Abstract
Endothelial progenitor cells (EPCs) have been isolated from circulating mononuclear cells in peripheral blood and shown to incorporate into foci of neovascularization, consistent with postnatal vasculogenesis. These circulating EPCs are derived from bone marrow and are mobilized endogenously in response to tissue ischemia or exogenously by cytokine stimulation. We show here, using a chemotaxis assay of bone marrow mononuclear cells in vitro and EPC culture assay of peripheral blood from simvastatin-treated animals in vivo, that the HMG-CoA reductase inhibitor, simvastatin, augments the circulating population of EPCs. Direct evidence that this increased pool of circulating EPCs originates from bone marrow and may enhance neovascularization was demonstrated in simvastatin-treated mice transplanted with bone marrow from transgenic donors expressing β-galactosidase transcriptionally regulated by the endothelial cell-specific Tie-2 promoter. The role of Akt signaling in mediating effects of statin on EPCs is suggested by the observation that simvastatin rapidly activates Akt protein kinase in EPCs, enhancing proliferative and migratory activities and cell survival. Furthermore, dominant negative Akt overexpression leads to functional blocking of EPC bioactivity. These findings establish that augmented mobilization of bone marrow–derived EPCs through stimulation of the Akt signaling pathway constitutes a novel function for HMG-CoA reductase inhibitors.
Introduction
The finding that circulating endothelial progenitor cells (EPCs) may home to sites of neovascularization and differentiate into mature endothelial cells (ECs) in situ is consistent with “vasculogenesis” (1), a critical paradigm for establishment of the primordial vascular network in the embryo. Our findings (2–7), together with the recent reports from other investigators (8–14), suggest that growth and development of new blood vessels in the adult is not restricted to angiogenesis but encompasses both vasculogenesis and angiogenesis. Although several studies have established angiogenic properties of EPCs (3–5, 15), including physiological mobilization (4) in response to the angiogenic growth VEGF (5), the involved signaling pathways have remained enigmatic.
Recently, Akt protein kinase has been shown to act downstream of the angiogenic growth factors VEGF and angiopoietin (16–18) to confer EC survival (18), migration (19), and production of endothelial cell NO (20, 21) in response to VEGF. These suggest a potentially important role for Akt signaling in mediating the response of ECs to angiogenic stimuli. Indeed Kureishi et al. have recently shown that the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor, simvastatin, rapidly activates Akt signaling in ECs, and that this stimulates EC bioactivity in vitro and enhances angiogenesis in vivo (22).
Statins inhibit the activity of HMG-CoA reductase, which catalyzes the synthesis of mevalonate, a rate-limiting step in cholesterol biosynthesis. The clinical application of statins has already led to important improvements in primary and secondary prevention of coronary artery disease (CAD) in subjects with and without elevated cholesterol levels. Preclinical studies suggest that statins may promote angiogenesis in ischemic limbs (22) and protect against ischemia-reperfusion injury of the heart (23), through mechanisms that may be mediated by activation of Akt signaling and endothelium-derived nitric oxide (NO) production in normocholesterolemic animals.
Accordingly, we investigated the hypothesis that Akt may constitute a key signaling pathway in the angiogenic activity of EPCs, specifically modulating EPC responsivity to statin therapy.
Methods
Human EPC culture and immunoblot analysis.
PBMCs were isolated from blood of human volunteers by density gradient centrifugation with Histopaque-1077 (Sigma Chemical Co., St. Louis, Missouri, USA) as described previously (2, 4, 5, 24). All experiments were performed with day 7 EPC cultures. Before immunoblot analysis, cells were serum depleted for 24 hours. Experiments were initiated by addition of the indicated amount of alkaline hydrolysis-activated simvastatin, 100 ng/ml vascular endothelial growth factor (VEGF; R&D Systems Inc., Minneapolis, Minnesota, USA) or vehicle control. Immunoblots were performed as described previously (22) using rabbit polyclonal anti-phosphorylated serine 473 residue of Akt1 antibody (Cell Signaling Technology Inc., Beverly, Massachusetts, USA) and goat polyclonal anti-Akt1 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA). Simvastatin was provided by Merck & Co. (West Point, Philadelphia, USA).
Mitogenic activity, migration assay, and chemotaxis assay.
Mitogenic activity was assayed using a previously validated colorimetric MTS assay (5). Simvastatin, VEGF, or vehicle was added to the culture plate wells for 24 hours before assay. Mitogenic activity was further evaluated by analysis of 5 × 105 day 7 EPCs seeded on a 35-mm plate and exposed to simvastatin, VEGF, or vehicle for 24 hours before manual counting on day 9.
EPC migration was evaluated using a modified Boyden chamber assay (5). Simvastatin, VEGF, or vehicle, in serum-free EBM-2 media with 0.5% lipid-free FBS (Sigma Chemical Co.), was placed in the lower compartment of the chamber. A total of 5 × 104 EPCs in 50 μl of EBM-2 supplemented with 0.5% BSA were seeded in the upper compartment of the chamber. Cell migration was quantified by counting cells in four randomly selected high-power fields (40×) (4, 5). All groups were studied in triplicate.
Chemotaxis was assayed as described previously (5). Briefly, 1 × 106 bone marrow (BM) cells were added to the upper chamber of a Coster Transwell (6.5-mm diameter, 3-μl pore), and 600 μl of chemotaxis buffer (without cells) was added to the lower chamber. Simvastatin was added to the chemotaxis buffer, and rhVEGF (100 ng/ml) and murine GM-CSF (50 ng/ml) were used for the positive chemoattractant response in this assay. Cells migrating into the lower chamber were collected in 50 μl of buffer and counted manually using a hemocytometer.
Expression of surface antigens of cultured human EPCs.
FACS was used to detect the expression of cell-surface integrins and endothelial lineage antigens on EPCs as described previously (24). Purified anti-KDR (Sigma Chemical Co.), antivascular endothelium cadherin (clone BV-6), anti-integrin αvβ5 (clone P1F6), antiendothelial P1H12 (all three from Chemicon International, Temecula, California, USA), the biotinylated anti-CD62E (E-selectin), the FITC-conjugated anti-αvβ3 (both from PharMingen, San Diego, California, USA), anti-CD3 (Becton Dickinson Labware, Franklin Lake, New Jersey, USA),anti-CD68, and the phycoerythrin-conjugated anti-CD31, anti-CD34, and anti-CD19 (Becton Dickinson Labware) were used for each epitope.
Mice.
All procedures were performed in accordance with the St. Elizabeth’s Institutional Animal Care and Use Committee. Wild-type (C57BL/6) mice and FVB/N mice (both from The Jackson Laboratory, Bar Harbor, Maine, USA) were used. Mice were fasted overnight, and blood obtained by heart puncture was collected into serum tubes. Sera from each mouse was centrifuged, following which cholesterol levels were determined.
Murine EPC culture assay.
Four days after culture, EPCs, recognized as attaching spindle-shaped cells, were assayed by costaining with acLDL-DiI and FITC-conjugated BS-1 lectin (Sigma Chemical Co.), each characteristic of endothelial lineage. Independent investigators used fluorescence microscopy to identify and manually count double positive cells as EPCs (4, 5, 24).
Murine BM transplantation model and cornea neovascularization assay.
A murine BM transplantation (BMT) model was used as described previously (3–5). The corneas of transplanted mice were collected at 6 days after corneal microsurgery for immunofluorescent detection of β-galactosidase. During these 6 days, simvastatin or control vehicle was continued. The pellets contained 180–200 ng of VEGF. The corneas of all mice were routinely examined by slit-lamp biomicroscopy to evaluate corneal neovascularization on the 6th postoperative day.
After completing the corneal examination, mice received 500 μg of BS-1–conjugated FITC (Vector Laboratories, Burlingame, California, USA) intravenously and were sacrificed 30 minutes later. The eyes were enucleated and fixed in 1% paraformaldehyde. After fixation, corneas were incubated with X-gal staining solution overnight and whole mounted, or they were incubated with β-gal antibody (Cortex, San Leandro, California, USA) at 1:500 dilution at 4°C overnight, and Cy3 goat anti-rabbit antibody (The Jackson Laboratory) as a secondary antibody at 1:300 dilution at room temperature for 1 hour. Otherwise they were whole mounted for detection by fluorescent microscopy or paraffin embedded and prepared for double-fluorescent immunohistochemistry as described below.
Fluorescent immunohistochemistry of BMT cornea model.
The corneas of transplanted mice were collected at 6 days after corneal microsurgery, and double staining was performed. β-Gal antibody (Chemicon International) was used at 1:200 dilution at 4°C overnight, and Cy3 goat anti rabbit antibody (Jackson Laboratories) was used as secondary antibody at 1:400 dilution at room temperature for 1 hour. Isolectin B4 (Vector Technologies Inc.) was used at 1:100 dilution at 4°C overnight. The resulting fluorescent signal was detected by fluorescence microscopy.
Apoptosis assays.
Annexin-V-FRUOS staining (Roche Molecular Biochemicals, Indianapolis, Indiana, USA) and Hoechst 33342 (Sigma Chemical Co.) staining were performed as described previously (22). Day 7 EPCs were seeded onto four-chamber slides (7.5 × 104 cells per well in 500 μl of EBM2). Annexin-V staining was performed after 24 hours of serum starvation; Hoechst 33342 staining was performed 48 hours after serum starvation. Resulting fluorescent signals were detected by fluorescence microscopy. Annexin-positive cells and Hoechst-stained pyknotic nuclei were counted as the percentage of 200 cells and nuclei in each well, respectively.
Dominant negative Akt/adenovirus infection.
Day 7 cultured EPCs were transduced with an adenoviral construct encoding dominant negative Akt1 (dnAkt) at an moi of 500 for 3 hours in EBM2 media containing 1% FBS (with growth factor). On the following day, cells were seeded in a 96-well plate (for MTS assay), Boyden chamber (for migration assay), or four-chamber slides (for apoptosis assays).
Statistical analysis.
All data are presented as mean ± SEM. Differences between group means were assessed by an unpaired Student’s t test for single comparisons and by ANOVA for multiple comparisons. Values of P < 0.05 were considered significant.
Results
Augmented in vitro angiogenic features of EPCs in response to simvastatin.
Ex vivo cultured EPCs were prepared as described previously (24). FACS analysis performed after 7 days in culture disclosed that the majority of the cells expressed EC-specific antigens, including VEGF receptor-2 (VEGFR-2 [KDR], 80.1% ± 4.1%), VE-cadherin (78.1% ± 8.2%), CD31 (77.5% ± 8.8%), and P1H12 (71.2% ± 5.7%). Further characterization excluded significant contamination by hematopoietic lineage cells such as T lymphocytes or macrophage/monocytes (data not shown).
To detect evidence of Akt signaling in EPCs after exposure to VEGF, Western immunoblot analysis of Akt phosphorylation was performed under various conditions. This disclosed that simvastatin treatment led to a dose-dependent increase in serine 473 Akt phosphorylation within 10 minutes, with maximal Akt phosphorylation occurring at 1 μM simvastatin (data not shown). Increased Akt phosphorylation was detected as early as 5 minutes after exposure to 1 μM simvastatin and peaked at approximately 1 hour (Figure 1a).
Figure 1.
Augmented angiogenic properties of EPCs in response to simvastatin via Akt signaling. (a) Representative Western immunoblots of Akt phosphorylation are shown as time-dependent changes in Akt phosphorylation at serine 473 after treatment with simvastatin (1 μM). Akt protein level was normalized by whole Akt1. Increased Akt phosphorylation was detected as early as 5 minutes. (b) Dose-dependent increase in cell number was observed in day 7 cultured EPCs. (d) MTS assay of human EPCs in response to simvastatin. A moderate dose-dependent mitogenic response to simvastatin was observed in day 7 cultured EPCs. (f) Migration assay of human EPCs in response to simvastatin. Migratory effect was augmented by simvastatin treatment with a peak at 1 μM concentration (c, e, and g). Cell number, mitogenic response, and migratory effect to simvastatin were precluded by dnAkt overexpression. –, simvastatin-negative; +, simvastatin-positive. *P < 0.01.
The effect of simvastatin on EPC proliferation was assayed using a previously validated colorimetric MTS assay with the electron coupling reagent phenazine methosulfate (CellTiter 96 AQ; Promega Corp., Madison, Wisconsin, USA). Simvastatin increased EPC proliferative activity (control versus 0.1 μM simvastatin, 0.47 ± 0.04 vs. 0.56 ± 0.03; control versus 1 μM simvastatin, 0.47 ± 0.04 vs. 0.62 ± 0.03, 490 nm light absorbance, respectively; P < 0.01) (Figure 1d). The increase in proliferative activity was confirmed by manual counting of EPCs (control versus 0.1 μM simvastatin, 6.18 ± 0.16 × 105 vs. 7.42 ± 0.20 × 105; control versus 1μM simvastatin, 6.18 ± 0.16 × 105 vs. 8.38 ± 0.13 × 105 cells per well; P < 0.01) (Figure 1b).
The effect of simvastatin on EPC migration was analyzed in a modified Boyden chamber assay. Simvastatin profoundly enhanced cell migration, maximal at 1 μM simvastatin (control versus 0.1 μM simvastatin, 5 ± 4 vs. 64 ± 26; control versus 1 μM simvastatin, 5 ± 4 vs. 213 ± 46; control versus 10 μM simvastatin, 5 ± 4 vs. 152 ± 36, cells per four high-powered [40×] fields, respectively; P < 0.01) (Figure 1f).
To determine the role of Akt in modulating these mitogenic and migratory responses to statin therapy, adenovirus gene transfer was used to overexpress dnAkt in EPCs. The impact of simvastatin on both proliferation and migration was abrogated in dnAkt-transfected cells, but not in cells transfected with adenovirus encoding β-gal (Figure 1, c, e, and g).
Increase in EPC survival induced by simvastatin.
Serum starvation was used to induce apoptosis in cultured EPCs. Annexin-V staining disclosed Akt-mediated reduction by simvastatin in the number of EPCs positive for Annexin-V or propidium iodide, a marker for cell death (Figure 2a). Tf/β-gal + no simvastatin versus Tf/β-gal + simvastatin was 29% ± 3% vs. 6% ± 1% (P < 0.01), and Tf/dnAkt + no simvastatin versus Tf/dnAkt + simvastatin was 31% ± 3% vs. 27% ± 1% (NS), Annexin-positive cells, respectively (Figure 2b).
Figure 2.
Increase in EPC survival induced by simvastatin via Akt signaling. (a) Apoptosis was evaluated after serum starvation of cultured EPCs. Annexin-V staining disclosed that simvastatin reduced the number of EPCs positive for Annexin-V or propidium odide, a marker for cell death. (b) Quantification of Annexin-V–positive cells: Tf/β-gal + no simvastatin versus Tf/β-gal + simvastatin, 29% ± 3% vs. 6% ± 1% Annexin-positive cells (*P < 0.01); Tf/dnAkt + no simvastatin versus Tf/dnAkt + simvastatin, 31% ± 3% vs. 27% ± 1% Annexin-positive cells (NS). (c) Hoechst 33342 staining was also performed to detect the frequency of apoptosis by counting pyknotic nuclei. Simvastatin attenuated apoptosis (control versus simvastatin, 24% ± 5% vs. 6% ± 1% pyknotic nuclei [**P < 0.02]). (d) Dominant negative Akt overexpression abolished positive cell survival effect of simvastatin (Tf/β-gal + no simvastatin versus Tf/β-gal + simvastatin, 21% ± 6% vs. 5% ± 1% pyknotic nuclei [*P < 0.01]; Tf/dnAkt + no simvastatin versus Tf/dnAkt + simvastatin, 26% ± 2% vs. 21% ± 2% pyknotic nuclei [NS]). –, simvastatin-negative; +, simvastatin-positive.
Hoechst 33342 staining was also performed to determine the proportion of apoptotic cells by manually counting pyknotic nuclei. Simvastatin reduced the percentage of apoptotic cells from 24% ± 5% in the controls to 6% ± 1% (P < 0.02) (Figure 2c). dnAkt overexpression again abolished the effect of simvastatin on cell survival (Tf/β-gal + no simvastatin versus Tf/β-gal + simvastatin, 21% ± 6% vs. 5% ± 1% [P < 0.01], and Tf/dnAkt + no simvastatin versus Tf/dnAkt + simvastatin, 26% ± 2% vs. 21% ± 2% [NS], pyknotic nuclei, respectively) (Figure 2d).
EPC mobilization induced by simvastatin in vitro and in vivo.
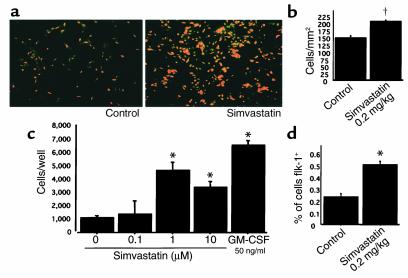
As demonstrated in previous reports (4, 5), mobilization of EPCs contributes to postnatal neovascularization and is enhanced by tissue ischemia or cytokine administration. To investigate EPC mobilization, we first studied the effect of simvastatin on EPC chemotactic activity in vitro in a transwell assay. Chemotactic activity was increased by simvastatin, with maximum chemotactic activity observed in the group treated with 1 μM simvastatin (control versus 1 μM simvastatin: 1,137 ± 148 vs. 4,681 ± 598; control versus 10 μM simvastatin: 1,137 ± 148 vs. 3387 ± 460, cells per 50 μl of lower chamber media; P < 0.01) (Figure 3c).
Figure 3.
EPC mobilization induced by simvastatin in vitro and in vivo. (a) Representative fluorescence photomicrographs of EPCs cultured 4 days after isolation from peripheral blood. EPCs are identified as double positive cells owing to uptake of acLDL-DiI (red) and BS-1 lectin reactivity (green). (b) Quantification of cultured EPC population isolated from mouse peripheral blood. EPC population increased after simvastatin administration. †P < 0.05. (c) The effect of simvastatin on EPC chemotactic activity in vitro was analyzed in a transwell assay. Chemotactic effect was augmented by simvastatin with a peak at 1 μM concentration. The potency of this effect reached 70% of chemotactic response to recombinant murine GM-CSF. *P < 0.01. (d) FACS analysis of mouse peripheral blood. Ratio of Flk-1–positive cells increased after simvastatin treatment. *P < 0.01.
To evaluate EPC mobilization in vivo, the murine EPC culture assay (4, 5) was used as a functional index of circulating EPCs. Peripheral blood samples from four animals of each treatment group were collected, and PBMCs were cultured as described above. The number of EPCs after 4 days in culture, confirmed by a combination of both acLDL uptake and BS-1 lectin reactivity, documented increased circulating EPCs in the peripheral blood of simvastatin-treated versus control mice (205 ± 5 vs. 147 ± 7 cells/mm2; P < 0.05) (Figure 3, a and b). FACS analysis confirmed the increase in EPCs, showing increased Flk-1–positive cells among peripheral mononuclear cells from simvastatin-treated versus control mice (0.50% ± 0.03% vs. 0.23% ± 0.03% of cells; P < 0.01) (Figure 3d). There was no statistically significant difference in the levels of serum cholesterol between treated and control mice (data not shown).
Enhanced contribution of BM-derived EPCs to corneal neovascularization.
To establish whether the in vitro and in vivo findings suggesting provasculogenic effects of simvastatin on EPCs were associated with augmented neovascularization, simvastatin therapy was studied in a murine model of corneal injury after BMT. Recipient mice were injected with BM from transgenic mice constitutively expressing β-galactosidase encoded by lacZ (LZ) under the transcriptional regulation of an EC-specific promoter, Tie-2 (25). Reconstitution of the transplanted BM yielded Tie-2/LZ/BMT mice in which expression of lacZ is restricted to BM-derived cells expressing Tie-2; lacZ expression is not observed in other somatic cells. The Tie-2/LZ/BMT mice then underwent corneal assay microsurgery (26, 27) after completing a 7-day course of simvastatin or control vehicle.
As shown in Figure 4, simvastatin treatment resulted in augmented corneal neovascularization (Figure 4a, right) and in more X-gal–positive cells (Figure 4b, right) than in the control group (Figure 4, a and b, left of each). Fluorescent immunohistochemistry performed on paraffin-embedded sections documented a marked increase in cells that were double positive for β-gal and the endothelial cell–specific marker isolectin B4 in the simvastatin group (Figure 4c). These findings were confirmed by in situ BS-1–lectin staining and fluorescent immunohistochemistry for β-gal using whole-mounted corneas. Sections from the simvastatin group documented more neovascularization and more extensive incorporation of β-gal–positive cells (Figure 4d) compared with controls. Quantitative analysis of incorporated β-gal–positive cells revealed that simvastatin enhanced vasculogenesis in neovascular foci of corneas of simvastatin-treated versus control mice (25.7% ± 4.0% versus 7.3% ± 2.0% incorporation of β-gal–positive cells; P < 0.05) (Figure 4e).
Figure 4.
Enhanced contribution of BM-derived EPCs to corneal neovascularization. (a) Representative photos show corneal neovascularization (left, vehicle; right, simvastatin). (b) Whole-mounted corneal X-gal staining. Blue dots show X-gal–positive cells (left, vehicle; right, simvastatin). (c) Representative photomicrograph after fluorescent histochemistry examination of paraffin-embedded corneas in neovascularization assay of Tie2/LacZ/BMT mice. Double positive cells indicate that Tie2-expressing BM-derived EPCs incorporated into foci of neovascularization. Red shows β-gal, and green shows isolectin B4 binding. Double positive cell (yellow) indicates BM-derived EPCs incorporated into neovasculature. (d) Representative photomicrograph after fluorescent histochemistry examination of whole-mounted corneas in neovascularization assay of Tie2/LacZ/BMT mice. Red shows β-gal–positive cells, and green shows BS-1–lectin–stained corneal neovasculature. (e) Quantification of BM-derived EPCs incorporated into neovasculature. Ratio indicates percentage of BM-derived EPCs among total endothelial cells comprising corneal neovasculature. †P < 0.05.
Discussion
These experiments establish a novel role for statins, analogous to that described for EPC-modulating cytokines, in the regulation of postnatal neovascularization. VEGF, as an example of a prototypical angiogenic growth factor, was initially considered to promote neovascularization solely via mitogenic and promigratory effects on fully differentiated endothelial cells; i.e., the classical paradigm of angiogenesis elaborated by Folkman et al. (28). Subsequent studies in animal models (5) and human subjects (6, 7), however, established that VEGF also acts to mobilize BM-derived EPCs that contribute to postnatal neovascularization via vasculogenesis. It is thus likely that this mechanism contributes in part to postnatal neovascularization recently documented in animals with hindlimb ischemia after statin therapy (22).
The documented chemotactic effect of simvastatin on EPCs supports the notion that simvastatin mobilizes EPCs from BM. This finding was directly confirmed in vivo by the demonstration, in mice transplanted with BM from transgenic donors expressing β-galactosidase transcriptionally regulated by the endothelial cell-specific Tie-2 promoter, that simvastatin augmented incorporation of EPCs mobilized from the BM into foci of corneal neovascularization.
Moreover, the Akt signaling pathway, shown here to mediate the proangiogenic effects of VEGF on EPCs in vitro, is also demonstrated to constitute the critical signaling pathway for statin-modulated vasculogenic properties of EPCs. The serine/threonine protein kinase Akt (also known as protein kinase B) was first identified as an oncogene owing to its ability to transform normal cells (29, 30). Subsequent studies, however, clarified that Akt functioned as an antiapoptosis protein, protecting against cell death induced by growth factor withdrawal (31–34). More recently, an expanded role for Akt has been established involving a variety of cardiovascular events (35). Akt, for example, has been shown to act downstream of the angiogenic growth factors VEGF and angiopoietin (16–18, 36, 37) to confer EC survival and ensure proper vessel development (18). Constitutive activation of Akt signaling also protects cardiomyocytes from apoptosis after ischemia-reperfusion injury in vivo (38). In addition to this cytoprotective role, Akt functions as an activator of endothelial cell NO production in response to VEGF and shear via phosphorylation of endothelial NO synthase (eNOS) on serine 1179 or 1177 (20, 21), thereby controlling vasomotor reactivity (39). Akt has also been shown to be essential for directed EC migration toward VEGF (19).
The evidence that Akt regulates VEGF-induced EC survival, NO production and migration suggests a potentially important role for Akt signaling in mediating the response of ECs to angiogenic stimuli. Indeed, Kureishi et al. have demonstrated that simvastatin rapidly activates Akt signaling in ECs, enhances phosphorylation of the endogenous Akt substrate eNOS, inhibits apoptosis, and accelerates formation of vascular structures in vitro in an Akt-dependent manner (22). Furthermore, augmented Akt signaling enhanced neovascularization in the rabbit ischemic hindlimb model.
In our experiments, simvastatin was shown to promote EPC proliferation, migration, and cell survival in vitro via the Akt signaling pathway, a finding that was confirmed by functional blocking with dominant negative Akt overexpression. The potential for similar systemic effects of statin on tissue regeneration have been previously demonstrated by statin-upregulated osteoblast activity leading to new bone formation (40).
The results from this study thus suggest indicate that simvastatin may have utility for therapeutic postnatal vasculogenesis of ischemic tissue, potentially including patient populations with normal cholesterol levels.
Acknowledgments
We thank M. Neely and T. Shiojima for secretarial assistance. Simvastatin was a generous gift from Samuel D. Wright (Merck & Co.). S. Murasawa is the recipient of a grant from the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad. J. Llevadot is the recipient of a grant from la Caixa (Spain) Foundation.
Footnotes
See the related Commentary beginning on page 365.
Joan Llevadot and Satoshi Murasawa contributed equally to this work.
References
- 1.Risau W, et al. Vasculogenesis and angiogenesis in embryonic stem cell-derived embryoid bodies. Development. 1988;102:471–478. doi: 10.1242/dev.102.3.471. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalka C, et al. Vascular endothelial growth factor165 gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86:1198–1202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 7.Kalka C, et al. Mobilization of endothelial progenitor cells following gene therapy with VEGF165 in patients with inoperable coronary disease. Ann Thorac Surg. 2000;70:829–834. doi: 10.1016/s0003-4975(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 8.Shi Q, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 9.Hatzopoulos AK, Folkman J, Vasile E, Eiselen GK, Rosenberg RD. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development. 1998;125:1457–1468. doi: 10.1242/dev.125.8.1457. [DOI] [PubMed] [Google Scholar]
- 10.Gunsilius E, et al. Evidence from a leukaemia model for maintenance of vascular endothelium by bone-marrow-derived endothelial cells. Lancet. 2000;355:1688–1691. doi: 10.1016/S0140-6736(00)02241-8. [DOI] [PubMed] [Google Scholar]
- 11.Gehling UM, et al. In vivo differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 12.Crosby JR, et al. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87:728–730. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 13.Moldovan NI, Goldschmidt-Clermont PJ, Parker-Thornburg J, Shapiro SD, Kolattukudy PE. Contribution of monocytes/macrophages to compensatory neovascularization. The drilling of metalloelastase-positive tunnels in ischemic myocardium. Circ Res. 2000;87:378–384. doi: 10.1161/01.res.87.5.378. [DOI] [PubMed] [Google Scholar]
- 14.Murohara T, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooke JP, et al. Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J Clin Invest. 1992;90:1168–1172. doi: 10.1172/JCI115937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim I, et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/akt signal transduction pathway. Circ Res. 2000;86:24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 17.Papapetropoulos A, et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 18.Carmeliet P, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 19.Morales-Ruiz M, et al. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase akt. Circ Res. 2000;86:892–896. doi: 10.1161/01.res.86.8.892. [DOI] [PubMed] [Google Scholar]
- 20.Fulton D, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimmeler S, et al. Activation of nitric oxide synthase in endothelial cells by akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 22.Kureishi Y, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefer AM, et al. Simvastatin preserves the ischemic-reperfused myocardium in normocholesterolemic rat hearts. Circulation. 1999;100:178–184. doi: 10.1161/01.cir.100.2.178. [DOI] [PubMed] [Google Scholar]
- 24.Kalka C, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlaeger TM, Qin Y, Fujiwara Y, Magram J, Sato TN. Vascular endothelial lineage-specific promoter in transgenic mice. Development. 1995;121:1089–1098. doi: 10.1242/dev.121.4.1089. [DOI] [PubMed] [Google Scholar]
- 26.Muthukkaruppan V, Auerbach R. Angiogenesis in the mouse cornea. Science. 1979;28:1416–1418. doi: 10.1126/science.472760. [DOI] [PubMed] [Google Scholar]
- 27.Asahara T, et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–240. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- 28.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 29.Chang HW, et al. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 30.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase b/akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the akt proto-oncogene product by phosphatidylinositol-3,4-bishosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 32.Eves EM, et al. Akt, a target of phosphatidylinositol 3-kinase, inhibits apoptosis in a differentiating neuronal cell line. Mol Cell Biol. 1998;18:2143–2152. doi: 10.1128/mcb.18.4.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crowder RJ, Freeman RS. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci. 1998;18:2933–2943. doi: 10.1523/JNEUROSCI.18-08-02933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulrich E, et al. Specific TrkA survival signals interfere with different apoptotic pathways. Oncogene. 1998;16:825–832. doi: 10.1038/sj.onc.1201842. [DOI] [PubMed] [Google Scholar]
- 35.Dimmeler S, Zeiher AM. Akt takes center stage in angiogenesis signaling. Circ Res. 2000;86:4–5. doi: 10.1161/01.res.86.1.4. [DOI] [PubMed] [Google Scholar]
- 36.Gerber HP, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 37.Kontos CD, et al. Tyrosine 1101 of tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and akt. Mol Cell Biol. 1998;18:4131–4140. doi: 10.1128/mcb.18.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walshi K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo Z, et al. Acute modulation of endothelial akt/PKB activity alters nitric oxide-dependent vasomotor activity in vivo. J Clin Invest. 2000;106:493–499. doi: 10.1172/JCI9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mundy G, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]