Abstract
p27Kip1 is an important regulator of cyclin-dependent kinases. Studies with p27 knockout mice have revealed abnormalities in proliferation and differentiation of multiple cell types. Here we show that primary hepatocytes isolated from livers of adult p27 knockout mice exhibit higher levels of DNA synthesis activity in culture than do wild-type cells. Interestingly, we found that, compared with control hepatocytes, p27 knockout hepatocytes proliferate better after transplantation into diseased livers to reverse liver failure. These results reveal an aspect of p27 that could be used to benefit cell-based therapy.
Introduction
p27Kip1 stably interacts with various cyclin/Cdk complexes and inhibits cyclin E/Cdk2 and cyclin A/Cdk2 kinases (1, 2). Consistent with its role as a negative regulator of cell proliferation, p27 knockout mice are about 25% larger than wild-type littermates (3–5). Most organs of p27 knockout mice have postmitotic and properly differentiated cells in greater numbers. The phenotypes of cells cultured from p27 knockout mice are cell-type specific. Mouse embryo fibroblasts from p27 knockout mice are normal in their ability to be serum starved and stimulated to reenter the cell cycle (3–5). In contrast, p27 knockout oligodendrocyte progenitors have a higher proliferative potential in culture before they exit the cell cycle and differentiate (6, 7). These in vivo and in vitro phenotypes establish that p27 plays an important role in cell cycle regulation of certain cell types. Since the lack of p27 led to an increased number of well-differentiated cells in many organs, we sought to investigate whether targeting p27 could be beneficial for cell-based therapies in which the success of the therapy depends on the extent of cell proliferation.
In this study, we investigated the role of p27 in hepatocyte proliferation in vitro and after transplantation into diseased livers. Our results show that in culture, primary hepatocytes purified from p27 knockout mice have increased DNA synthesis and Cdk2-kinase activities. Interestingly, when transplanted into a mouse model of liver failure, p27 knockout cells are better able to proliferate and rescue the animals from liver failure. These results reveal an important role of p27 in hepatocyte proliferation regulation that may be useful in improving hepatocyte-based therapy of liver disease.
Methods
Mice.
The p27+/– mice (129/Sv × C57BL/6) were a kind gift from Andrew Koff (Memorial Sloan-Kettering Cancer Center, New York, New York, USA) (4) and were bred to generate p27 knockout and wild-type mice. Fumarylacetoacetate hydrolase (FAH) knockout mice (129/Sv) were maintained with the drug 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC; a gift from Swedish Orphan AB, Stockholm, Sweden) in the drinking water (7.5 μg/ml). Mouse colonies were maintained in a pathogen-free environment with 12-hour light/dark cycles. Animal care and experiments were conducted in accordance with US federal guidelines and under protocols approved by the Animal Care Use Committee of the Albert Einstein College of Medicine.
Liver perfusion.
Livers from 2- to 3-month-old p27 wild-type and p27 knockout littermates were perfused with a standard two-step perfusion protocol (8). This procedure typically yielded 1 × 107 to 2 × 107 viable hepatocytes (>90% pure after isodensity Percoll centrifugation) per mouse.
Western blots and kinase assays.
Livers were homogenized by 15 strokes in a Dounce homogenizer in ELB (50 mM HEPES, pH 7.0, 250 mM NaCl, 5 mM EDTA, 0.1% NP-40, 1 mM DTT, with protease and phosphatase inhibitors) and sonicated. Lysates were clarified by centrifugation for 10 minutes at 12,000 g. Purified hepatocytes were plated on either Primaria (Sigma Chemical Co., St. Louis, Missouri, USA) or rat tail collagen-coated plates in attachment media (DMEM with 10% FBS). After attachment for 2–4 hours, cells were washed once with PBS and changed to DMEM/F12, 10–7 M dexamethasone, and ITS (5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenium; Biofluids Inc., Rockville, Maryland, USA). At various time points, cells were harvested and lysed in ELB. For Western blot analysis, 20 μg per sample was boiled in Laemmli sample buffer, run on 6% (for p130 phosphorylation) or 10% SDS gels, blotted to PVDF, and probed with Ab’s to p27 (C19), cyclin A (H432), cyclin E (M20), Cdk2 (M2), and p107 (C18; all from Santa Cruz Biotechnology Inc., Santa Cruz, California, USA). Anti-FAH Ab was kindly provided by Robert M. Tanguay (University of Laval, Ste-Foy, Quebec, Canada). Equal loading was confirmed by uniform India ink staining of the membranes and equal intensities of bands cross-reacting with the various Ab’s. For Cdk2-kinase assays, 80 μg of protein was mixed with 300 ng anti-Cdk2 Ab (M2) in 200 μl ELB with protease and phosphatase inhibitors for 1 hour on ice. Ab’s were collected with protein A beads for 1 hour. Beads were washed three times in ELB with inhibitors, and twice in Cdk2-kinase buffer A (50 mM HEPES, pH 7.0, 10 mM MgCl2, 5 mM MnCl2, 1 mM DTT), and kinase assays were performed in 20 μl buffer A containing 10 μCi 32P-γ-ATP, 2 μg histone H1, and 10 μM cold ATP for 20 minutes at 30°C. Kinase assays were stopped with Laemmli sample buffer, boiled, and loaded on 12% SDS gels.
DNA synthesis determination.
Tritiated thymidine incorporation was determined by scintillation counting of trichloroacetic acid–precipitable materials, and DNA synthesis activity was presented as counts per minute per microgram of total cellular protein. For bromodeoxyuridine (BrdU) incorporation, hepatocytes were labeled with 10 μM BrdU for 24 hours and fixed in 0.2% formaldehyde. Cells were permeabilized with 70% ethanol, and BrdU incorporation was detected by indirect immunofluorescence with anti-BrdU mAb NA-20 (Calbiochem-Novabiochem Corp., San Diego, California, USA) and a FITC-labeled secondary Ab (Pierce Chemical Co., Rockford, Illinois, USA). Nuclei were stained with Hoechst 33258. For each sample, 300–400 nuclei were counted and scored for BrdU incorporation.
Hepatocyte transplantation and tissue processing.
Hepatocytes (105 cells in 50 μl) were injected into the spleens of 2- to 3-month-old male FAH knockout mice using a Hamilton gas-tight syringe fitted with a 26-gauge needle. The injection site was ligated with silk suture to prevent spillage of the transplanted cells out of the spleen. NTBC treatment was discontinued on the day of the transplantation. The weights and general conditions of the animals were monitored 2–3 times a week. Livers were fixed in 10% buffered formalin and processed for staining with hematoxylin and eosin (H&E). Anti-FAH immunostaining was performed as described previously (9) using the IHC AEC kit (InnoGenex, San Ramon, California, USA).
Quantitation of repopulation extent.
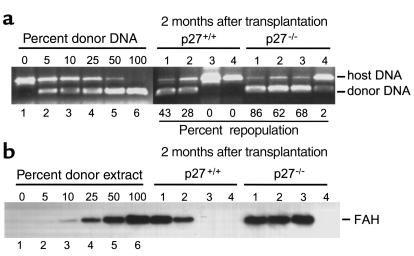
Both FAH DNA and protein quantitations were performed on material isolated from the same piece of liver tissue. Livers were homogenized in ELB as described above and clarified by centrifugation. Supernatants were removed and prepared for Western blot analysis for FAH protein as described above. A standard curve was generated by mixing FAH–/– liver extracts with p27+/+ liver extracts. Pellets containing nuclei were resuspended and digested overnight in digestion buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 20 mM EDTA, 0.5% SDS, and 0.5 mg/ml proteinase K). DNA was isolated by isopropanol precipitation, and 150 ng genomic DNA was used in PCR reactions for FAH genomic sequences as described previously (10). For quantitation of repopulation extent, ratios of donor/host (FAH wild-type/FAH mutant) PCR bands, as determined by ImageQuant software, were interpolated against a standard curve generated by PCR of artificially mixed donor and host genomic DNA (see Figure 5a, lanes 1–6). Since hepatocytes in a normal liver constitute about 60% of the cells, the value obtained was further multiplied by 1.67 to yield the actual repopulation percentage used in the text.
Figure 5.
Quantitation of repopulation efficiencies. (a) Semi-quantitative PCR analysis of FAH genomic sequences. Primers for FAH mouse genotyping (10) were used in PCR on genomic DNA from livers of transplanted animals. In lanes 1–6, known amounts of wild-type and FAH knockout genomic DNA were premixed (the percentages of FAH wild-type donor DNA are indicated) for PCR to serve as reference points. The ratio of host/donor DNA bands was used to calculate repopulation percentages (see Methods) indicated under each animal (four mice for each genotype). (b) Western blot for FAH protein in transplanted livers as indicated. Similar to a, known amounts of extracts from wild-type and FAH knockout livers were mixed in lanes 1–6 at the indicated ratios. The four animals from each genotype were the same as those in a.
Results
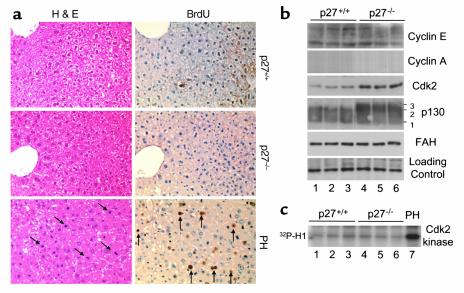
We first determined the proliferation status of hepatocytes in the livers of adult p27 knockout mice. Livers of 2-month-old wild-type and p27 knockout mice (4) contained similarly rare S-phase hepatocytes determined by in vivo BrdU labeling, and mitotic hepatocytes, determined by chromosome condensation morphology (Figure 1a). Western blot analysis (Figure 1b) showed equally low levels of cyclins E and A in the two types of livers. Cdk2 content was elevated about threefold in p27 knockout livers, but the CAK phosphorylated, faster-migrating species was not present. Consistently, Cdk2-associated kinase activity was equally low in both types of livers compared with the amount of Cdk2-kinase activity present in the remnant liver 2 days after 70% partial hepatectomy of the wild-type mice (Figure 1c). Livers of p27 knockout mice contained slightly higher levels of p130, which was, most notably, more hyperphosphorylated, as demonstrated by the conversion of form 2 to form 3 (Figure 1b). It is known that phosphorylation form 2 of p130 is specific for quiescent cells and form 3 appears in early G1 (11, 12). Several serine and threonine residues in the spacer region and B pocket have been identified recently as the sites of phosphorylation in G0 and early G1 (13). Although these residues exist in consensus Cdk phosphorylation sequences, the identities of the kinases responsible for their phosphorylation are not clear since known cyclin-dependent kinases are not active at these times. Taken together, these results reveal that, although hepatocytes and possibly nonparenchymal liver cells of the adult p27 knockout livers do exhibit some differences in Cdk2 levels and p130 phosphorylation compared with wild-type livers, they are not proliferative.
Figure 1.
Proliferation status of p27 wild-type and knockout livers. (a) Histological (H&E) and BrdU immunohistochemistry analyses for wild-type and p27 knockout livers as indicated. Wild-type liver 2 days after partial hepatectomy (PH) is shown for comparison. Diagonal and vertical arrows denote mitotic figures and BrdU-positive cells, respectively. All photographs are at ×400. (b) Western blots on quiescent liver extracts. Three animals from each genotype are shown (lanes 1, 2, and 3, wild-type; lanes 4, 5, and 6, p27 knockout). Hyperphosphorylated forms of p130 are indicated. (c) Cdk2 immunoprecipitation-kinase assay on quiescent liver extracts. Three animals from each genotype are shown, and wild-type liver 2 days after partial hepatectomy (PH) is shown for comparison.
We next determined whether the lack of p27 had an effect on hepatocyte proliferation in primary culture. Primary hepatocytes were isolated using a two-step perfusion protocol and cultured in defined media (see Methods section). It is known that primary mouse hepatocytes isolated with this protocol can be stimulated to enter S phase by hepatocyte mitogens, such as EGF. In this study, we used two methods to measure DNA synthesis activity. Tritiated thymidine incorporation of the whole culture measures the total DNA synthesis activity, while BrdU labeling by immunofluorescence staining reveals the actual number of cells that are progressing through S phase.
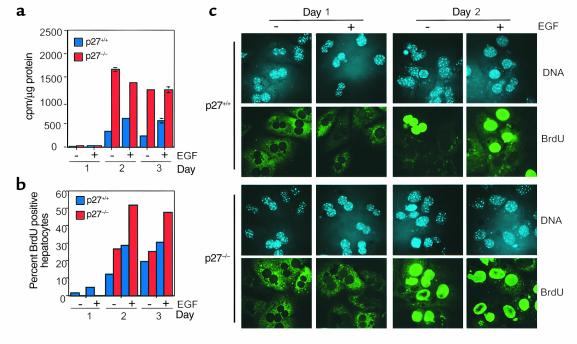
After 1 day in culture, no significant DNA synthesis activities (as determined by both tritiated thymidine incorporation and BrdU labeling) were observed in either wild-type or p27 knockout hepatocytes in either the absence or presence of EGF (Figure 2, a and b). This result was consistent with the above finding that p27 knockout hepatocytes were not proliferative in adult liver. At day 2, wild-type hepatocytes contained low DNA synthesis activities in the absence of EGF (12% BrdU positive; Figures 2, a and b). Addition of EGF stimulated DNA synthesis activities (tritiated thymidine incorporation increased 1.8-fold and 29% of the cells were BrdU positive). In comparison, p27 knockout hepatocytes contained significantly elevated DNA synthesis activity at day 2, even in the absence of EGF (tritiated thymidine incorporation increased fivefold over wild-type hepatocytes, and 27% of the knockout hepatocytes were BrdU positive). Addition of EGF further stimulated BrdU incorporation 1.9-fold to 51%. At day 3 of the primary culture, DNA synthesis activities subsided somewhat in both wild-type and p27 knockout cultures, but were still higher in p27 knockout hepatocytes. Representative fields of BrdU staining shown in Figure 2c demonstrated that most of the p27 knockout hepatocyte clusters in day-2 culture are positive for BrdU staining. Thus, p27 knockout hepatocytes were better able to enter S phase in primary culture than wild-type hepatocytes.
Figure 2.
DNA synthesis in cultured hepatocytes. Hepatocytes of the indicated genotypes were cultured in the presence and absence of EGF as indicated and labeled with 5 μCi tritiated thymidine or 10 μM BrdU for 24 hours before harvest on different days in culture. (a) Tritiated thymidine incorporation. The data presented are means ± SEM and are representative of five separate experiments with similar results. (b) BrdU incorporation. For each sample, 300–400 nuclei were scored for BrdU incorporation. (c) Immunofluorescence staining for BrdU incorporation in hepatocytes. Nuclei were stained with Hoechst 33258, and BrdU was detected with anti-BrdU primary and FITC-labeled secondary Ab’s. Cells were photographed at ×400 with an Olympus IX70 inverted fluorescence microscope (Olympus America Inc., Melville, New York, USA) and digital camera (cooled CCD camera Photometrics PXL; Roper Instruments, Tuscon, Arizona, USA). All data presented are from the same animals.
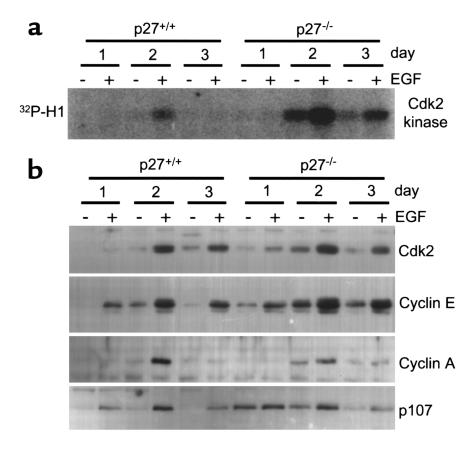
Biochemical analysis showed that Cdk2-kinase activity was increased in primary p27 knockout hepatocytes. Initially, both wild-type and p27 knockout hepatocytes contained no detectable Cdk2-kinase activity at day 1 in culture (Figure 3a), consistent with the results obtained from the liver samples (Figure 1c). At day 2, in the absence of EGF, wild-type hepatocytes lacked detectable Cdk2-kinase activity that was then stimulated by the presence of EGF. Since day 2 hepatocyte cultures in the absence of EGF contained low levels of DNA synthesis activity (Figures 2, a and b), the amount of Cdk2-kinase activity necessary for S-phase entry is likely below the level of sensitivity of our assay conditions. The lack of p27 led to significantly elevated Cdk2-kinase activity in p27 knockout hepatocytes at day 2 even in the absence of EGF. Addition of EGF further elevated Cdk2-kinase activity. Since BrdU incorporation (Figure 2b), but not tritiated thymidine incorporation (Figure 2a), was further increased by EGF treatment on day 2, it appears that Cdk2-kinase activity correlated more closely with BrdU incorporation than with tritiated thymidine incorporation. At day 3 of primary culture, Cdk2-kinase activities returned to baseline levels in wild-type hepatocytes, but remained significantly elevated in p27 knockout hepatocytes. These results demonstrated that, in the absence of p27, Cdk2 was more easily activated in either the presence or absence of EGF.
Figure 3.
Cdk2-kinase activity and expression of late G1/S-phase cell cycle regulators in cultured hepatocytes. (a) Cdk2 immunoprecipitation-kinase activity in hepatocytes cultured with or without EGF for the indicated times after isolation from the liver. (b) Western blots on extracts from cultured hepatocytes with the indicated Ab’s. The same extracts were analyzed in a and b and were derived from the same animals used for Figure 2.
During cell cycle entry, after growth factor stimulation of many cell types, levels of Cdk2, cyclin E, and the pocket protein p107 increase during the progression from G0 to S phase, and cyclin A is expressed at the G1/S transition and thus serves as a marker of S phase. Cdk2 (including the CAK phosphorylated, faster-migrating form resulting in a tight doublet in our gels), cyclin E, and p107 were higher in p27 knockout hepatocytes than in wild-type hepatocytes at parallel time points, consistent with the above finding that p27 knockout hepatocytes entered S phase more actively than wild-type hepatocytes. Compared with the more significant increases in Cdk2-kinase activity (Figure 3a), the relatively smaller differences in protein levels (Figure 3b) indicated that the lack of the p27 inhibitor was the primary mechanism for the activation of Cdk2 kinase.
It is known that in the culture conditions we used for DNA synthesis analysis, primary mouse hepatocytes are able to enter S phase but unable to proliferate even in the presence of hepatocyte mitogens (14). Rather, they show signs of morphological changes (spreading) and cell death after a few days in culture. In this respect, p27 knockout hepatocytes did not differ from wild-type hepatocytes. Cell numbers in both types of hepatocyte cultures increased 1.4-fold over a period of 5 days (data not shown), consistent with previous reports on wild-type hepatocytes (15). Starting from day 4 in culture, both wild-type and p27-null hepatocytes suffered from gradual cell death as judged by the gradual appearance of floating cells in culture. Results from measurements of DNA synthesis and cell population expansion demonstrate that lack of p27 led to increased S-phase entry but did not render primary hepatocytes with increased potential to actually divide and proliferate in the culture conditions used.
We next determined the proliferation potential of p27 knockout hepatocytes after transplantation into livers of p27 wild-type mice with liver failure. We used mice deficient in FAH as a model (10). The lack of FAH leads to the accumulation of tyrosine degradation intermediates that are toxic to hepatocytes. FAH knockout mice treated with NTBC, which inhibits an upstream enzyme in the tyrosine degradation pathway, can live to adulthood (16), but die from liver failure after discontinuation of NTBC treatment. Transplantation of hepatocytes from congenic wild-type male mice into female FAH knockout recipients led to repopulation of diseased livers with transplanted cells and rescue of the animals after NTBC withdrawal (17).
To determine whether p27 knockout hepatocytes have a better ability to proliferate and rescue FAH knockout mice, we exploited two new aspects of the FAH transplantation model. First, we have found that it is possible to repopulate the livers of FAH knockout mice with allogeneic hepatocytes in the absence of immunosuppressive drugs (M. Grompe, unpublished observations). This likely is due to the immunosuppressive action of succinylacetone (18), the key metabolite accumulated in tyrosinemia. We postulated that allogeneic hepatocytes with more active proliferation potentials may have an advantage in rescuing liver failure under this less-optimal transplantation condition, which more closely resembles human hepatocyte transplantation where only allogeneic donor cells are available. With these considerations, we used wild-type or p27 knockout mice on a 129/Sv × C57BL/6 hybrid background as donors and FAH knockout mice on a 129/Sv background as recipients. Second, we have observed that male FAH knockout mice suffer from more acute liver failure and die faster than female FAH knockout mice after NTBC withdrawal (M. Grompe, unpublished observations). Since it is more difficult to rescue acute, rapidly progressing liver failure (19), we chose to use male FAH mice as recipients to determine whether p27 knockout hepatocytes are better at rescuing liver failure.
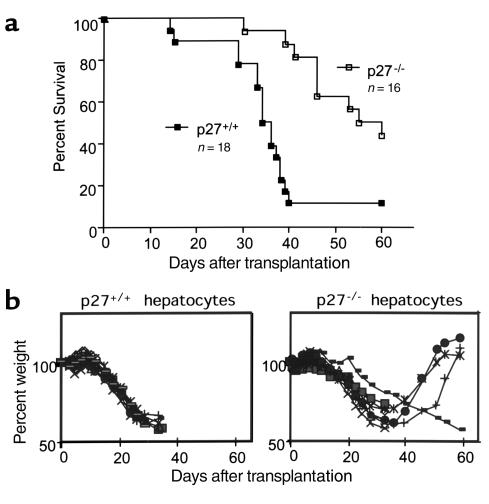
Twenty-three mice were transplanted with male wild-type hepatocytes and 25 with male p27 knockout hepatocytes in a total of six separate perfusion-transplantation experiments. Five mice transplanted with wild-type hepatocytes died within 6 days of surgery and were not included in the data analysis to avoid confounding results from postoperative complications (death from liver failure should take place between 1 and 2 months after NTBC withdrawal; ref. 17 and see Figure 4a). Several mice from each group were sacrificed at 1 month after transplantation for histological analysis. The survival curves for the 2-month period after transplantation demonstrated that it was indeed difficult to rescue male FAH knockout mice with wild-type hepatocytes. However, mice transplanted with p27 knockout hepatocytes had significantly better survival than those transplanted with wild-type hepatocytes (Figure 4a). The median survival of animals transplanted with wild-type and p27 knockout hepatocytes was 35.0 and 57.5 days, respectively (P = 0.0003), and the survival rates at 2 months after transplantation were 11% (2/18) and 50% (8/16), respectively. Mice sacrificed at 1 month after transplantation, and therefore not included in the survival curve, were moribund in the group receiving wild-type hepatocytes, but were generally in good condition (i.e., displayed normal appearance, physical activity, and reactions to handling) in the group receiving p27 knockout hepatocytes, suggesting that the difference in rescue could be bigger had these mice not been sacrificed for analysis.
Figure 4.
Rescue of male FAH knockout mice by hepatocytes. (a) Kaplan-Meier survival curve of FAH knockout mice after hepatocyte transplantation and withdrawal of NTBC treatment. The graph does not include animals that were excluded before their deaths or sacrificed (see text). The difference in the two curves is statistically significant (P = 0.0003). (b) Body weight measurements of transplanted animals. The results are from one pair of donors (wild-type and p27 knockout), each transplanted into eight hosts. Body weight at the time of transplantation is indicated as 100%. In this transplantation experiment, one animal in the wild-type hepatocyte group was sacrificed before its imminent death (square), and four generally healthy animals in the p27 knockout hepatocyte group (square, diamond, cross, and open circle) were sacrificed at 30 days for histological analysis. All the remaining animals in the wild-type hepatocyte group died before day 40, but survived to 2 months in knockout hepatocyte group. One animal in the knockout hepatocyte group (rectangle) survived without regaining body weight.
Figure 4b shows body weights of the two groups of mice that received wild-type or p27 knockout hepatocytes in the same experiment (i.e., each group received hepatocytes from one donor mouse). Weight loss after transplantation (and withdrawal of NTBC) followed similar kinetics in both groups, the body weights decreasing to about 60% of the original body weight by 1 month. After that, most of the mice transplanted with p27 knockout hepatocytes (except one) started to regain body weight, whereas mice transplanted with wild-type hepatocytes died.
Next, we performed semiquantitative PCR for FAH genomic sequences to measure the extent of liver repopulation by transplanted hepatocytes. Mixing known amounts of genomic DNA from wild-type and FAH-null liver demonstrated that the detection threshold was about 5% repopulation (Figure 5a, lanes 1 to 6). At the 1-month time point, repopulation was not detectable for mice transplanted with either wild-type or p27 knockout hepatocytes (not shown). We then examined four mice from each transplantation group at the 2-month time point, including four survivors from the wild-type transplanted mice (all the mice that survived) and four from the p27 knockout transplanted mice (out of eight that survived). Of the four mice transplanted with wild-type hepatocytes, two did not contain detectable FAH sequences, one had 28% repopulation, and one had 43% repopulation (Figure 5a). In contrast, the four mice transplanted with p27 knockout hepatocytes contained 2%, 62%, 68%, and 86% repopulation. These results showed that survival up to 2 months did not necessarily indicate successful repopulation by the transplanted hepatocytes. In fact, every mouse that survived to 2 months, but did not regain its body weight, lacked detectable repopulation (such as the one mouse in Figure 4b). Thus, of the mice that survived to the 2-month time point, livers transplanted with p27 knockout hepatocytes were repopulated to a greater extent overall.
We also examined FAH protein levels in these same livers. As shown in Figure 5b (lanes 1 to 6), the sensitivity of FAH protein detection is between 5% and 10% repopulation. Of the eight mice examined with PCR in Figure 5a, levels of FAH protein were proportional to the corresponding levels of FAH sequences. This result suggested that p27 knockout hepatocytes expressed similar amounts of FAH protein, consistent with the finding that livers of p27 knockout mice contained the same amount of FAH protein as wild-type livers (Figure 1b).
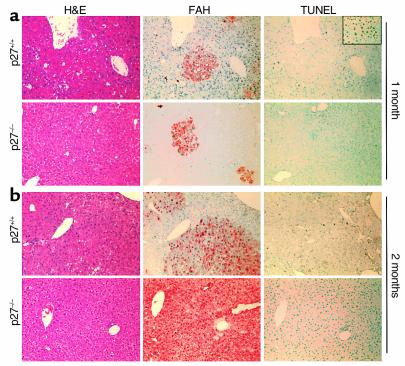
We performed FAH staining to visualize the presence of transplanted hepatocytes in host livers. Livers from animals sacrificed at 1 month after transplantation with either wild-type or p27 knockout hepatocytes showed scattered clusters of FAH-positive hepatocytes (Figure 6a), indicating engraftment and proliferation of both types of cells in the recipient livers. As a reference to the PCR and Western blot analyses at 2 months after transplantation, the representative animals analyzed in Figure 6b (2–month time point) correspond to no. 2 of the wild-type transplanted group and no. 3 of the p27 knockout transplanted group presented in Figure 5. As shown in Figure 6b, the liver that received p27 knockout hepatocytes contained large areas of FAH-positive hepatocytes after 2 months, demonstrating that transplanted hepatocytes had proliferated significantly to repopulate the recipient liver. In the liver that received wild-type hepatocytes and survived to 2 months, clusters of FAH-positive cells also became bigger than those at the 1-month time point, but many of the clusters were isolated and had not become confluent with adjacent clusters. Furthermore, FAH-positive cells in the p27 knockout hepatocyte transplanted liver were found in platelike structures in proper relationship with sinusoids, indicating the presence of architectural remodeling of the donor cells. Histological examination of the livers revealed similarly low levels of lymphocytic infiltration in areas containing FAH-positive cells in both types of livers at both 1-month and 2-month time points.
Figure 6.
Immunohistochemical analysis of host livers after transplantation. Liver sections from animals sacrificed at 1 month (a) and 2 months (b) after transplantation were processed for H&E staining, FAH immunostaining for transplanted cells, and TUNEL staining for apoptotic cells. The insert in TUNEL staining represents a wild-type liver treated with DNase I to serve as a positive control of TUNEL staining. All photographs were taken at ×200.
In parallel with FAH staining, we also performed TUNEL staining to measure the presence of apoptosis in the FAH-positive nodules. As shown in Figure 6, both the host parenchyma and the FAH-positive nodules contained very few and scattered TUNEL-positive cells, at both 1-month and 2-month time points. Taken together, histochemical studies of recipient livers demonstrated that, in this transplantation system, p27 knockout hepatocytes proliferated to a greater extent than wild-type hepatocytes to rescue a higher percent of animals from liver failure.
Discussion
We have demonstrated that hepatocytes lacking p27 are better able to activate Cdk2 and enter S phase in primary culture, and, in vivo, p27 knockout hepatocytes are better able to repopulate the livers of transplanted FAH-null mice and rescue them from liver failure. Importantly, this study is, to our knowledge, the first demonstration of enhanced transplantation success using genetically modified primary hepatocytes. Our results suggest a new concept that p27 could be a potential target to benefit hepatocyte-based therapies. Hepatocyte transplantation is a logical means to treat acute liver failure due to hepatocyte injury (20, 21). Although hepatocytes have been shown to have almost unlimited proliferative potential in serial transplantation experiments (22), one obstacle to the successful treatment of liver failure is that the number of hepatocytes that can engraft at one time is often insufficient for treating severe and acute liver failures (19). Transplantation of a greater number of cells runs the risk of occluding liver circulation (19, 23). Another logical, yet less-explored approach is to modify the donor hepatocytes so that they can proliferate better in the recipient liver.
One major concern about the manipulation of p27 levels is the associated tumor risk, since p27 has been shown to be a haploinsufficient tumor suppressor in mice (24). Manipulation of p27, however, should pose less of a risk of hepatocellular carcinogenesis than other means of stimulating the hepatocyte cell cycle, such as transgenic expression of hepatocyte mitogens HGF (25) and TGF-α (26). Whereas both TGF-α transgenic and p27-deficient hepatocytes display increased DNA synthesis in primary culture (27), only TGF-α transgenic mice develop hepatocellular carcinomas (26, 28). The incidence of liver adenomas in p27 knockout mice after injection of carcinogen N-ethyl-N-nitrosourea was also not different from that of wild-type mice (24). Likewise, there has been little evidence that p27 alterations are involved in hepatocellular carcinogenesis in humans. Our findings suggest that p27 could be a potential target for ex vivo modification for improving the efficacy of hepatocyte transplantation in the treatment of liver failure.
Since our studies have used the original p27 knockout strain on a 129/Sv × C57BL/6 hybrid background (4), our results, in theory, could be complicated by contributions from other genes that are functionally different in the two strains. Our use of six separate pairs of wild-type and p27 knockout littermates from different parents as donors minimized such complications since a particular modifier gene is unlikely to consistently cosegregate with the p27 knockout locus. We nevertheless cannot rule out the possibility that a modifier gene closely linked to the p27 locus may have contributed to the hepatocyte proliferation phenotypes. However, it was shown that p27 knockout mice on the 129/Sv background had an equivalent extent of increased body weight during development as the hybrid strain (5). Since organomegaly (including the liver) is proportional to body weight increase, it is unlikely that the observed phenotypes of p27 knockout hepatocytes were the result of a p27 locus-linked modifier.
With respect to the utility of targeting p27 in human hepatocyte transplantation, we also need to consider the possibility that hepatocytes in livers of p27 knockout mice may not have been truly quiescent. If this was the case, targeting p27 in wild-type hepatocytes, which are truly quiescent, may not achieve the same effects as p27 knockout in the germline. This possibility can be experimentally tested in the future with ex vivo modification to reduce p27 levels in wild-type hepatocytes. We consider this scenario less likely, however, since we did not see any differences in the baseline DNA synthesis states of wild-type and p27 knockout adult hepatocytes during the first day of culture.
Finally, after the completion of this work, Cheng and coworkers published their study showing that p27 knockout hematopoietic stem cells had a repopulation advantage after bone marrow transplantation (29) to rescue lethally irradiated mice. Their results with blood stem cells are consistent with results from this work, demonstrating that the potential benefit of targeting p27 is not restricted to hepatocytes.
Acknowledgments
We thank Andrew Koff for p27 mice, Staffan Ekberg for NTBC, and Robert M. Tanguay (University Laval) for the FAH Ab. We thank Charles Rogler, Erwin Bottinger, Milton Finegold, and Harmeet Malhi for their help with hepatocyte transplantation experiments; Jayanta Roy Chowdhury, Sanjeev Gupta, and Charles Rogler for critical reading of the manuscript; and the Einstein Analytical Imaging Facility for assistance with imaging. This work was supported by NIDDK Digestive Diseases Core Center Grant Pilot and Feasibility Study (P30 DK-41296) and the Howard Hughes Medical Institute–Research Resource Program for Medical Schools. A.N. Karnezis is supported by the Medical Scientist Training Program, M. Dorokhov is supported by General Medical Services Training Program in Pharmacological Sciences at the Albert Einstein College of Medicine, and L. Zhu is a Leukemia & Lymphoma Society Scholar.
Footnotes
See the related Commentary beginning on page 367.
References
- 1.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 2.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 3.Nakayama K, et al. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 4.Kiyokawa H, et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 5.Fero ML, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 6.Casaccia-Bonnefil P, et al. Oligodendrocyte precursor differentiation is perturbed in the absence of the cyclin-dependent kinase inhibitor p27Kip1. Genes Dev. 1997;11:2335–2348. doi: 10.1101/gad.11.18.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durand B, Fero ML, Roberts JM, Raff MC. p27Kip1 alters the response of cells to mitogen and is part of a cell-intrinsic timer that arrests the cell cycle and initiates differentiation. Curr Biol. 1998;8:431–440. doi: 10.1016/s0960-9822(98)70177-0. [DOI] [PubMed] [Google Scholar]
- 8.Ponder KP, et al. Mouse hepatocytes migrate to liver parenchyma and function indefinitely after intrasplenic transplantation. Proc Natl Acad Sci USA. 1991;88:1217–1221. doi: 10.1073/pnas.88.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labelle Y, Puymirat J, Tanguay RM. Localization of cells in the rat brain expressing fumarylacetoacetate hydrolase, the deficient enzyme in hereditary tyrosinemia type 1. Biochim Biophys Acta. 1993;1180:250–256. doi: 10.1016/0925-4439(93)90046-4. [DOI] [PubMed] [Google Scholar]
- 10.Grompe M, et al. Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev. 1993;7:2298–2307. doi: 10.1101/gad.7.12a.2298. [DOI] [PubMed] [Google Scholar]
- 11.Mayol X, Garriga J, Grana X. Cell cycle-dependent phosphorylation of the retinoblastoma-related protein p130. Oncogene. 1995;11:801–808. [PubMed] [Google Scholar]
- 12.Mayol X, Garriga J, Grana X. G1 cyclin/CDK-independent phosphorylation and accumulation of p130 during the transition from G1 to G0 lead to its association with E2F-4. Oncogene. 1996;13:237–246. [PubMed] [Google Scholar]
- 13.Canhoto AJ, Chestukhin A, Litovchick L, DeCaprio JA. Phosphorylation of the retinoblastoma-related protein p130 in growth-arrested cells. Oncogene. 2000;19:5116–5122. doi: 10.1038/sj.onc.1203893. [DOI] [PubMed] [Google Scholar]
- 14.Guguen-Guillouzo, C. 1992. Culture of epithelial cells. R.I. Freshney, editor. Wiley-Liss Inc. New York, New York, USA. 197–273.
- 15.Kao C-Y, Factor VM, Thorgeirsson SS. Reduced growth capacity of hepatocytes from c-myc and c-myc/TGF-α transgenic mice in primary culture. Biochem Biophys Res Commun. 1996;222:64–70. doi: 10.1006/bbrc.1996.0698. [DOI] [PubMed] [Google Scholar]
- 16.Grompe M, et al. Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1995;10:453–459. doi: 10.1038/ng0895-453. [DOI] [PubMed] [Google Scholar]
- 17.Overturf K, et al. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1996;12:266–273. doi: 10.1038/ng0396-266. [DOI] [PubMed] [Google Scholar]
- 18.Tschudy DP, Hess RA, Frykholm BC, Blaese RM. Immunosuppressive activity of succinylacetone. J Lab Clin Med. 1982;99:526–532. [PubMed] [Google Scholar]
- 19.Braun KM, Degen JL, Sandgren EP. Hepatocyte transplantation in a model of toxin-induced liver disease: variable therapeutic effect during replacement of damaged parenchyma by donor cells. Nat Med. 2000;6:320–326. doi: 10.1038/73179. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S, Gorla GR, Irani AN. Hepatocyte transplantation: emerging insights into mechanisms of liver repopulation and their relevance to potential therapies. J Hepatol. 1999;30:162–170. doi: 10.1016/s0168-8278(99)80022-1. [DOI] [PubMed] [Google Scholar]
- 21.Strom SC, Chowdhury JR, Fox IJ. Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis. 1999;19:39–48. doi: 10.1055/s-2007-1007096. [DOI] [PubMed] [Google Scholar]
- 22.Overturf K, al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997;151:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 23.Groth CG, Arborgh B, Bjorken C, Sundberg B, Lundgren G. Correction of hyperbilirubinemia in the glucuronyltransferase-deficient rat by intraportal hepatocyte transplantation. Transplant Proc. 1977;9:313–316. [PubMed] [Google Scholar]
- 24.Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177–180. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakata H, et al. Hepatocyte growth factor/scatter factor overexpression induces growth, abnormal development, and tumor formation in transgenic mouse livers. Cell Growth Differ. 1996;7:1513–1523. [PubMed] [Google Scholar]
- 26.Jhappan C, et al. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990;61:1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- 27.Wu JC, Merlino G, Cveklova K, Mosinger B, Fausto N. Autonomous growth in serum-free medium and production of hepatocellular carcinomas by differentiated hepatocyte lines that overexpress transforming growth factor α. Cancer Res. 1994;54:5964–5973. [PubMed] [Google Scholar]
- 28.Lee G-H, Merlino G, Fausto N. Development of liver tumors in transforming growth factor α transgenic mice. Cancer Res. 1992;52:5162–5170. [PubMed] [Google Scholar]
- 29.Cheng T, Rodrigues N, Dombkowski D, Stier S, Scadden DT. Stem cell repopulation efficiency but not pool size is governed by p27Kip1. Nat Med. 2000;6:1235–1240. doi: 10.1038/81335. [DOI] [PubMed] [Google Scholar]