In 1992 a new adhesion deficiency syndrome was described in two unrelated Arab boys, then 3 and 5 years old, each the offspring of a consanguineous mating (1, 2). Both subjects showed severe psychomotor and growth retardation and a distinctive facial appearance characterized by a flat face with a depressed nasal bridge, antiverted nostrils, and long eyelashes. They also had recurrent infections with marked neutrophilia and periodontitis. The children were found to have the rare Bombay blood phenotype, wherein the red cells express a nonfucosylated variant of the H antigen. Also missing from their red cells was another set of fucose-containing surface molecules, the Lewis blood group antigens, such as sialyl Lewis X, which is an important ligand for selectins on leukocyte surfaces.
Because the features observed differed from those of a previously described leukocyte adhesion deficiency (LADI, which arises from mutations in the gene encoding the integrin β2 subunit), the new syndrome was designated LADII. The infectious symptoms in LADI are much more serious, but the patients do not suffer from the severe psychomotor and growth retardation seen in LADII. In vivo and in vitro studies also showed differences between the two LAD syndromes. Leukocytes from LADII roll on activated endothelial cells at only 20% the rate of control and LADI cells. Transmigration through the endothelium, conversely, is normal in LADII but severely impaired in LADI. These studies showed the importance of selectin interactions with sialyl Lewis X in leukocyte rolling, the first phase of the adhesion cascade. See Becker and Lowe for a detailed review of the clinical symptoms in LADII (3).
More recently, LADII has been described in a Turkish child whose parents are from the same small village and therefore may be distantly related (4). This new subject, too, showed an absence of fucosylated macromolecules and shared many clinical symptoms seen in the Arab children with LADII, although he differed in exhibiting severe intrauterine growth retardation and a more pronounced immunodeficiency, which required continuous antibiotic prophylaxis (4).
A global defect in fucose-glycoconjugates in LADII
Fucose, bound via different linkages, is present on multiple blood group antigens, some of which are known to play a role in endothelial cell adhesion. Because the various fucosyltransferases, the enzymes that carry out fucose transfer to sugars, are highly specific for the structure of the linkage they generate, the global defect seen in LADII patients is unlikely to result from simultaneous defects in multiple transferases. Indeed, there is direct evidence that these enzymes are normal in LADII cells, suggesting that the defect in fucose metabolism lies upstream (4, 5).
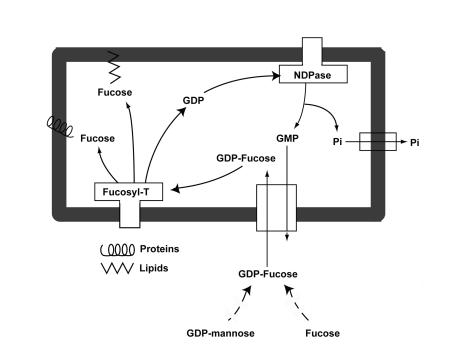
As can be seen in Figure 1, the events in this pathway that lie upstream of fucosylation include the synthesis of the fucose donor, GDP-fucose, and the transport of this nucleotide sugar from its site of synthesis in the cytosol into the lumen of the Golgi apparatus, where fucose is transferred to macromolecules. GDP-fucose may arise either from GDP-mannose, probably the major pathway, or from free fucose. In LADII, the GDP-D-mannose 4,6 dehydratase, an enzyme involved in de novo GDP-fucose synthesis from GDP-mannose, is normal in sequence and expression level (6, 7). GDP-fucose synthesis from free fucose via a so-called salvage pathway also appears to be normal in LADII patients (8). Thus, the most attractive model for a molecular defect in fucosylation involves decreased availability of GDP-fucose within the Golgi lumen.
Figure 1.
Mechanism of the Golgi membrane GDP-fucose antiporter. GDP-fucose is synthesized in the cytosol from either GDP-mannose (probably the major pathway) or from free fucose. The nucleotide sugar is then transported via the GDP-fucose transporter into the lumen of the Golgi apparatus, where specific fucosyltransferases add fucose to a variety of glycoproteins and glycolipids. GDP, the other reaction product, is converted by a lumenal nucleoside diphosphatase to GMP and inorganic phosphate. The former is exported to the cytoplasm through an antiport mechanism that is coupled with GDP-fucose transport, whereas the latter is postulated to leave the Golgi lumen via a specific channel, GOLAC (23). Fucosyl-T, fucosyltransferases; Pi, inorganic phosphate.
As can be seen in Figure 1, two proteins are critical for the nucleotide sugar transport cycle that provides GDP-fucose to enzymes in the lumen of the Golgi. These are the Golgi membrane GDP-fucose transporter itself and the intrinsic Golgi membrane protein nucleoside diphosphatase, which generates GMP from the released GDP. The transporter couples the import of GDP-fucose transport into the Golgi lumen with the export of GMP into the cytoplasm. Luebke et al. (9) confirmed that Golgi GDP-fucose transport activity is specifically defective in the cells of the Turkish child, a result that has been confirmed in one of the Arab LADII subjects (8); nucleoside diphosphatase activity is not affected in the cells from these patients.
The biochemistry of Golgi nucleotide transport
The Golgi membrane GDP-fucose transporter is part of a group of novel Golgi nucleotide transporters. A requirement for transporters of nucleotide sugars, nucleotide sulfate, and ATP in the membrane of the Golgi apparatus became apparent with the realization that glycosylation, sulfation, and phosphorylation of most secreted and membrane proteins, proteoglycans, and glycosphingolipids occurs in the Golgi lumen. Because most nucleotides used for the above posttranslational reactions are synthesized in the cytosol, they must be transported into the Golgi lumen prior to their use as substrates in posttranslational modifications. Studies in vitro, mostly with Golgi vesicles from rat liver, mammary gland, and yeast, have shown that transport of the nucleotide solutes is organelle-specific and that the entire nucleotide derivative enters the Golgi lumen through transporters with Km’s in the range of 1–10 μM (10, 11). The nucleotide derivatives, including GDP-fucose, become concentrated in the lumen of the Golgi vesicles. Nucleotide import does not require ATP for energy but is linked to the export of the corresponding nucleoside monophosphate, as is seen in the pathway in Figure 1, where the transporter is specific for GDP-fucose and GMP.
The human GDP-fucose transporter was recently cloned by complementation of cells derived from LADII patients (12, 13) and the molecular defect of LADII was localized to the transporter protein per se. This highly hydrophobic protein is predicted to contain 364 amino acids and to span the membrane multiple times. This predicted size is in good agreement with that of a rat liver protein of 39 kDa that had previously been purified as the GDP-fucose transporter (14).
Sequencing of transcripts of one patient of Turkish and two of Arab origin (12, 13) showed different point mutations in the coding regions. In the Turkish patient, a C-T transition at nucleotide 1048 caused a change of arginine to cysteine at amino acid 147, in the putative fourth transmembrane domain. In the Arab patients, a C-G transversion at nucleotide 1532 leads to a threonine-to-arginine change at amino acid 308 in the putative ninth transmembrane domain. In a separate study (15) an additional patient of Arab origin was found to have the same mutation as the above Arab patients. Consistent with a simple recessive Mendelian inheritance, all patients were homozygous for their mutation, whereas all parents were heterozygous.
The fact that the mutations of the Turkish and Arab children are different may help explain the clinical differences between the two groups of patients, particularly their response to oral fucose therapy (8, 16, 17). Whereas the Turkish child showed increased expression of certain fucosylated ligands and improved in clinical symptoms except mental retardation following fucose therapy (16), the Arab children did not, even when treated with the same protocol as the Turkish child (8, 17). One might speculate that the mutation in the Turkish child decreases the transporter’s affinity for GDP-fucose. Increasing the cytosolic concentration of fucose following administration of this sugar leads to increased synthesis of the nucleotide sugar, perhaps allowing for some transport of GDP-fucose despite the decreased affinity of the mutated transporter for this substrate (Figure 1). The disease allele seen in the Arab children encodes a protein with wild-type affinity for GDP-fucose but decreased activity as a transporter, which may explain why fucose supplementation is not beneficial for these individuals (8, 17).
Cellular consequences of Golgi transport defects
Two lines of evidence suggest that transport of nucleotide derivatives into the Golgi lumen regulates which macromolecules undergo posttranslational modifications in the Golgi lumen. First, my group has characterized a mutant Madin-Darby canine kidney (MDCK) cell line with a 2–5% residual transport activity of UDP-galactose and a consequent loss of galactose incorporation into glycoproteins, glycolipids, and keratan sulfate proteoglycans, which contain large quantities of galactose in their associated glycosaminoglycan (GAG) chains. Chondroitin sulfate and heparan sulfate proteoglycans also contain galactose, but the sugar is found only in the linkage region to the protein, not in the disaccharide repeats that make up the bulk of the GAG chains, and these classes of proteoglycans are not reduced in the mutant cell line (18). One hypothesis for this phenotype holds that the Km’s for galactosyltransferases of the latter proteoglycans are lower than those for other galactosylation reactions: this would then lead to their preferential galactosylation when supply of nucleotide sugar becomes limiting. We speculate that a similar effect permits some degree of selective fucosylation of macromolecules when cells from LADII patients are grown in the presence of fucose — and when the Turkish child was administered fucose orally. In this instance, some fucosylated epitopes, including leukocyte P-selectin ligands, reappeared, while others, such as the H blood group epitope, did not (16).
A second line of evidence supports regulation of posttranslational modifications at the level of the transporter/antiport cycle. Because transport of nucleotide derivatives into the Golgi lumen is coupled to countertransport of the corresponding nucleoside monophosphate (19), the cycle depends on the continuous production of nucleoside monophosphates. Although earlier studies had detected nucleoside diphosphatases in the lumen of mammalian Golgi apparatus, direct evidence for the in vivo role of the nucleotide sugar transport/nucleoside monophosphate antiport cycle comes from experiments with Sacchasomyces cerevisiae in which the Golgi GDPase gene (GDA1) was deleted. The null mutant showed severely decreased in vitro transport into Golgi vesicles of GDP-mannose (20), a nucleotide sugar that is transported into Golgi vesicles of some eukaryotes, but not mammals. While all glycoproteins and glycolipids in the S. cerevisiae gdal null mutant showed impaired mannosylation of the Golgi mannan chains, some proteins had chains that were considerably shorter than others, indicating differential mannosylation. Again, this is probably the result of different affinities of the various mannosyltransferases for GDP-mannose, an effect that is only observed because, with low levels of lumenal GMP and reduced antiporter function, the nucleotide sugar becomes limiting for certain of the biosynthetic enzymes (20).
Mammalian mutant cells defective in transport of specific nucleotide sugars have been isolated based on their resistance to lectin cytotoxicity (10). Subsequently, nucleotide sugar transport mutants were found in other eukaryotes including yeast, plants, Leishmania donovani (21), and, more recently, Caenorhabditis elegans (22) and Drosophila melanogaster (23). All these mutants have a severe reduction of the corresponding sugar in their macromolecules, due to defective transport of a sugar nucleotide (10). The L. donovani mutant is deficient in transport of GDP-mannose into the Golgi apparatus, a function which does not occur in mammals (10). The mutation results in a deficiency in lipophosphoglycan, a complex mannosylated glycolipid on the cell surface, whose absence renders the mutant avirulent. The C. elegans mutants have point mutations in the SQV-7 gene, which encodes a novel transporter for UDP-glucuronic acid, UDP-N-acetylgalactosamine, and UDP-galactose (22). SQV-7 plays a pivotal role in epithelial invagination and early embryogenesis. The mutants, which have a squashed vulva phenotype, accumulate reduced levels of chondroitin and heparan sulfate, due to a deficiency in Golgi transport of the nucleotide sugar substrates required for their synthesis. In D. melanogaster the fringe connection gene (frc) encodes a nucleotide sugar transporter for UDP-glucuronic acid and UDP-N-acetylglucosamine (23). These nucleotide substrates are essential for the synthesis of heparan sulfate chains involved in Wnt/Wingless, Hedgehog, and FGF signaling, as well as for the fringe protein-dependent glycosylation of Notch, a modification that is involved in several signaling pathways in metazoans. These studies in lower and higher eukaryotes demonstrate that posttranslational modifications of most secreted and membrane proteins and glycosphingolipids can be profoundly affected by genes encoding proteins of the transport cycle (10) and illustrate their functional conservation during evolution.
Structure and function of the Golgi nucleotide transporters
To date several of the Golgi nucleotide transporters have been cloned. They are all multitransmembrane, hydrophobic proteins that appear to assemble as homodimers. These proteins can share as much as 50% or 60% primary amino acid sequence identity while exhibiting distinct substrate specificities. On the other hand, transporters from different organisms that share as little as 20% amino acid sequence identity can exhibit identical nucleotide sugar transport specificities. For example, the transporter for UDP-N-acetylglucosamine from MDCK cells was cloned by correcting a yeast mutant in this transport, indicating that targeting of these proteins to the Golgi and functionality are conserved through evolution (11). Yeast, which expresses few endogenous transporters, provide a useful expression system in which to determine the substrate specificity of putative transporters. The Golgi vesicles from the heterologously transformed yeast can be then assayed for transport of different nucleotide sugars (11).
Searching for novel diseases of nucleotide transport
Predicting the cellular or organismal phenotype of a defect in transporter function is complicated for several reasons. First, as seen in the case of LADII, the effects may be pleiotropic, leading to major pathological consequences in many organ systems. Conversely, some nucleotide derivatives may be imported into Golgi apparatus of different tissues by more than one transporter, in which case mutations in specific transporters may be difficult to detect.
Assuming that GDP-fucose transport is only due to one transporter in humans and that there is only one transporter for CMP–sialic acid, UDP-N-acetylgalactosamine, and PAPS, we speculate that mutants deficient in any of these activities will lead to a clear defect in glycan synthesis, and thus may be somewhat more easily detected than mutants in other transporters. For example, mutants partially deficient in CMP–sialic acid transport may show abnormal patterns of isoelectrofocusing mobility of transferrin, may have reduced sialyl Lewis X antigens, and may have reduced levels of other sialoglycoproteins and/or gangliosides and lower serum half-lives of the former. Should the above phenotypes be observed, it would be reasonable first to assay CMP–sialic acid levels and then (absent evidence for a defect in the biosynthesis of this molecule) to focus on the relevant nucleotide sugar transporters. Similarly, a partial defect in UDP-N-acetylgalactosamine transport may be suspected in individuals with H blood group antigens (which lack N-acetylgalactosamine) if either of their parents is of the AA or AB blood group type, since the A antigen has N-acetylgalactosamine. The synthesis of the N-acetylgalactosamine–containing Forssman antigen and of the secreted polymer mucin would also likely be deficient in such individuals. Similarly, a decrease in PAPS transport should result in a general hyposulfation, including a decrease in sulfo-Lewis structures as well as decreased sulfated proteoglycans, including but not limited to heparan sulfate, heparin, and chondroitin sulfate. Possible coagulation defects are also likely to occur because of the role of sulfation in heparin function.
The availability of the complete human genomic sequence may provide complementary means to identify possible transporter defects. Once functions are attributed to the different putative Golgi nucleotide transporters of the human genome, sequencing of these genes in patients with broad clinical symptoms such as LADII may help in identifying additional cases of Golgi nucleotide transporter diseases — diseases that are likely to occur and perhaps to be treatable with simple dietary supplementation, but that have been impossible to diagnose so far.
Acknowledgments
I thank Jacques Baenziger, Amos Etzioni, John Lowe, Claudia Abeijon, and Patricia Berninsone for helpful editorial suggestions, and Jacqueline Guinyard and Troy Tyree for expert editorial assistance. The research in the author’s laboratory was supported by NIH grants GM-30365 and -34396.
References
- 1.Etzioni A, et al. Brief report: recurrent severe infections caused by a novel leukocyte adhesion deficiency. N Engl J Med. 1992;327:1789–1792. doi: 10.1056/NEJM199212173272505. [DOI] [PubMed] [Google Scholar]
- 2.Frydman M, et al. Rambam-Hasharon syndrome of psychomotor retardation, short stature, defective neutrophil motility, and Bombay phenotype. Am J Med Genet. 1992;44:297–302. doi: 10.1002/ajmg.1320440307. [DOI] [PubMed] [Google Scholar]
- 3.Becker DJ, Lowe JB. Leukocyte adhesion deficiency type II. Biochim Biophys Acta. 1999;1455:193–204. doi: 10.1016/s0925-4439(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 4.Marquardt T, et al. Leukocyte adhesion deficiency II syndrome, a generalized defect in fucose metabolism. J Pediatr. 1999;134:681–688. doi: 10.1016/S0022-3476(99)70281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karsan A, et al. Leukocyte adhesion deficiency type II is a generalized defect of de novo GDP-fucose biosynthesis. J Clin Invest. 1998;101:2438–2445. doi: 10.1172/JCI905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturla L, et al. Defective intracellular activity of GDP-D-mannose-4,6-dehydratase in leukocyte adhesion deficiency type II syndrome. FEBS Lett. 1998;429:274–278. doi: 10.1016/s0014-5793(98)00615-2. [DOI] [PubMed] [Google Scholar]
- 7.Koerner C, et al. Decreased availability of GDP-L-fucose in a patient with LAD II with normal GDP-D-mannose dehydratase and FX protein activities. J Leukoc Biol. 1999;66:95–98. doi: 10.1002/jlb.66.1.95. [DOI] [PubMed] [Google Scholar]
- 8.Sturla L, et al. Impairment of the golgi gdp-l-fucose transport and unresponsiveness to fucose replacement therapy in lad II patients. Pediatr Res. 2001;49:537–542. doi: 10.1203/00006450-200104000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Luebke T, Marquardt T, von Figura K, Korner C. A new type of carbohydrate-deficient glycoprotein syndrome due to a decreased import of GDP-fucose into the golgi. J Biol Chem. 1999;274:25986–25989. doi: 10.1074/jbc.274.37.25986. [DOI] [PubMed] [Google Scholar]
- 10.Hirschberg CB, Robbins PW, Abeijon C. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Ann Rev Biochem. 1998;67:49–69. doi: 10.1146/annurev.biochem.67.1.49. [DOI] [PubMed] [Google Scholar]
- 11.Berninsone PM, Hirschberg CB. Nucleotide sugar transporters of the Golgi apparatus. Curr Opin Struct Biol. 2000;10:542–547. doi: 10.1016/s0959-440x(00)00128-7. [DOI] [PubMed] [Google Scholar]
- 12.Luehn K, Wild MK, Eckhardt M, Gerardy-Schahn R, Vestweber D. The defective gene in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat Genet. 2001;28:69–72. doi: 10.1038/ng0501-69. [DOI] [PubMed] [Google Scholar]
- 13.Luebke T, et al. Complementation cloning identifies CDG-Iic, a new type of congenital disorders of glycosylation, as a GDP-fucose transporter deficiency. Nat Genet. 2001;28:73–76. doi: 10.1038/ng0501-73. [DOI] [PubMed] [Google Scholar]
- 14.Puglielli L, Hirschberg CB. Reconstitution, identification and purification of the rat liver Golgi membrane GDP-fucose transporter. J Biol Chem. 1999;274:35596–35600. doi: 10.1074/jbc.274.50.35596. [DOI] [PubMed] [Google Scholar]
- 15.Etzioni, A., et al. 2001. Leukocyte adhesion deficiency (LAD) type II/carbohydrate deficient glycoprotein (CDG)IIc-founder effect and genotype/phenotype correlation. J. Pediatrics. In Press. [DOI] [PubMed]
- 16.Marquardt T, et al. Correction of leukocyte adhesion deficiency type II with oral fucose. Blood. 1999;94:3976–3985. [PubMed] [Google Scholar]
- 17.Etzioni A, Tonetti M. Fucose supplementation in leukocyte adhesion deficiency type II. Blood. 2000;95:3641–3643. [PubMed] [Google Scholar]
- 18.Toma L, Pinhal MA, Dietrich CP, Nader HB, Hirschberg CB. Transport of UDP-galactose into the Golgi lumen regulates the biosynthesis of proteoglycans. J Biol Chem. 1996;271:3897–3901. doi: 10.1074/jbc.271.7.3897. [DOI] [PubMed] [Google Scholar]
- 19.Capasso JM, Hirschberg CB. Mechanisms of glycosylation and sulfation in the Golgi apparatus: evidence for nucleotide sugar/ nucleotide monophosphate and nucleotide sulfate/ nucleoside monophosphate antiporters in the Golgi apparatus membrane. Proc Natl Acad Sci USA. 1984;81:7051–7055. doi: 10.1073/pnas.81.22.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abeijon C, et al. Guanosine diphosphatase is required for protein and sphingolipid glycosylation in the Golgi lumen of Saccharomyces cerevisiae. J Cell Biol. 1993;122:307–323. doi: 10.1083/jcb.122.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma D, Russell DG, Beverly SM, Turco SJ. Golgi GDP-mannose uptake requires Leishmania LPG2. A member of a eukaryotic family of putative nucleotide-sugar transporters. J Biol Chem. 1997;272:3799–3805. [PubMed] [Google Scholar]
- 22.Berninsone PM, Hwang HY, Zemtseva I, Horvitz HR, Hirschberg CB. SQV-7, a protein involved in Caenorhabditis elegans epithelial invagination and early embryogenesis, transports UDP-glucuronic acid, UDP-N- acetylgalactosamine, and UDP-galactose. Proc Natl Acad Sci USA. 2001;98:3738–3743. doi: 10.1073/pnas.061593098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selva, E.M., et al. 2001. Dual role of the fringe connection gene in both wingless and fringe-dependent signaling events. Nat. Cell Biol. In press. [DOI] [PubMed]
- 24.Nordeen MH, Jones SM, Howell KE, Caldwell JH. GOLAC: an endogenous anion channel of the Golgi complex. Biophys J. 2000;78:2918–2928. doi: 10.1016/S0006-3495(00)76832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]