It has recently been documented that survival and homeostasis of peripheral mature T cells depend on self-recognition. Thus, contrary to previous assumptions, naive T cells apparently require a constant subthreshold signal provided by their engagement with self-MHC/peptide ligands to persist in a quiescent state. In a lymphopenic state, this self-MHC/peptide recognition provides a proliferation-inducing signal and leads to T cell expansion until the T cell pool is reestablished to a nearly normal size. Here, we postulate that homeostatic anti-self T cell proliferation may, depending on additional background genes, contribute to systemic autoimmune disease pathogenesis.
Systemic autoimmunity, of which lupus is the prototypic disease, has been extensively investigated, particularly in spontaneous mouse lupus models. Despite key advances in several areas (reviewed in ref. 1), our understanding of the mechanisms leading to a breakdown of tolerance to a wide spectrum of self-molecules remains unclear. Disappointingly, early efforts to demonstrate central T cell tolerance defects in lupus mice, either with regard to deletions of endogenous superantigen-recognizing T cells or to conventional self-peptide–recognizing transgenic antigen receptor–expressing CD4+ or CD8+ cells, were unsuccessful — even in lupus mice with defects in the proapoptotic Fas pathway, by which activated T cells are eventually deleted. Although peripheral T cells of Fas-defective mice show reduced activation-induced cell death, the reasons for the excessive activation of T cells therein has not been identified. We argue below that in this and other seemingly unrelated cases, autoimmune manifestations can result from disturbances in T cell homeostasis that lead to continuous or intermittent T cell stimulation by self-MHC/peptide ligands.
Homeostatic T cell anti-self proliferation
During intrathymic development, a small fraction of T cells expressing receptors of sufficient affinity for self-MHC/peptide ligands is positively selected, survives, and differentiates, whereas the majority of T cells with little or no affinity for such ligands undergoes apoptotic death by neglect. Thereafter, those positively selected cells with dangerously high affinity for self-MHC/peptide ligands are also deleted by apoptosis (negative selection), and only a small fraction (3–5%) is released to the periphery to constitute the mature T cell pool (reviewed in ref. 2).
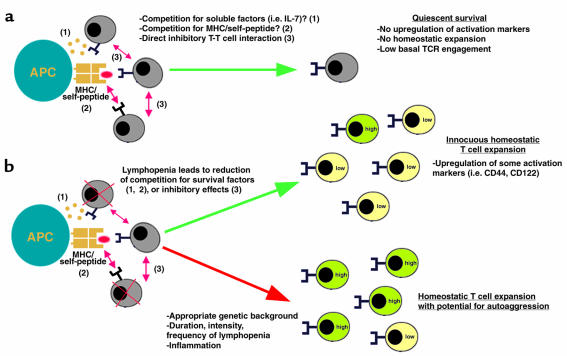
According to previously accepted views, naive T cells that survive these somatic selection processes persist in a dormant state within the confines of the secondary lymphoid organs, unaware of self-antigens until engaged by activated antigen-presenting cells displaying foreign antigenic peptides. This assumption has recently been challenged by several groups, who have shown that low-grade recognition of self-MHC/peptide ligands and delivery of covert signals — those that do not induce proliferation, as do conventional antigen-provoked, overt signals — are requirements for long-term survival of naive T cells in the periphery (Figure 1). Thus, in MHC class II–deficient mice manipulated to express the class II molecules only in the thymus, exported CD4+ cells do not survive long in the class II–barren periphery (3, 4), although this population can be rescued upon transfer of class II+ dendritic cells (5). Similarly, naive H-Y–specific T cell receptor transgenic (TCR transgenic) CD8+ cells do not survive after adoptive transfer into an MHC class I–deficient host or in a host carrying an MHC class I molecule different from the one on which these cells had been selected (6, 7). The molecular events associated with survival-promoting interactions have not been fully defined, but work with naive T cells derived from MHC class II+/+ environments shows partial phosphorylation of the T cell receptor complex protein CD3-ς (pp21) as well as recruitment of the ZAP-70 kinase to this complex (8). In contrast to proliferation-inducing signals, however, there is no recruitment of the p56lck kinase (9). It should be noted that, despite considerable supporting evidence, some recent studies have called into question the strict necessity of self-MHC/peptide ligand recognition for the survival of naive CD4+ cells in the periphery (10, 11).
Figure 1.
Regulation of naive T cell homeostasis in the periphery and the potential relevance for the development of autoimmunity. Positive and negative selection in the thymus creates a repertoire of T cells with a broad spectrum of affinities to self, from low self-affinity (just strong enough to escape from death by neglect) to comparatively high self-affinity, which is, however, not strong enough to undergo negative selection. In a T cell–sufficient individual, the bulk of naive T cells needs continuous signals by self-MHC/self-peptide complexes to survive in a quiescent state. How the presence of surrounding T cells prevents naive T cells from uncontrolled proliferation is currently unknown. Competition for soluble stimulatory factors (labeled “1” in figure), for contact with self-MHC/self-peptide complexes (labeled “2”), or a direct inhibitory effect between T cells themselves (labeled “3”) are possible mechanisms. Even while resting, a certain degree of T cell receptor engagement is measurable. Reduction of T cell numbers leads to spontaneous homeostatic T cell expansion, either by increased availability of stimulatory factors (labeled “1,” “2”) or by reduction of inhibitory impulses (labeled “3”). Homeostasis-driven proliferation leads to upregulation of some activated/memory markers. This proliferation usually occurs innocuously, without harm to normal individuals under physiologic circumstances. Either through constitutive T cell depletion or repeated episodes of T cell depletion, a T cell repertoire might be skewed to more T cells with high self-affinity. In this scenario, when homeostatic T cell expansion occurs, such cells, in conjunction with the appropriate genetic background and/or coexisting inflammation, might be overstimulated. In some models, such T cells have been shown to hyper-respond and acquire effector function. This provides the basis for the development of autoimmunity
Further studies have also established that self-recognition is important to the homeostatic process that maintains the near-constant overall size of the peripheral T cell pool, which is established at a young age. Homeostatic adjustment of T cell numbers is necessary at the final stage of an immune response, during which time the vast majority of the specific effector cells die by apoptosis. Such an adjustment will also occur after the onset of lymphopenia induced by a variety of insults (e.g., viral infections, toxic agents, irradiation, cytotoxic drugs). Restoration of the size of the original T cell pool following severe T cell deficiency has long been known to be accomplished by spontaneous expansion of the spared T cells, even in the absence of any contribution from the thymus (12). Accordingly, homeostatic proliferation of T cells has been observed upon adoptive transfer of small numbers of cells into syngeneic lymphopenic nu/nu, SCID, RAG-deficient, TCR α-chain–deficient, or sublethally irradiated hosts (13–15). Homeostatic proliferation of T cells is polyclonal and occurs in the absence of deliberate immunization.
Recent studies have now made it clear that homeostatic proliferation of T cells is mediated by, and dependent upon, recognition of self-MHC/peptide ligands. Accordingly, naive CD4+ cells from wild-type B6 mice do not undergo efficient homeostatic proliferation when transferred to H-2M–deficient, syngeneic T cell–depleted mice, which express MHC class II molecules loaded almost exclusively with a single species of peptide (16, 17). In contrast, naive CD4+ cells from H-2M–deficient mice that were positively selected to this peptide proliferated well in T cell–depleted H-2M–deficient hosts, again arguing that lymphopenia-induced homeostatic T cell proliferation in the periphery depends on recognition of self-peptides identical to those used in their positive selection. Additional support for this model comes from experiments involving T cell–depleted mice engineered to express MHC class I molecules that were loaded with specific peptides: TCR transgenic CD8+ cells transfused into these animals proliferated only when the host expressed a peptide that induced the positive selection of the T cells, but not when the host expressed an irrelevant peptide (18).
Together, these findings indicate that the weak interaction mediated by self-MHC/peptide recognition provides a p56lck-independent signal that is necessary for survival in a resting state, but that under lymphopenic conditions, this signal instead becomes p56lck-dependent, is translated differently, and leads to strong proliferation (reviewed in 19–21; Figure 1). In another recent study (22), it was shown that T cells have to enter intact T cell compartments in secondary lymphoid tissues in order for homeostatic proliferation to occur. Experiments to clarify why homeostatic proliferation does not occur in a nonlymphopenic state found that a large number of “bystander” T cells coinjected with a small number of syngeneic T cells (differentiated by allelic markers and intracellular dyes) can inhibit expansion of the latter in a lymphopenic host. Of interest, naive T cells can act as bystander inhibitors, whereas activated/memory phenotype T cells are inefficient inhibitors. Crucially, bystander naive T cells in these experiments did not require signaling through the TCR, nor did they need to undergo homeostatic proliferation themselves to inhibit homeostatic proliferation of the small numbers of T cells that were coinfused. These findings suggest that T cell homeostasis is likely regulated either by non–MHC-stimulating factors, such as stromal cell–derived cytokines (i.e., IL-7, IL-12) (23, 24), or by direct intercellular contact. Several studies (6, 16, 17, 24–26) have also shown that although all typical T cells are thought to require positive selection by self-MHC/peptide ligands in the thymus, only a fraction (∼30%) can undergo homeostatic proliferation in the periphery. The reasons for this selective response remain unclear, but it may be that some positive-selecting peptides are absent from the periphery or that expansion is restricted to cells with higher self-affinity. Recent experiments with transgenic T cells favor the second possibility (27).
The relevance of homeostatic anti-self proliferation of naive T cells to autoimmunity rests to a great extent on whether the proliferating cells acquire effector function and differentiate to the activated/memory phenotype. Several studies in which TCR transgenic CD8+ or CD4+ cells were transfused to lymphopenic syngeneic hosts appear to provide an affirmative answer (11, 25–29). Proliferating TCR transgenic CD8+ cells kill target cells ex vivo in a peptide- and TCR-dependent manner, and express IFN-γ after stimulation with anti-CD3. In vivo, they also acquire several, but not all, phenotypic markers of activated/memory T cells (upregulation of CD44, CD122, and CD132, and downregulation of CD45RB, but no upregulation of conventional antigen-induced early activation markers CD69, CD71, and CD25 or downregulation of CD62L). Whereas acquisition of CD44 is slower in homeostatically proliferating T cells than in cognate antigen-driven proliferation, expression of CD122 and CD132 depends strictly on the number of divisions, regardless of whether they are homeostasis- or antigen-driven (29). In two of these studies (11, 28), even polyclonal CD8+ or CD4+ cells undergoing homeostasis-driven proliferation also acquired activated/memory phenotypes, and such polyclonal CD8+ cells were able to rapidly secrete IFN-γ after anti-CD3 stimulation, and to kill ConA-coated syngeneic targets; control naive CD8+ cells were devoid of such activities.
Following reestablishment of nearly normal numbers of T cells and cessation of proliferation, the majority of the expanded cells retain the activated/memory phenotype, but a small percentage revert to the naive phenotype (11, 25, 26, 29). Maintenance of or reversion to the activated/memory phenotype may depend on the initial affinity for self-MHC/peptide complexes. Thus, cells with high affinity may retain the activated/memory phenotype, while those with low affinity lose it (20). Therefore, it has been concluded that “memory” phenotype T cells in an individual may not all be true foreign antigen-experienced cells, but could include naive cells “masquerading” as memory cells (29).
Homeostatic T cell proliferation in models of induced systemic autoimmunity
Several findings support the role of homeostatic anti-self T cell proliferation in the pathogenesis of autoimmunity, including autoimmune disease manifestations in mice and rats following neonatal thymectomy, discontinuation of cyclosporin treatment, irradiation, retroviral infections, or exposure to certain xenobiotics. Other examples may be spontaneous autoimmunity in genetically lymphopenic BB rats, and autoimmunity upon transfer of lymphocytes into RAG-deficient, nu/nu, or SCID mice (Table 1).
Table 1.
Conditions in which homeostatic anti-self T cell proliferation may contribute to the appearance of autoimmune manifestations
Neonatally thymectomized mice of several, but not all, backgrounds develop a strain-dependent spectrum of autoimmune reactions against multiple organs, as judged by the presence of T cell infiltrates, inflammation, and appearance of autoantibodies (reviewed in refs. 30, 31). There is a strict temporal relationship between the day of thymectomy (third to seventh day of life) and the development of autoimmunity. Importantly, inoculation of T cells from adult euthymic animals can prevent disease in thymectomized syngeneic mice. This inhibition appears to be mediated primarily by a thymus-derived, naturally unresponsive (anergic) CD4+CD25+ (IL-2R α-chain) population. These cells comprise approximately 5–10% of thymic and peripheral T cells, are functionally distinct from other activated T cells, and mediate suppression in an antigen-nonspecific manner. Activation of CD4+CD25+ regulatory T cells does not seem to require CD28 costimulation, but their generation appears to depend on CD40–CD40L interactions. Recent studies suggest that these cells suppress inflammatory and autoimmune manifestations by a constitutive expression of CTLA-4, a molecule that inhibits CD28/B7-mediated costimulation (32, 33). It is of interest that CTLA-4–deficient mice develop a severe lymphoproliferative disorder and die from autoimmune-like disease within 1 month of birth (34). Autoimmunity with multiorgan involvement and even lupus-like manifestations have also been reported in adult athymic nu/nu mice following inoculation with either spleen cell suspension from 3-day-old euthymic nu+/– mice or CD4+CD25+-depleted spleen suspension from adult nu+/– mice (30, 31).
Autoimmunity associated with neonatal thymectomy has been attributed to the absence of the regulatory CD4+CD25+ subset (which appears to develop later in ontogeny), inefficient deletion of self-reactive T cells due to low MHC expression in the neonatal thymus, and/or late developmental thymic expression of certain self-molecules. In view of the findings pertaining to self-MHC/peptide–mediated homeostatic expansion of T cells, we propose that another contributing factor in autoimmunity associated with neonatal thymectomy and related models may be a dearth of peripheral T cells, leading to a strong anti-self homeostatic proliferation that fills this “empty space.” We also suggest that inhibition of neonatal thymectomy–induced autoimmunity by supplementation with large numbers of adult T cells may depend on the restoration of the T cell pool and the consequent curtailment of homeostatic proliferation. The regulatory CD4+CD25+ T cells may also exert their autoimmunity-inhibiting effect by suppressing homeostatic anti-self T cell proliferation.
Another potential example of autoimmunity caused by disturbance of lymphocyte homeostasis and subsequent attempts to reestablish the immune system is observed in certain rat and mouse strains treated neonatally with cyclosporin (CsA), or as adults with lethal irradiation and bone marrow reconstitution followed by treatment with, and subsequent withdrawal of, CsA (reviewed in ref. 35). Development of this disease requires the presence of a thymus and the elimination of peripheral T cells by irradiation. The disease is mediated by self-MHC class II/peptide-reactive IL-2 and IFN-γ–producing T cells, and can be transferred to syngeneic irradiated hosts. Once again, transfer of the disease can be prevented by coinfusion of large numbers of spleen T cells from normal adult mice. The precise mechanisms by which this immunosuppressive drug can paradoxically induce autoimmunity remain largely unexplained. Although some studies have reported interference with apoptosis and intrathymic negative selection, others have failed to confirm such a defect, but report inhibition of suppressor T cell action or prevention of peripheral T cell clonal anergy (35, 36). We suggest that a more appropriate explanation may be homeostatic anti-self T cell proliferation triggered by the severe lymphopenia that follows irradiation and cyclosporin treatment. Indeed, cyclosporin has been shown to cause thymic involution and a severe block in the maturation of double-positive (CD4+CD8+) thymocytes and a consequent drop in the output of recent T cell emigrants to the periphery (37).
Several other instances of autoimmune disease development, presumably caused by perturbations in lymphocyte homeostasis, have been reported. For example, high-dose, fractionated, total lymphoid irradiation of mice can induce multiorgan autoimmunity with characteristics dependent on radiation dose, extent of lymphoid irradiation, and the genetic background of the mouse (38). Radiation-induced tissue damage is not the primary cause of these disorders, because irradiation of the target organs alone fails to elicit autoimmunity, and shielding them from irradiation does not prevent it. This syndrome is also prevented by inoculation of splenic CD4+ cells from syngeneic nonirradiated mice, a treatment that probably inhibits homeostatic proliferation by restoring the T cell pool. Thus, this disease appears to result from lymphopenia and subsequent elicitation of anti-self homeostatic responses. Similar phenomena have been reported with certain mouse strains infected neonatally with a CD4+-depleting mouse T lymphotropic virus (39). As with the other models discussed above, inoculation of peripheral CD4+ cells from syngeneic, noninfected adult mice prevented the development of autoimmunity. A similar mechanism may be operative in the unexplained autoimmune manifestations characteristic of many HIV-infected individuals with AIDS (reviewed in ref. 40). Certain xenobiotics, such as mercuric chloride (HgCl2) and gold, also induce autoimmunity resembling lupus in some rat and mouse strains (reviewed in ref. 41). The mechanism of xenobiotic-induced autoimmunity is unknown, but this condition is characterized by excessive in vivo T cell proliferation and anti–self-MHC class II reactivity; therefore it is likely that disease in this model may also be attributed to disturbances in T cell homeostasis.
A few final examples in which low levels of T cells appear to result in autoimmune diseases: First, as a result of an as-yet undefined genetic defect, BB/W rats exhibit excessive T cell apoptosis and lymphopenia associated with autoimmune manifestations and diabetes (42). Likewise, SCID mice transplanted with coisogenic CD4+CD45RBhi naive lymph node T cells develop autoimmune colitis, which can be prevented by coinjection of high IL-10– and low IL-2–producing CD4+ T cells (43). Finally, a similar inflammatory bowel disease has been reported in RAG-deficient mice injected with CD4+CD45RBhi cells (44). The transferred cells acquired an activated phenotype and show high proliferative capacity.
Homeostatic T cell proliferation in spontaneous systemic autoimmunity
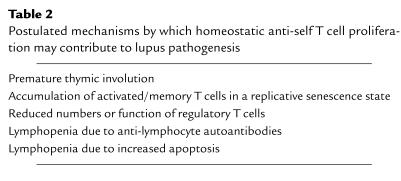
Several findings in predisposed mice appear to support the concept that primary or secondary homeostatic lymphocyte perturbations contribute to the pathogenesis of spontaneous lupus. These mice show premature thymic involution, which can lead to reduced export of naive T cells, excessive T cell activation leading to replicative senescence, reduced number and/or function of regulatory T cells, and in some strains, lymphopenia induced by anti-lymphocyte autoantibodies or increased apoptosis (Table 2).
Table 2.
Postulated mechanisms by which homeostatic anti-self T cell proliferation may contribute to lupus pathogenesis
Thymic atrophy in lupus-prone mouse strains is first evident as a loss of cortical thymocytes, which may later be accompanied by medullary degeneration (reviewed in ref. 1). The thymic atrophy associated with abnormal fine structure appears by the age of 3–4 months in female (NZB × NZW)F1 mice, which, by 6–7 months of age, have lost 70–90% of their cortices. In BXSB males and MRL-Faslpr mice of both sexes, thymic atrophy and cystic necrosis appear by the age of 2 months and progress to a complete loss of the cortical areas by 4.5 and 3.5 months, respectively. Ultrastructural studies and immunocytochemistry with mAb’s to thymic constituents have shown considerable abnormalities, including dramatic alterations in microarchitecture, decreases in subcapsular and medullary epithelia, free spaces (“cortical holes”), decreased thymocyte frequencies, and altered composition (45). Although these changes have been considered manifestations of premature aging, they differ considerably from those seen in normal aging mice. The relevance of these findings to our hypothesis lies in the possibility that intrinsic thymic defects in T cell maturation may lead to a lymphocyte deficit in the periphery, and consequently, to the initiation of a homeostatic, yet deleterious, anti-self response.
Another possible means of homeostatic disturbance in lupus is through continuous T cell stimulation by ever-present self-antigens, and attainment of a state of replicative senescence wherein such cells may be unable to act as efficient inhibitors of homeostatic proliferation. Indeed, mice (and humans) with lupus show an expedited accumulation of activated/memory phenotype cells with clonal expansions (46). Moreover, most of the accumulated activated/memory phenotype CD4+CD44hi cells of male BXSB mice and double-negative (DN CD4–CD8–) CD44hi cells of MRL-Faslpr mice are arrested at the G1 phase of the cell cycle, express high levels of cyclin kinase inhibitors, and are refractory to apoptosis (46), all characteristics of cells in replicative senescence (47). Such cells may be inefficient inhibitors of naive T cell homeostatic proliferation, since such proliferation can be inhibited by large numbers of naive, but not activated, T cells (22).
Lymphopenia, which occurs in a high percentage of lupus patients in the acute phases, may also trigger homeostatic anti-self T cell proliferation. The absolute levels of lymphocytes are reduced in this disorder, as are the percentages of T and B cells. Patients not undergoing prednisolone treatment also show a marked reduction of CD4+ cells. Lymphopenia in lupus has primarily been attributed to anti-lymphocyte autoantibodies and/or increased activation-induced apoptosis via the Fas/FasL system. Anti-lymphocyte autoantibodies are also present in several of the lupus-predisposed mouse strains.
Apart from lupus, homeostatic anti-self proliferation of T cells may play a role in other autoimmune conditions wherein the composition of the peripheral repertoire and other factors may dictate a different clinical outcome. Recent studies have shown that patients with rheumatoid arthritis exhibit a premature decline in thymic output and a compensatory expansion of peripheral T cells (48). It has therefore been hypothesized that these phenomena may lead to a repertoire contraction that may favor T cells with autoreactive potential (49).
Summary
The exact mechanisms by which homeostatic anti-self proliferation of peripheral T cells is controlled are not yet well understood. Nevertheless, evidence exists that self-MHC/peptide recognition, necessary for T cell survival in a resting state, may become overt under certain conditions associated with lymphopenia and lead to T cell expansion and generation of effector cells (Figure 1). Several factors may influence whether this expansion is beneficial or detrimental to the host, including genetic background, intensity, frequency and duration of lymphocyte disturbances, and previous inflammatory damage of the target organs. We further postulate that if the T cell repertoire and expanded clones primarily have low self-affinity, then the expansion will be largely innocuous, whereas if composed of cells with high self-affinity, clinically evident autoaggression may be the outcome. Validation of this hypothesis may provide a new paradigm for understanding autoimmune syndromes, including lupus.
Acknowledgments
This is publication number 13818-IMM from the Department of Immunology, The Scripps Research Institute (La Jolla, California, USA). The authors thank Charles Surh for manuscript review and M. Kat Occhipinti-Bender for editorial assistance. Space limitations necessitated citing reviews rather than original articles in many instances.
References
- 1.Theofilopoulos, A.N., and Kono, D.H. 1998. Murine lupus models: gene-specific and genome-wide studies. In Systemic lupus erythematosus. 3rd edition. R.G. Lahita, editor. Academic Press Inc. San Diego, California, USA. 145–181.
- 2.Kisielow P, von Boehmer H. Development and selection of T cells: facts and puzzles. Adv Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- 3.Takeda S, Rodewald HR, Arakawa H, Bluethmann H, Shimizu T. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity. 1996;5:217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 4.Kirberg J, Berns A, von Boehmer H. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex-encoded molecules. J Exp Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brocker T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J Exp Med. 1997;186:1223–1232. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 7.Nesic D, Vukmanovic S. MHC class I is required for peripheral accumulation of CD8+ thymic emigrants. J Immunol. 1998;160:3705–3712. [PubMed] [Google Scholar]
- 8.Witherden D, et al. Tetracycline-controllable selection of CD4(+) T cells: half-life and survival signals in the absence of major histocompatibility complex class II molecules. J Exp Med. 2000;191:355–364. doi: 10.1084/jem.191.2.355. [DOI] [PubMed] [Google Scholar]
- 9.Seddon B, Legname G, Tomlinson P, Zamoyska R. Long-term survival, but impaired homeostatic proliferation, of naive T cells in the absence of p56lck. Science. 2000;290:127–131. doi: 10.1126/science.290.5489.127. [DOI] [PubMed] [Google Scholar]
- 10.Dorfman JR, Stefanova I, Yasutomo K, Germain RN. CD4+ T cell survival is not directly linked to self-MHC-induced TCR signaling. Nat Immunol. 2000;1:329–335. doi: 10.1038/79783. [DOI] [PubMed] [Google Scholar]
- 11.Clarke SR, Rudensky AY. Survival and homeostatic proliferation of naive peripheral CD4+ T cells in the absence of self peptide:MHC complexes. J Immunol. 2000;165:2458–2464. doi: 10.4049/jimmunol.165.5.2458. [DOI] [PubMed] [Google Scholar]
- 12.Mackall CL, Hakim FT, Gress RE. Restoration of T-cell homeostasis after T-cell depletion. Semin Immunol. 1997;9:339–346. doi: 10.1006/smim.1997.0091. [DOI] [PubMed] [Google Scholar]
- 13.Bell EB, Sparshott SM, Drayson MT, Ford WL. The stable and permanent expansion of functional T lymphocytes in athymic nude rats after a single injection of mature T cells. J Immunol. 1987;139:1379–1384. [PubMed] [Google Scholar]
- 14.Rocha B, Dautigny N, Pereira P. Peripheral T lymphocytes: expansion potential and homeostatic regulation of pool sizes and CD4/CD8 ratios in vivo. Eur J Immunol. 1989;19:905–911. doi: 10.1002/eji.1830190518. [DOI] [PubMed] [Google Scholar]
- 15.Tanchot C, Rosado MM, Agenes F, Freitas AA, Rocha B. Lymphocyte homeostasis. Semin Immunol. 1997;9:331–337. doi: 10.1006/smim.1997.0090. [DOI] [PubMed] [Google Scholar]
- 16.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 17.Viret C, Wong FS, Janeway CA., Jr Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity. 1999;10:559–568. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- 18.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 20.Surh CD, Sprent J. Homeostatic T cell proliferation. How far can T cells be activated to self-ligands? J Exp Med. 2000;192:F9–F14. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrack P, et al. Homeostasis of alpha beta TCR+ T cells. Nat Immunol. 2000;1:107–111. doi: 10.1038/77778. [DOI] [PubMed] [Google Scholar]
- 22.Dummer W, Ernst B, LeRoy E, Lee D-S, Surh CD. Autologous regulation of naive T cell homeostasis within the T cell compartment. J Immunol. 2001;166:2460–2468. doi: 10.4049/jimmunol.166.4.2460. [DOI] [PubMed] [Google Scholar]
- 23.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 24.Kieper WC, Prlic M, Schmidt CS, Mescher MF, Jameson SC. IL-12 enhances CD8 T cell homeostatic expansion. J Immunol. 2001;166:5515–5521. doi: 10.4049/jimmunol.166.9.5515. [DOI] [PubMed] [Google Scholar]
- 25.Oehen S, Brduscha-Riem K. Naive cytotoxic T lymphocytes spontaneously acquire effector function in lymphocytopenic recipients: a pitfall for T cell memory studies? Eur J Immunol. 1999;29:608–614. doi: 10.1002/(SICI)1521-4141(199902)29:02<608::AID-IMMU608>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 26.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge Q, Rao VP, Cho BK, Eisen HN, Chen J. Dependence of lymphopenia-induced T cell proliferation on the abundance of peptide/MHC epitopes and strength of their interaction with T cell receptors. Proc Natl Acad Sci USA. 2001;98:1728–1733. doi: 10.1073/pnas.98.4.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. J Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 30.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 31.Shevach EM. Regulatory T cells in autoimmunity. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 32.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi T, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tivol EA, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 35.Bucy RP, Xu XY, Li J, Huang G. Cyclosporin A-induced autoimmune disease in mice. J Immunol. 1993;151:1039–1050. [PubMed] [Google Scholar]
- 36.Prud’homme GJ, Vanier LE. Cyclosporine, tolerance, and autoimmunity. Clin Immunol Immunopathol. 1993;66:185–192. doi: 10.1006/clin.1993.1024. [DOI] [PubMed] [Google Scholar]
- 37.Beijleveld LJ, Damoiseaux JG, Van Breda Vriesman PJ. Differential effects of X-irradiation and cyclosporin-A administration on the thymus with respect to the generation of cyclosporin-A-induced autoimmunity. Dev Immunol. 1995;4:127–138. doi: 10.1155/1995/18495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakaguchi N, Miyai K, Sakaguchi S. Ionizing radiation and autoimmunity. Induction of autoimmune disease in mice by high dose fractionated total lymphoid irradiation and its prevention by inoculating normal T cells. J Immunol. 1994;152:2586–2595. [PubMed] [Google Scholar]
- 39.Morse SS, Sakaguchi N, Sakaguchi S. Virus and autoimmunity: induction of autoimmune disease in mice by mouse T lymphotropic virus (MTLV) destroying CD4+ T cells. J Immunol. 1999;162:5309–5316. [PubMed] [Google Scholar]
- 40.Ende N, Schwartz RA. Autoimmunity and AIDS. A commentary. J Exp Med. 1997;28:273–274. [PubMed] [Google Scholar]
- 41.Pollard KM, Hultman P. Effects of mercury on the immune system. Met Ions Biol Syst. 1997;34:421–440. [PubMed] [Google Scholar]
- 42.Zadeh HH, Greiner DL, Wu DY, Tausche F, Goldschneider I. Abnormalities in the export and fate of recent thymic emigrants in diabetes-prone BB/W rats. Autoimmunity. 1996;24:35–46. doi: 10.3109/08916939608995355. [DOI] [PubMed] [Google Scholar]
- 43.Groux H, et al. A CD4+ T cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 44.Annacker O, Burlen-Defranoux O, Pimenta-Araujo R, Cumano A, Bandeira A. Regulatory CD4 T cells control the size of the peripheral activated/memory CD4 T cell compartment. J Immunol. 2000;164:3573–3580. doi: 10.4049/jimmunol.164.7.3573. [DOI] [PubMed] [Google Scholar]
- 45.Takeoka Y, et al. Thymic microenvironmental abnormalities in MRL/MP-lpr/lpr, BXSB/MpJ Yaa and C3H HeJ-gld/gld mice. J Autoimmun. 1995;8:145–161. doi: 10.1006/jaut.1995.0012. [DOI] [PubMed] [Google Scholar]
- 46.Sabzevari H, Propp S, Kono DH, Theofilopoulos AN. G1 arrest and high expression of cyclin kinase and apoptosis inhibitors in accumulated activated/memory phenotype CD4+ cells of older lupus mice. Eur J Immunol. 1997;27:1901–1910. doi: 10.1002/eji.1830270813. [DOI] [PubMed] [Google Scholar]
- 47.Campisi J. Replicative senescence: an old lives’ tale. Cell. 1996;84:497–500. doi: 10.1016/s0092-8674(00)81023-5. [DOI] [PubMed] [Google Scholar]
- 48.Koetz K, et al. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 2000;97:9203–9208. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goronzy JJ, Weyand CM. Thymic function and peripheral T-cell homeostasis in rheumatoid arthritis. Trends Immunol. 2001;22:251–255. doi: 10.1016/s1471-4906(00)01841-x. [DOI] [PubMed] [Google Scholar]