was blocked by 20 μM of SP600125 (Figure 2). It is of interest that 100 μM of PD98059 partially inhibited this activity, but SB203580 was inactive at concentrations that completely blocked p38. Both the JNK inhibitor and the p38α/β inhibitor blocked phosphorylation of GST-ATF2 by the extracts, consistent with the ability of both JNK and p38 to phosphorylate ATF2 (17). No cytotoxicity was observed at the concentrations of SP600125 tested in these assays.
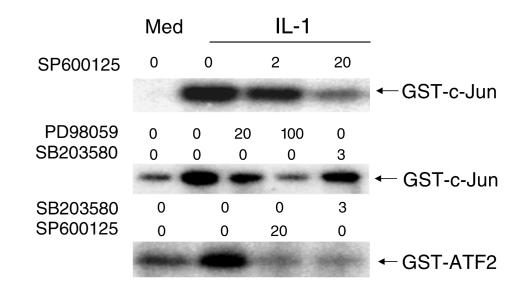
Figure 2.
Effect of SP600125 on c-Jun phosphorylating activity. Cultured FLS were stimulated with medium (Med) or 2 ng/ml of IL-1 for 15 minutes in the presence of increasing concentrations of SP600125 (0–20 μM; JNK inhibitor), PD98059 (0–100 μM; MEK/ERK inhibitor), or SB203580 (0–3 μM; p38 inhibitor). The ability of cell lysates to phosphorylate GST-c-Jun or GST-ATF2 was determined. SP600125 inhibited c-Jun and ATF2 phosphorylation in vitro.
Effect of MAPK inhibitors on c-Jun phosphorylation in RA FLS.
Having demonstrated that c-Jun phosphorylating activity was inhibited in vitro by SP600125 in RA FLS extracts, we then examined effect of the JNK inhibitor on accumulation of phospho-c-Jun in IL-1–stimulated FLS. Western blot analysis showed that SP600125 and, to a lesser extent PD98059, interfered with JNK activity, while SB203580 had no effect (n = 5) (Figure 3). Additional studies showed that SP600125 blocked c-Jun phosphorylation at both serine 63 and serine 73 (data not shown). While SP600125 is known to inhibit JNK, it was possible that its effect on c-Jun phosphorylation could result from nonspecific inhibition of the upstream kinases JNKK1/MKK4 or JNKK2/MKK7. However, SP600125, PD98059, and SB203580 did not block phosphorylation of JNK in IL-1–stimulated RA FLS (n = 3; data not shown). Therefore, SP600125 functions by directly inhibiting JNK.
Figure 3.
Effect of MAP kinase inhibitors on phospho-c-Jun (P-c-Jun) levels. Cultured FLSs were stimulated with medium or 2 ng/ml of IL-1 for 15 minutes in the presence of SP600125 (20 μM), PD98059 (100 μM), or SB203580 (3 μM). Phospho-c-Jun and total c-Jun protein levels were determined by Western blot analysis. SP600125 inhibited intracellular c-Jun phosphorylation. The phospho-c-Jun/total Jun protein ratios were medium = 0.18, IL-1 = 1.00, IL-1 + SP600125 = 0.23, IL-1 + PD98059 = 0.73, IL-1 + SB203580 = 1.09.
Gene expression of c-jun and c-fos in RA FLS.
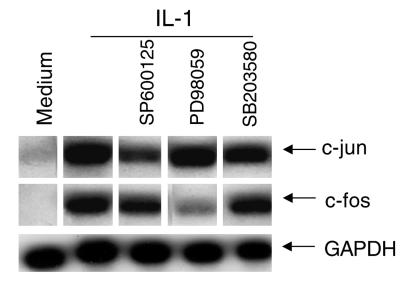
MAPKs are thought to be involved in regulation of c-jun and c-fos genes (18). The role of JNK in c-jun and c-fos gene regulation in FLSs was therefore determined using the three MAP kinase inhibitors. We observed that SP600125 blocked c-jun mRNA accumulation in IL-1–treated cells, while PD98059 primarily blocked c-fos expression (Figure 4). SB203580 had no effect on c-fos or c-jun expression at a concentration that completely inhibits p38 function. Nonspecific toxicity of the JNK inhibitor is not likely since the compound had little effect on either c-fos or GAPDH expression.
Figure 4.
Effect of MAP kinase inhibitors on c-jun and c-fos gene expression. Cultured FLS were stimulated with medium or 2 ng/ml of IL-1 for 15 minutes in the presence of SP600125 (20 μM), PD98059 (100 μM), or SB203580 (3 μM). The figure is representative of four FLS lines examined. The c-jun and c-fos mRNA levels were determined by Northern blot analysis. RNA loading is shown in the bottom row with GAPDH. JNK inhibition decreased c-jun expression, while ERK inhibition decreased c-fos expression.
Collagenase and AP-1 expression in RA synoviocytes.
Collagenase (MMP1) plays a critical role in irreversible matrix degradation in RA by cleaving native type II collagen (19). AP-1 is an important IL-1–inducible transcription factor that regulates collagenase gene expression (20). Figure 5a shows that SP600125 suppressed AP-1 binding in IL-1–stimulated FLS. The ERK/MEK inhibitor also had a modest effect, but the p38 inhibitor did not alter AP-1 binding. Because SP600125 inhibits AP-1 activation, its effect on collagenase gene expression was examined. Northern blot analysis indicated that the JNK inhibitor blocked IL-1–induced collagenase expression in RA FLS, although SP600125 (20 μM) did not reduce levels of MMP1 gene expression below baseline (see Figure 5b). This is consistent with the Western blot experiments (Figure 3), indicating that the JNK inhibitor decreased IL-1–induced phospho-c-Jun levels to baseline. SP600125 also inhibited IL-1–induced MMP3 expression in FLS (data not shown). Weak inhibition was observed in cells treated with high concentrations of PD98059, but SB203580 had no effect. Therefore, JNK blockade interferes with transcription factor activation and collagenase gene expression in cultured RA FLS.
Figure 5.
Effect of MAP kinase inhibitors on AP-1 expression and collagenase gene. Cultured FLS were stimulated with medium or 2 ng/ml of IL-1 for 18 hours (Northern blot analysis) and 1 hour (electrophoretic mobility shift assay [EMSA]) in the presence of medium, SP600125, PD98059, or SB203580. In EMSA experiments (a), SP600125 (20 μM), PD98059 (100 μM), or SB203580 (3 μM) were tested (n = 3; two separate experiments are shown). Note that the JNK inhibitor SP600125 decreased AP-1 binding in nuclear extracts of IL-1–stimulated FLS. The ERK/MEK inhibitor had a modest effect, and the p38 inhibitor did not alter AP-1 binding. (b) Northern blot analysis in which increasing concentrations of the inhibitors were tested for the ability to inhibit collagenase gene expression: SP600125 (0–20 μM; JNK inhibitor), PD98059 (0–100 μM; MEK/ERK inhibitor), or SB203580 (0–3 μM; p38 inhibitor) (n = 3). SP600125 and, to a lesser extent, PD98059 decreased MMP1 gene expression. GAPDH shows equal loading of RNA in each lane. Here, 20 μM of SP600125 inhibited IL-1–induced MMP1 induction (GAPDH-normalized MMP1 gene expression for medium = 0.66; IL-1 = 1.70; IL-1 + SP600125 10 μM = 1.28; IL-1 + SP600125 20 μM = 0.63; IL-1 + SP600125 50 μM = 0.49). However, PD98059 did not decrease MMP1 expression to baseline (GAPDH-normalized MMP1 gene expression for medium = 0.43; IL-1 = 1.40; IL-1 + PD98059 10 μM = 1.15; IL-1 + PD 98059 100 μM = 0.86).
MMP13 and MMP3 expression and regulation in JNK1 and JNK2 knockout synoviocytes.
To prove a direct role of JNK in the regulation of collagenase gene expression, a genetic approach to evaluate the role of JNK in regulation of collagenase gene expression was taken. FLS were isolated from mice with homozygous disruption of the jnk1 or jnk2 locus. We examined IL-1–induced MMP3 and MMP13 (collagenase 3) expression instead of MMP1 because mice do not express the MMP1 gene. IL-1 induced significant accumulation of MMP13 and MMP3 mRNA in wild-type FLS. However, MMP13 and MMP3 gene induction was suppressed in FLS isolated from JNK1 and JNK2 knockout mice (n = 3) (Figure 6a). Both the JNK1- and JNK2-deficient cells had lower levels of AP-1 binding activity than wild-type cells (Figure 6b).
Figure 6.
Metalloproteinase gene and AP-1 expression in JNK1 and JNK2 knockout (KO) FLS. Cultured FLS from JNK1 KO FLS lines, JNK2 KO FLS lines, and wild-type FLS lines were stimulated with medium or 2 ng/ml of IL-1 for 18 hours (Northern blot analysis) and 1 hour (EMSA) in the presence of medium or 20 μM SP600125. (a) MMP3 and MMP13 mRNA levels were determined by Northern blot analysis in two separate lines for each strain (line 1 and line 2). JNK1 KO and especially JNK2 KO FLS had lower MMP expression. Residual MMP expression was further suppressed by 20 μM SP600125 (see MMP3 experiment). The ethidium-stained gel shows equal loading of RNA in each lane. (b) AP-1 binding was shown for two JNK2 KO, one wild-type, and one JNK1 KO line. The far left part of the gel shows a positive control and cold competition of the positive control (cold oligo). JNK KO FLS lines have decreased basal and IL-1–stimulated AP-1 activation compared with wild-type cells.
Effect of SP600125 on JNK function in vivo.
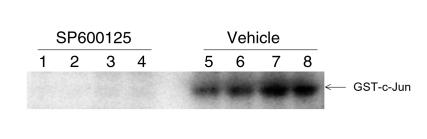
To determine if SP600125 inhibits JNK function in vivo, adjuvant arthritis was induced in Lewis rats. Beginning on day 8, rats were treated subcutaneously with either 30 mg/kg/d or vehicle. The rats were sacrificed after the onset of arthritis (day 14) 2 hours after drug administration. Ankle joint extracts were subsequently prepared for in vitro kinase assays to determine JNK activity. As seen in Figure 7, the extracts from vehicle-treated rats were able to phosphorylate GST-c-Jun substrate, but no kinase activity was detected in the animals that received SP600125. Therefore, systemic administration of SP600125 inhibited JNK function in the joints of rats with adjuvant arthritis.
Figure 7.
Effect of SP600125 on JNK activity in adjuvant arthritis. Rats with adjuvant arthritis were treated with SP600125 or vehicle beginning on day 8 and sacrificed on day 14. The ability of joint lysates to phosphorylate GST-c-Jun was determined as described in Methods. Vehicle-treated rats had high levels of JNK kinase activity, while no activity was detected in the SP600125-treated rats (n = 4 each).
Effect of JNK inhibition in adjuvant arthritis.
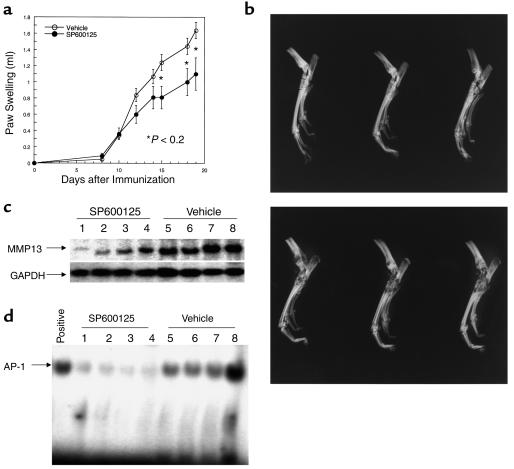
To determine the effect of JNK inhibition on matrix destruction in an animal model of arthritis, rats were immunized on day 0 with complete Freund’s adjuvant and treated with SP600125 (30 mg/kg/d, subcutaneously) or vehicle beginning on day 8 (n = 8 animals/group). Paw volumes were measured daily and, as shown in Figure 8a, the JNK inhibitor modestly decreased swelling in treated rats. Histologic evaluation of synovial inflammation showed significant beneficial effect in animals treated with SP600125 (histologic score for SP600125 = 9.0 ± 2.1; vehicle = 14.4 ± 0.6; P < 0.05). Most importantly, in SP600125-treated animals, radiographic analysis at the conclusion of the study demonstrated a marked decrease in bone and cartilage damage (Figure 8b; radiographic score for SP600125 = 1.5 ± 0.7; vehicle = 4.5 ± 0.6; P < 0.01). Furthermore, significantly lower amounts of collagenase mRNA were detected in the joint extracts of SP600125-treated animals (Figure 8c). AP-1 binding was also significantly decreased in the joints of SP600125-treated animals compared with vehicle-treated controls (Figure 8d). Therefore, JNK inhibition had a modest anti-inflammatory effect but had a marked protective effect on joint destruction.
Figure 8.
(a) Effect of SP600125 on adjuvant arthritis in rats. Rats were immunized with complete Freund’s adjuvant on day 0 and treated with vehicle or 30 mg/kg/d of SP600125 subcutaneously beginning on day 8. Significantly less paw swelling was observed in the treated animals. (b) Effect of SP600125 on radiographic damage in adjuvant arthritis. Representative examples of ankle radiographs demonstrate markedly less destruction in the rats treated with SP600125 (top) compared with vehicle (bottom). (c) Effect of SP600125 on synovial collagenase gene expression. Northern blot analysis was performed on joint extracts of vehicle and SP600125-treated rats. Each lane contains the extract of an ankle joint from a control or treated rat (n = 4 for each). Note the lower levels of MMP13 in the SP600125-treated animals (G3PDH-normalized MMP13 mRNA levels for SP600125 = 0.23 ± 0.086 and vehicle = 0.822 ± 0.131; P < 0.01). (d) Effect of SP600125 on synovial AP-1 activation. EMSA analysis was performed on joint extracts of vehicle and SP600125-treated rats with adjuvant arthritis. Positive control is shown on the far left lane of the gel. Note lower levels of AP-1 binding in the SP600125-treated rats (SP600125 = 2.89 ± 0.43 and vehicle = 12.6 ± 2.5, P < 0.01; data presented as arbitrary density units).
Discussion
Based on the importance of metalloproteinases in RA (5, 16, 19), we hypothesized that MAPKs serve as key regulators of matrix remodeling in this disease through their effects on AP-1. Of the three MAPK families, JNK likely plays a central role in this process by virtue of its ability to activate AP-1–mediated transcription (21). AP-1 function is regulated both through changes in the abundance of its Jun and Fos components and posttranslational modification by protein phosphorylation (22, 23). The various JNK isoforms, including JNK1, JNK2, and JNK3, phosphorylate two N-terminal serines (amino acids 63 and 73) on c-Jun and subsequently enhance transcriptional activity. In addition to c-Jun, JNK can regulate the expression of other genes through the phosphorylation of ATF2 and Elk1 (24). JNK2 binds c-Jun with greater avidity than the other JNKs and may be the most physiologically relevant isoform, especially in FLS where it is the dominant JNK protein. Our focus on JNK was stimulated by previous studies demonstrating that JNK phosphorylation is greater in RA than osteoarthritis synoviocytes and correlated with increased MMP expression (9, 25). Furthermore, phospho-JNK has also been observed in intact RA synovium using both immunohistochemistry and Western blot analysis (9, 26).
Previous studies of JNK function in vitro and in vivo have been limited by the lack of a selective inhibitor. Using a novel inhibitor of JNK (SP600125), we evaluated JNK biology in cultured synoviocytes and in an animal model of arthritis. Initial in vitro experiments showed that SP600125 blocked JNK function in FLS. The inhibitor suppressed IL-1–induced phospho-c-Jun accumulation in synoviocytes as well as c-Jun phosphorylating activity in cell extracts. SP600125 did not suppress c-Jun phosphorylation below baseline, suggesting that other pathways contribute to basal AP-1 activation. Inhibitors of the other MAPK pathways were also evaluated to determine the relative contributions of each to c-Jun phosphorylation and AP-1 activation. Although JNK played a primary role in c-Jun phosphorylation, ERK also appeared to contribute. However, p38 had no effect on any of the JNK-related functions. SP600125 also inhibited c-jun mRNA accumulation in synoviocytes, whereas ERK was more important in c-fos regulation.
JNK inhibition also decreased the induction of AP-1 DNA-binding activity in synoviocytes, which is primarily dependent on new Jun and Fos protein synthesis in synovial fibroblasts. Of particular interest, SP600125 suppressed the induction of collagenase gene expression, which contains a key AP-1 site in its promoter that is critical for cytokine-induced transcription (18). As with c-Jun phosphorylation, the JNK inhibitor reduced MMP1 to baseline levels. Similar, but less dramatic, results were produced by knockout of the jnk1 or jnk2 loci. The absence of either gene suppressed MMP expression in mouse synoviocytes, although JNK2 appeared to be more important. The relatively greater decrease in the JNK2 knockout mice suggests that this kinase plays a key role. This is consistent with our previous observation that the JNK2 is more abundant than either JNK1 or JNK3 in RA synoviocytes (9). SP600125 further decreased MMP expression in the knockout synoviocytes, suggesting that blockade of both JNKs is needed for complete inhibition of MMP production. This is especially an important consideration when assessing murine models of inflammation in JNK knockout mice where only a single locus can be deleted.
The role of individual MAPKs in MMP expression varies with cell type and stimulus. For instance, p38 might play an important role in phorbol ester–induced type IV collagenase production by a squamous cell carcinoma cell line as well as in cytokine-stimulated chondrocytes (27, 28). ERK and JNK have been implicated in the regulation of collagenase gene expression in cultured fibroblasts (29). In contrast to FLS, collagenase and stromelysin gene expression in endothelial cells are dependent on p38 (30). Using both selective inhibitors and genetically modified cells, our data indicate that cytokine-induced MMP1 expression is independent of p38 in synoviocytes, which are the primary source of MMPs in the rheumatoid synovial membrane. While ERK can contribute to collagenase gene expression, JNK is a key MAPK pathway that regulates this process in RA synoviocytes.
After demonstrating a key role of JNK in the regulation of AP-1 and MMP1 expression in vitro, we then evaluated the effect of the compound in vivo. The JNK inhibitor markedly decreased JNK functional activity in arthritic rats treated with the compound. The JNK inhibitor also had a beneficial effect in rat adjuvant arthritis, which is a polyarticular, destructive arthritis that serves as a model for RA (16). SP600125 modestly decreased paw swelling in this model. However, the most striking benefit was on joint destruction. Radiographic evaluation of animals treated with the JNK inhibitor showed significantly less joint damage and remodeling than vehicle-treated controls. Hence, JNK activation is a primary mediator of joint destruction in arthritis.
The mechanism of joint protection in adjuvant arthritis was investigated by determining MMP and AP-1 activation in rats treated with SP600125. As with cultured synoviocytes, the JNK inhibitor suppressed synovial MMP13 gene expression. It is therefore likely that JNK blockade interfered with a cascade of events, beginning with c-Jun phosphorylation and including c-jun gene expression, AP-1 binding, and MMP1 gene transcription. These data indicate that the JNK pathway lies at a critical convergent point in the regulation of extracellular matrix regulation in arthritis. Alternatively, JNK blockade could potentially alter the immune responses since these kinases play a role in Th1/Th2 balance and T cell activation (13, 31, 32). However, treatment with SP600125 was delayed in order to minimize its effect on the primary T cell responses. Anti-inflammatory and matrix protection actions of therapeutic agents can be readily assessed with delayed treatment (16).
Based on the modest effects on paw swelling, the inflammatory components of arthritis appear to be less dependent on AP-1. This differs somewhat from NF-κB inhibitors, which have tended to demonstrate more prominent anti-inflammatory effects (33–35). We have also observed that suppression of NF-κB activation in the joints of rats with adjuvant arthritis leads to decreased joint inflammation but no change in bone destruction (36). These data suggest that AP-1 and NF-κB might complement each other by primarily regulating destruction or inflammation, respectively. These two aspects of RA are not always linked, and different mechanisms appear to regulate their progression in human RA as well as various animal models (1). Hence, JNK inhibition is a potential therapeutic approach for prevention of matrix destruction. In combination with approaches that suppress other pathways such as NF-κB, both inflammation and joint damage could suppressed in RA.
Acknowledgments
These studies were supported by grants from the Arthritis Foundation and from the Signal Research Division of Celgene Inc.
Footnotes
See the related Commentary beginning on page 181.
References
- 1.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–185. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 2.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 3.Fanger GR, Gerwins P, Widmann C, Jarpe MB, Johnson GL. MEKKs, GCKs, MLKs, PAKs, TAKs, and tpls: upstream regulators of the c-Jun amino-terminal kinases? Curr Opin Genet Dev. 1997;7:67–76. doi: 10.1016/s0959-437x(97)80111-6. [DOI] [PubMed] [Google Scholar]
- 4.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2149–2160. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 5.Firestein GS. Rheumatoid arthritis. Shaun Ruddy, editor. Scientific American Inc. New York, New York, USA. Scientific American Medicine. 1998;15:2.1–2.14. [Google Scholar]
- 6.Firestein GS, Paine MM, Littman BH. Gene expression (collagenase, tissue inhibitor of metallproteinases, complement, and HLA-DR) in rheumatoid arthritis and osteoarthritis synovium. Quantitative analysis and effect of intraarticular corticosteroids. Arthritis Rheum. 1991;34:1094–1105. doi: 10.1002/art.1780340905. [DOI] [PubMed] [Google Scholar]
- 7.Devary Y, Karin M. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 1992;71:1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- 8.Kallunki T, et al. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes Dev. 1994;8:2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- 9.Han Z, et al. Jun-N-Terminal kinase in rheumatoid arthritis. J Pharmacol Exp Ther. 1999;291:124–130. [PubMed] [Google Scholar]
- 10.Bennett, B.L., et al. 2001. SP600125, a novel inhibitor of Jun N-terminal kinase. In press. [DOI] [PMC free article] [PubMed]
- 11.Alvaro-Gracia JM, Zvaifler NJ, Firestein GS. Cytokines in chronic inflammatory arthritis. V. Mutual antagonism between interferon-gamma and tumor necrosis factor-alpha on HLA-DR expression, proliferation, collagenase production, and granulocyte macrophage colony-stimulating factor production by rheumatoid arthritis synoviocytes. J Clin Invest. 1990;86:1790–1798. doi: 10.1172/JCI114908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnett FC, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 13.Sabapathy K, et al. JNK2 is required for efficient T-cell activation and apoptosis but not for normal lymphocyte development. Curr Biol. 1999;9:116–125. doi: 10.1016/s0960-9822(99)80065-7. [DOI] [PubMed] [Google Scholar]
- 14.Chang, L., et al. 2001. JNK1 is required for neuropeptide corticotropin releasing hormone responses to emotional stress. In press.
- 15.Han Z, Boyle DL, Manning AM, Firestein GS. AP-1 and NF-kB regulation in rheumatoid arthritis and murine collagen-induced arthritis. Autoimmunity. 1998;28:197–208. doi: 10.3109/08916939808995367. [DOI] [PubMed] [Google Scholar]
- 16.Boyle DL, et al. Anti-inflammatory effects of ABT-702, a novel non-nucleoside adenosine kinase inhibitor, in rat adjuvant arthritis. J Pharmacol Exp Ther. 2001;296:495–500. [PubMed] [Google Scholar]
- 17.Raingeand J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 19.Firestein GS. Mechanisms of tissue destruction and cellular activation in rheumatoid arthritis. Curr Opin Rheumatol. 1992;3:348–354. doi: 10.1097/00002281-199206000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Angel P, et al. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987;6:729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- 21.Tibbles LA, Woodgett JR. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55:1230–1254. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kallunki T, et al. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes Dev. 1994;8:2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- 23.Minden A, et al. c-Jun N-terminal phosphorylation correlates with activation of the JNK subgroup but not the ERK subgroup of mitogen-activated protein kinases. Mol Cell Biol. 1994;14:6683–6688. doi: 10.1128/mcb.14.10.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S, et al. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- 25.Firestein GS, Manning AM. Signal transduction and transcription factors in rheumatic disease. Arthritis Rheum. 1999;42:609–621. doi: 10.1002/1529-0131(199904)42:4<609::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 26.Schett G, et al. Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum. 2000;43:2501–2512. doi: 10.1002/1529-0131(200011)43:11<2501::AID-ANR18>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 27.Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801–811. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Simon C, Goepfert H, Boyd D. Inhibition of the p38 mitogen-activated protein kinase by SB 203580 blocks PMA-induced Mr 92,000 type IV collagenase secretion and in vitro invasion. Cancer Res. 1998;58:1135–1139. [PubMed] [Google Scholar]
- 29.Reunanen N, et al. Enhancement of fibroblast collagenase (matrix metalloproteinase-1) gene expression by ceramide is mediated by extracellular signal-regulated and stress-activated protein kinase pathways. J Biol Chem. 1998;273:5137–5145. doi: 10.1074/jbc.273.9.5137. [DOI] [PubMed] [Google Scholar]
- 30.Ridley SH, et al. Actions of IL-1 are selectively controlled by p38 mitogen-activated protein kinase: regulation of prostaglandin H synthase-2, metalloproteinases, and IL-6 at different levels. J Immunol. 1997;158:3165–3173. [PubMed] [Google Scholar]
- 31.Dong C, et al. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–2095. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- 32.Yang DD, et al. Differentiation of CD4+ T cells to Th1 cells requires MAP kinase JNK2. Immunity. 1998;9:575–585. doi: 10.1016/s1074-7613(00)80640-8. [DOI] [PubMed] [Google Scholar]
- 33.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;15:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 34.Miagkov AV, et al. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci USA. 1998;95:13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aupperle KR, et al. NF-kB regulation by IκB kinase in primary fibroblast-like synoviocytes. J Immunol. 1999;163:427–433. [PubMed] [Google Scholar]
- 36.Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]