Abstract
We studied the distribution of 5-hydroxytryptamine– (5-HT-) containing cells in the guinea pig pancreas and examined the effects of 5-HT on fluid secretion by interlobular pancreatic ducts. The 5-HT–immunoreactive cells with morphological characteristics of enterochromaffin (EC) cells were scattered throughout the duct system and were enriched in islets of Langerhans. The fluid secretory rate in the isolated interlobular ducts was measured by videomicroscopy. Basolateral applications of 5-HT strongly but reversibly reduced HCO3-dependent, as well as secretin- and acetylcholine- (ACh-) stimulated, fluid secretion, whereas 5-HT applied into the lumen had no such effects. Secretin-stimulated fluid secretion could be inhibited by a 5-HT3 receptor agonist, but not by agonists of the 5-HT1, 5-HT2, or 5-HT4 receptors. Under the stimulation with secretin, 5-HT decreased the intracellular pH (pHi) and reduced the rate of pHi recovery after acid loading with NH4+, suggesting that 5-HT inhibits the intracellular accumulation of HCO3–. The elevation of intraductal pressure in vivo reduced secretin-stimulated fluid secretion, an effect that could be attenuated by a 5-HT3 receptor antagonist. Thus, 5-HT, acting through basolateral 5-HT3 receptors, strongly inhibits spontaneous, secretin-, and ACh-stimulated fluid secretion by guinea pig pancreatic ducts. 5-HT released from pancreatic ductal EC cells on elevation of the intraductal pressure may regulate fluid secretion of neighboring duct cells in a paracrine fashion.
Introduction
The gastrointestinal tract is the major source of serotonin, 5-hydroxytryptamine (5-HT); a large amount of 5-HT is concentrated in the enterochromaffin (EC) cells and the submucosal and myenteric neurons (1, 2). Mechanical distension of the intestinal mucosa and a variety of neurohormonal stimuli have been shown to release 5-HT from the EC cells mainly into the interstitial space (3), but also into the gut lumen (4, 5). The released 5-HT binds various subtypes of 5-HT receptors on enteric neurons, smooth muscle cells, and epithelial cells and regulates intestinal fluid transport and peristalsis (2, 4, 6).
In the pancreas, 5-HT is mainly present in β cells of the islet of Langerhans (7, 8) and in serotonergic pancreatic nerve fibers (2, 9). The presence of the EC cells in the exocrine pancreas has also been noticed but their exact localization and function have not been described (8). In the present investigation we examined the distribution of the EC cells in the guinea pig pancreas by immunohistochemical methods and found that they were present throughout the pancreatic duct system. To understand a regulatory function of the EC cells on the neighboring duct cells, we examined the effect of 5-HT on the isolated pancreatic ducts, where the functions of the pancreatic duct in vivo are well preserved (10).
Methods
The following study was approved by the Ethical Committee of Nagoya University on Animal Use for Experiment.
Immunohistochemistry.
Under anesthesia with pentobarbital, female Hartley guinea pigs (300–350 g) were killed by intracardiac perfusion with Ringer’s solution for 1 minute followed by 4% paraformaldehyde for 10 minutes, as described previously (11). The dissected specimens of the pancreas were further dipped in the fixative overnight. They were cryoprotected in graded concentration of sucrose, embedded in OCT compounds, and then frozen. Sections of 4-μm thickness were cut by a cryostat and mounted on albumin-coated glass slides. Endogenous peroxidase activity of the sections was blocked with 0.3% H2O2 in methanol for 30 minutes at room temperature. The section was incubated with 10% normal goat serum for 30 minutes and then with mouse anti-serotonin mAb (clone 5HT-H209, 1:200 dilution) for 1–2 days at 4°C. It was then incubated with biotinylated anti-mouse IgG (1:200) at room temperature for 2 hours followed by the avidin-biotin-peroxidase complex for 2 hours. The immunoreaction was visualized by 0.02% diaminobenzidine tetrahydrochloride in 0.05 M Tris-HCl containing 0.01% H2O2 and was examined with an interference contrast microscope (Olympus AX; Olympus Optical Co., Tokyo, Japan).
Isolated interlobular ducts.
Guinea pigs were killed by cervical dislocation. The pancreas was removed and interlobular ducts were isolated as described previously (12). The duct segments were placed in McCoy’s 5A tissue culture medium supplemented with 10% FCS, 2 mM glutamine, 0.1 mg/ml soybean trypsin inhibitor, 0.1 IU/ml insulin and 4 μg/ml dexamethazone. They were cultured at 37°C in 5% CO2 in air for 3 hours, during which time both ends of the interlobular duct segments sealed spontaneously, thus isolating the luminal space from the bathing medium.
Solutions.
The standard HEPES-buffered solution contained (in mM): 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 D-glucose, and 10 HEPES, and was equilibrated with 100% O2. The standard HCO3–-buffered solution contained (in mM): 115 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 D-glucose, and 25 NaHCO3, and was equilibrated with 95% O2–5% CO2. In the solution containing NH4Cl (20 mM), NaCl was reduced to 95 mM. The solutions were adjusted to pH 7.4 at 37°C.
Measurement of the fluid secretory rate in isolated ducts.
The cultured ducts were stored at 4°C in the standard HEPES-buffered solution before use. The fluid secretory rate into the closed luminal space was measured by a modification of the methods described previously (10, 13). The ducts were attached to the glass coverslips pretreated with Cell-Tak (Becton Dickinson Labware, Bedford, Massachusetts, USA) and were superfused at 37°C on the stage of an inverted Olympus IX microscope (Olympus Optical Co.). The bright-field images of the duct were obtained at 1-minute intervals using a charge coupled device (CCD) camera. To determine the fluid secretory rate, the initial values for the length (L0), diameter (2R0) and image area (A0) of the duct lumen were measured in the first image of the series. The initial volume (V0) of the duct lumen was calculated, assuming cylindrical geometry, as πR02L0. The values of L0, R0, and V0 of the ducts used for experiments were 270 ± 10 μm, 60 ± 4 μm, and 3.2 ± 0.4 nl (mean ± SEM, n = 48), respectively. The luminal surface area of the epithelium was taken to be 2πR0L0. In subsequent images of the series, the luminal image area (A) was expressed as the relative area (A/A0). The relative volume (V/V0) was estimated from the relative area assuming V/V0 = (A/A0)3/2. The rate of fluid secretion was calculated at 1-minute intervals from the increment in duct volume and expressed as the secretory rate per unit luminal area of epithelium (nanoliters per minute per square millimeter.)
Micropuncture of the lumen.
To investigate the effects of luminal 5-HT, the lumen of the cultured ducts was micropunctured with a double-barreled micropipette as described previously (10). The luminal fluid was withdrawn and replaced by the standard HEPES-buffered solution with or without 5-HT (1 μM).
Measurement of [Ca2+]i.
Intracellular free Ca2+ concentration ([Ca2+] i) was estimated by microfluorometry in duct cells loaded with fura-2 as described previously (14). The cultured duct segments were incubated for 90 minutes at room temperature with the acetoxymethyl ester fura-2/AM (3 μM). Microfluorometry was performed on a small area of the ductal epithelium (10–20 cells).
Measurement of intracellular pH (pHi).
Intracellular pH (pHi) was estimated by microfluorometry in duct cells loaded with 2′7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) as described previously (12). The cultured duct segments were incubated for 10 minutes at room temperature with the acetoxymethyl ester BCECF-AM (2 μM). Microfluorometry was performed on the ductal epithelium illuminated alternately at 430 nm and 480 nm. Values of pHi were calculated from the fluorescence ratio (F480/F430) measured at 530 nm. In situ calibration was performed using the high-K+/nigericin technique.
Pancreatic fluid secretion in anesthetized guinea pigs.
Guinea pigs were anesthetized by an intraperitoneal injection of pentobarbital. The trachea was cannulated for artificial ventilation. Saline was infused intravenously at 5 ml/hour via a polyethylene tube placed in the external jugular vein throughout the experiments. The abdomen was opened through a midline incision, and the main pancreatic duct was cannulated with a polyethylene tube. The cannula was connected with a silastic tube (1 mm, inner diameter) to a 26G needle attached to a manipulator placed over an electric balance. Pancreatic juice was allowed to drop from the needle so that fluid secretion was measured continuously by a gravimeter using a computer-controlled electric balance (14). The outlet of the needle was positioned at the level of the pancreas (0 cmH2O) using a manipulator. Secretin (1 μg/kg/h) and granisetron (40 μg/kg/h) were infused intravenously with saline. Hydrostatic pressure of 3.0 cmH2O above the initial level was applied to the duct system for 5 minutes by raising the position of the needle.
Materials.
Antiserotonin mAb (clone 5HT-H209) was obtained from Biomeda Corp. (Foster City, California, USA); biotinylated anti-mouse IgG from Amersham International (Little Chalfont, United Kingdom); avidin-biotin-peroxidase complex from Vector Laboratories (Burlingame, California, USA); secretin from the Peptide Institute (Minoh-shi, Osaka, Japan); acetylcholine (ACh), 5-HT, and 5-carboxyamidotryptamine (5-CT) from Sigma Chemical Co. (St. Louis, Missouri, USA); 2-methyl-5-hydroxytryptamine (2-methyl-5-HT), 5-methoxytryptamine (5-MeOT), and α-methyl-5-hydroxytryptamine (α-methyl-5-HT) from Research Biochemical International (Natick, Massachusetts, USA); granisetron from Nippon Roche k.k (Tokyo, Japan); fura-2/AM from Dojindo Laboratories (Kumamoto, Japan); and BCECF-AM from Molecular Probes Inc. (Eugene, Oregon, USA).
Statistics.
Data are presented as means ± SEM. Statistical analysis was carried out by ANOVA followed by Student’s t test for paired or unpaired data. P values less than 0.05 was considered significant.
Results
5-HT–containing cells in the pancreas.
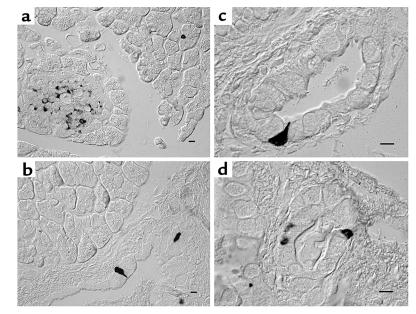
Numerous 5-HT–immunoreactive cells were found in the islet of Langerhans (Figure 1a), and 5-HT–immunoreactive cells were scattered in the epithelium of the main duct (Figure 1b), interlobular ducts (diameter 25–500 μm) (Figure 1, c and d), intralobular ducts, and in acini (Figure 1a). They were pyramid shaped and had a slender process toward the lumen.
Figure 1.
Immunohistochemistry for 5-HT. The distribution of 5-HT–positive cells in the guinea pig pancreas was examined with mouse anti-serotonin mAb. The majority of 5-HT–immunoreactive cells are present in the islet of Langerhans (a). They are also present in the epithelium of the main (b), larger (c), and smaller (d) interlobular ducts and in acini (a). Bar, 5 μm.
Effects of 5-HT on spontaneous (HCO3–-dependent) fluid secretion.
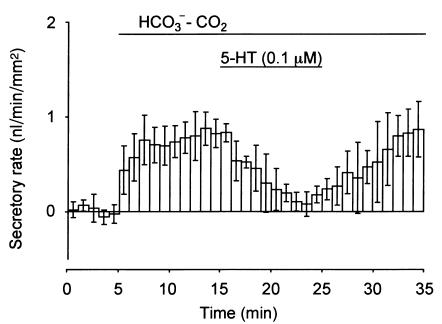
There was little ductal fluid secretion (0.01 ± 0.13 nl/min/mm2; n = 4) during superfusion with HCO3–-CO2–free HEPES-buffered solution (Figure 2). The fluid secretory rate increased to 0.78 ± 0.02 nl/min/mm2 when the bath solution was switched to the standard HCO3-CO2–buffered solution. The application of 5-HT (0.1 μM) reduced spontaneous (HCO3-dependent) fluid secretion significantly (P < 0.01) to 0.22 ± 0.04 nl/min/mm2 in 7 minutes. When the application of 5-HT was halted, fluid secretion recovered in 10 minutes.
Figure 2.
Effects of 5-HT on basal fluid secretion in interlobular duct segments isolated from guinea pig pancreas. The closed ducts were initially superfused with HCO3–-CO2-free HEPES-buffered solution. After a 5-minute period, the bath solution was switched to the standard HCO3–-CO2–buffered solution. After a further 10 minutes, 0.1 μM 5-HT was added to the bath in the presence of HCO3–-CO2. The fluid secretory rates are shown as means ± SEM of four experiments.
Effects of 5-HT on secretin-stimulated fluid secretion.
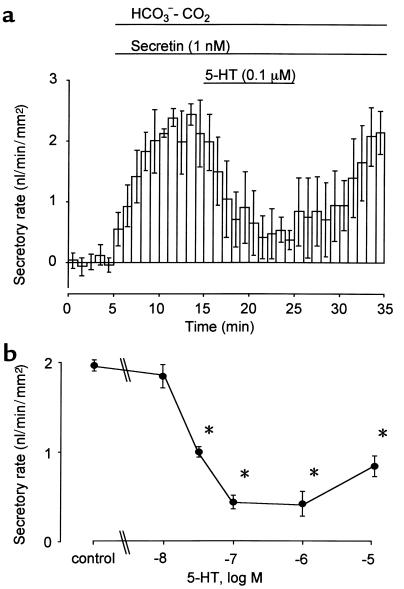
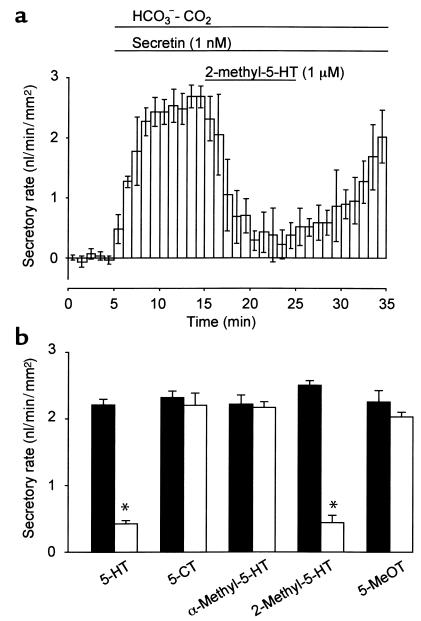
Secretin (1 nM) increased the fluid secretory rate to 2.23 ± 0.05 nl/min/mm2 (n = 24), which remained constant over 15 minutes. When 5-HT at concentrations of 0.01, 0.03, 0.1 (Figure 3a), 1, or 10 μM was added to the bath, 5-HT (> 0.03 μM) significantly (P < 0.01) inhibited the secretin-stimulated fluid secretion. The inhibitory effect was concentration dependent at a range of 0.01 to 0.1 μM, and IC50 was approximately 0.03 μM (Figure 3b).
Figure 3.
Effects of 5-HT on secretin-stimulated fluid secretion. (a) Ducts were stimulated with 1 nM secretin in the presence of HCO3–-CO2. 5-HT (0.1 μM) was added to the bath during stimulation with 1 nM secretin. (b) Concentration-response curve for the effects of 5-HT on secretin-stimulated fluid secretion. The fluid secretory rates are shown as means ± SEM of four experiments. Asterisks indicate significant (P < 0.01) differences.
Effects of 5-HT on ACh-stimulated fluid secretion.
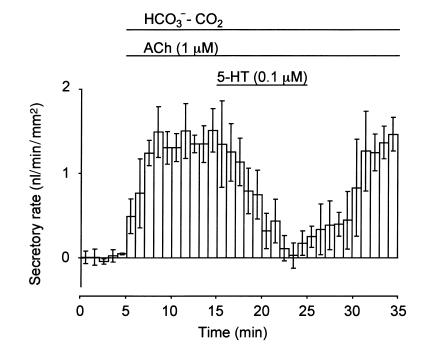
ACh (1 μM) increased the fluid secretory rate to 1.42 ± 0.04 nl/min/mm2, which remained constant for over 20 minutes (n = 4). The application of 0.1 μM 5-HT to the bath reversibly reduced ACh-stimulated fluid secretion by 73.4% ± 0.7% (P < 0.01, Figure 4).
Figure 4.
Effects of 5-HT on ACh-stimulated fluid secretion. Ducts were stimulated with 1 μM ACh in the presence of HCO3–-CO2. 5-HT (0.1 μM) was added to the bath during stimulation with 1 μM ACh. The fluid secretory rates are shown as means ± SEM of four experiments.
Effects of 5-HT receptor agonists on secretin-stimulated fluid secretion.
To identify the subtypes of 5-HT receptors that are responsible for the inhibition of fluid secretion, the effects of 5-HT receptor agonists on secretin-stimulated (1 nM) fluid secretion were examined. The application of 1 μM 2-methyl-5-HT (5-HT3 receptor agonist) significantly (P < 0.01) reduced fluid secretion by 82.4% ± 4.1% (n = 4; Figure 5a). However, 5-CT (5-HT1 receptor agonist), α-methyl-5-HT (5-HT2 receptor agonist), and 5-MeOT (5-HT4 receptor agonist) failed to inhibit secretin-stimulated fluid secretion (Figure 5b).
Figure 5.
Effects of 5-HT receptor agonists on secretin-stimulated fluid secretion. (a) One micrommolar of 2-methyl-5-HT (5-HT3 receptor agonist) was added to the bath during stimulation with 1 nM secretin. (b) Effects of 1 μM of 5-HT, 5-CT (5-HT1 receptor agonist), α-methyl-5-HT (5-HT2 receptor agonist), 2-methyl-5-HT (5-HT3 receptor agonist), and 5-MeOT (5-HT4 receptor agonist) on secretin-stimulated fluid secretion. Filled bars, the secretory rate before the application of 5-HT agonists. Open bars, the secretory rate in the presence of 5-HT agonists. The fluid secretory rates are shown as mean ± SEM of four experiments. Asterisks indicate significant (P < 0.01) differences.
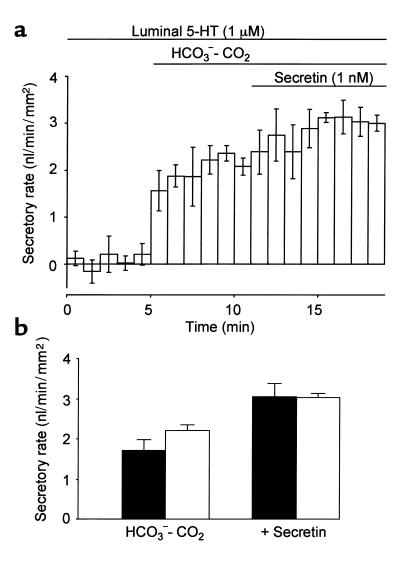
Effects of luminal 5-HT on secretin-stimulated fluid secretion.
To examine the effects of luminal 5-HT on fluid secretion, the ducts were filled with the standard HCO3–-CO2–free HEPES-buffered solution with or without 1 μM of 5-HT. In the presence of 5-HT in the lumen, the introduction of HCO3–-CO2 to the bath evoked fluid secretion of 2.22 ± 0.14 nl/min/mm2 (n = 4; Figure 6a), which was not significantly different from that without 5-HT (Figure 6b). Secretin-stimulated fluid secretion (3.04 ± 0.10 nl/min/mm2) was also unaffected by the luminal application of 5-HT (Figure 6b).
Figure 6.
Effects of luminal 5-HT on fluid secretion. The lumen of the cultured ducts was micropunctured and the luminal fluid was replaced by the HEPES-buffered solution with or without 5-HT (1 μM). The ducts were initially superfused with HEPES-buffered solution for 5 minutes. The bath solution was switched to the HCO3–-CO2-buffered solution. After a 6-minute period, 1 nM secretin was added to the bath. (a) The fluid secretory rate in ducts injected with 5-HT (1 μM). Mean ± SEM of four experiments. (b) Spontaneous (HCO3–-CO2–dependent) and secretin-stimulated (1 nM) fluid secretion in the absence (filled bars) and presence (open bars) of luminal 5-HT (1 μM). Mean ± SEM of four experiments.
The effect of 5-HT on [Ca2+]i in duct cells.
ACh (1 μM) induced a sustained increase in [Ca2+]i, while 5-HT (1 μM) caused a smaller transient increase in [Ca2+]i as compared with ACh (data not shown). When 5-HT (1 μM) was applied during the stimulation with ACh (1 μM), [Ca2+]i showed a transient increase over the plateau response to ACh.
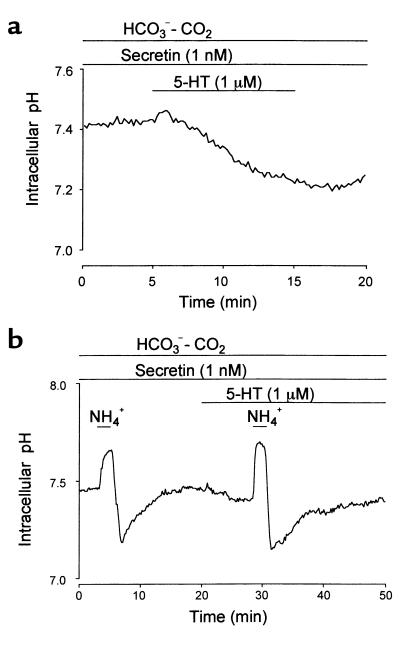
The effect of 5-HT on pHi in duct cells.
When 5-HT (1 μM) was applied to the bath during the stimulation with secretin (1 nM), pHi started to decrease gradually (Figure 7a). The initial rate of acidification was 0.024 ± 0.004 pH unit per minute (n = 6). When duct cells were acid loaded with the NH4+ pulse technique, the initial rate of the pHi recovery (0.099 ± 0.030 pH unit per minute) was significantly (P < 0.01) reduced to 0.033 ± 0.024 pH unit per minute in the presence of 5-HT (1 μM) (n = 5; Figure 7b).
Figure 7.
Effect of 5-HT on intracellular pH. Ducts were superfused with the standard HCO3–-buffered solution in the presence of secretin (1 nM). (a) One micrometer 5-HT was applied to the bath. Representative of six experiments. (b) Duct cells were acid loaded by exposure to 2-minute pulses of 20 mM NH4+ in the absence and presence of 5-HT (1 μM). Representative of five experiments.
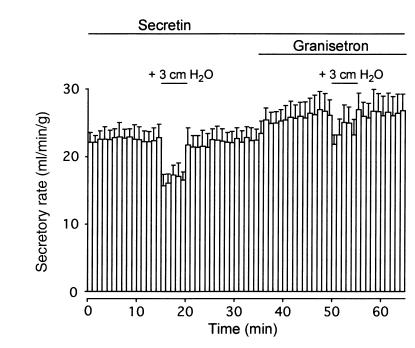
Effects of intraductal pressure on secretin-stimulated fluid secretion in vivo.
An intravenous infusion of secretin (1 μg/kg/h) induced steady pancreatic fluid secretion (22.5 ± 1.8 μl/min/g wet weight of pancreas; n = 4) for over 1 hour (Figure 8). When a hydrostatic pressure of +3.0 cmH2O was applied to the pancreatic duct system for a 5-minute period, the secretory rate dropped significantly (P < 0.01) by 5.9 ± 0.9 μl/min/g. The secretory rate quickly returned to the initial levels when the intraductal pressure was reduced to the initial zero level. An intravenous administration of granisetron (40 μg kg/h), an antagonist of 5-HT3 receptor, increased the secretory rate by 4.1 ± 1.4 μl/min/g (P < 0.05). In the presence of granisetron, the reduction of the secretory rate (2.8 ± 1.4 μl/min/g) by the elevation of intraductal pressure (+3.0 cmH2O) was significantly (P < 0.05) smaller than that of control (without a 5-HT3 receptor antagonist).
Figure 8.
Effects of intraductal pressure on secretin-stimulated fluid secretion in anesthetized guinea pigs. Pancreatic fluid secretion was stimulated with an intravenous infusion of secretin (1 μg/kg/h). A hydrostatic pressure of +3.0 cmH2O was applied to the duct system by elevating the position of the outlet of the needle connected to the pancreatic duct without or with an intravenous infusion of granisetron (40 μg/kg/h), a 5-HT3 receptor antagonist. Mean ± SEM of four experiments in four guinea pigs.
Discussion
5-HT–containing cells in the guinea pig pancreas.
The present study confirmed earlier studies that many 5-HT–immunoreactive cells are present in the islet of Langerhans in guinea pigs (8). Insulin and 5-HT are costored within the secretory granules in β cells (7); 5-HT that is coreleased with insulin (15) may regulate pancreatic ductal functions. However, it is not known whether a sufficient amount of 5-HT reaches pancreatic duct cells via the efferent vessels from the islet capillary network that communicate the ductal capillary plexus (16).
Previous studies in vivo suggest that serotonergic enteropancreatic nerves inhibit pancreatic fluid and protein secretion via presynaptic receptors on cholinergic nerves (17, 18). More recently, Li et al. (19) reported that 5-HT released from the intestinal EC cells activated neuronal 5-HT3 and 5-HT4 receptors on vagal afferent fibers to mediate luminal factor-stimulated pancreatic secretion in rats. This evidence indicates that 5-HT regulates pancreatic secretion indirectly by controlling cholinergic nerves.
The present study clearly showed that 5-HT–immunoreactive cells with morphological characteristics of EC cells were present throughout the duct system, i.e., the main, intra-, and interlobular ducts. The EC cells in the exocrine pancreas, in contrast to 5-HT–immunoreactive cells in the islets, are unreactive for the pancreatic hormones (8). Since intra- and interlobular ducts are thought to be responsible for most of HCO3– and water secretion in the pancreas (20), this finding leads us to a hypothesis that 5-HT may regulate pancreatic duct cell function locally in a paracrine fashion.
Direct inhibition of ductal fluid secretion by 5-HT.
In the isolated interlobular duct segments 5-HT strongly but reversibly inhibited secretin- and ACh-stimulated fluid secretion as well as spontaneous (HCO3–-dependent) secretion (Figures 2–4). Because the subepithelial tissues were carefully removed in our preparations, this finding strongly suggests that 5-HT exerted the inhibitory effect by directly acting on pancreatic duct cells.
The concentration of 5-HT required for a half-maximal inhibition was approximately 30 nM (Figure 3b), which is 10 to 100 times lower compared with those reported in other systems. In isolated rat pancreatic lobules, the maximal inhibition of veratridine-stimulated amylase secretion was obtained by 5-HT at 5–10 μM (17). In the guinea pig distal colon, 5-HT at 0.1–1 μM stimulated Cl– transport via cholinergic nerves (21, 22). Direct stimulation of epithelial Cl– transport by 5-HT was observed at 0.05–1 μM in rat choroid plexus (23), at 1–100 μM in rat trachea (24), and at 0.1–1 μM in rat epididymis (25).
Neurohormonal agents that have been shown to inhibit pancreatic ductal secretion directly are substance P, arginine vasopressin, and ATP. Substance P (0.1–10 nM) inhibited secretin-stimulated fluid secretion by 60–80% in isolated rat pancreatic ducts (26). In guinea pig ducts, secretin-stimulated fluid secretion was inhibited with vasopressin (100 nM) by 30% (14) and with ATP (1 μM) by 40% (27). In the present study, 5-HT, irrespective of stimuli, reduced ductal fluid secretion by about 75%. Thus, 5-HT is a new candidate agent that directly regulates pancreatic ductal fluid secretion.
5-HT3 receptor on duct cell mediates the inhibition.
Several subtypes of 5-HT receptors have been identified in the gastrointestinal tract (6). The 5-HT1A receptor is present in the enteric nerves and ganglia and inhibits the release of ACh. 5-HT1P receptor is a major neuronal receptor that mediates neurotransmission in submucosal and myenteric plexuses. The 5-HT2A and 5-HT2B receptors are present on vascular and intestinal smooth muscle and epithelial cells and are coupled to phosphoinositol metabolism. The 5-HT3 receptor is a ligand-gated nonspecific cation channel (28, 29) and is present on submucosal and myenteric neurons. The 5-HT4 receptor is present on neurons, smooth muscle cells, and epithelial cells and is coupled to adenylate cyclase.
In guinea pig pancreatic ducts, secretin-stimulated fluid secretion was inhibited by the specific agonist of 5-HT3 receptor but not by 5-HT1, 5-HT2, and 5-HT4 receptors’ agonists (Figure 5b), suggesting that 5-HT3 receptor mediates the inhibition. Although 5-HT3 receptor is believed to be exclusively located on neurons (30), some previous studies showed weak binding of zacopride, a high-affinity ligand for detecting 5-HT3 binding sites at the luminal side of the intestinal mucosa (31, 32). A recent immunolabeling study also indicated that 5-HT3 receptor was present on both enterocytes and nerve processes (33).
Luminal 5-HT does not inhibit fluid secretion.
In a previous study, we demonstrated that spontaneous ductal fluid secretion was largely dependent on the presence of Cl– within the lumen (10). Since the solution injected into the lumen contains high concentration of Cl– (149 mM), the rate of spontaneous fluid secretion was higher than that in nonpunctured ducts, where the luminal fluid probably contained higher concentration of HCO3– and lower concentration of Cl–.
In the gastrointestinal EC cells, most of 5-HT is secreted from the basolateral membrane by exocytosis (3), but it is also released into the lumen (4, 5). When 5-HT was applied to the intestinal mucosa, it evoked Cl– secretion (5). In the present study, previous administration of 5-HT into the lumen failed to affect basal and secretin-stimulated fluid secretion, suggesting that only the basolateral, but not luminal, 5-HT receptor mediates the inhibition of fluid secretion.
Cellular mechanisms for 5-HT–induced inhibition.
In pancreatic duct cells, secretin and ACh stimulates HCO3– and fluid secretion by elevating intracellular cAMP and Ca2+, respectively (20). We have shown previously that in secretin-stimulated ducts isolated from guinea pig pancreas HCO3– is actively accumulated in the cell, mainly by Na+-HCO3– cotransport (12). HCO3– is transported into the lumen via a cAMP-sensitive pathway (34). ACh also stimulated HCO3– secretion in this preparation (34), but the transport mechanisms have not been fully investigated. Because 5-HT inhibited both secretin- and ACh-stimulated fluid secretion in this study, it is unlikely that 5-HT exerts the inhibitory effect via its action on receptors of the secretagogues or their intracellular messengers.
The 5-HT3 receptor that mediates the ductal fluid inhibition is a member of the ligand-gated ion channel family (28, 29). Although a transient increase of [Ca2+]i by 5-HT per se may not be directly related to the inhibition of fluid secretion, it is probably caused by the opening of serotonin-gated nonselective cation channels on pancreatic duct cells (35). Activation of this channel by 5-HT would also cause Na+ influx into the cell. This would reduce the inward gradient of Na+, which is necessary for the accumulation of HCO3– via the basolateral Na+-HCO3– cotransport. Indeed, the application of 5-HT decreased pHi and reduced the rate of pHi recovery from acid loading during stimulation with secretin. Moreover, the initial rate of acidification by 5-HT (0.024 pH unit per minute) was comparable to that observed in the presence of H2DIDS (0.027 pH unit per minute), a potent blocker of Na+-HCO3– cotransport (12). These results support a view that 5-HT inhibits fluid and HCO3– secretion by abolishing the activity of Na+-HCO3– cotransport almost completely.
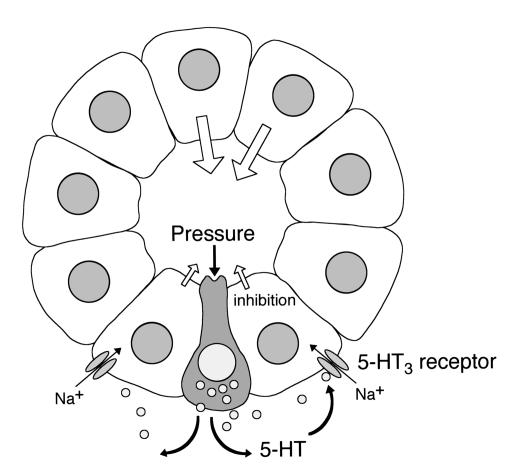
A hypothetical function of ductal EC cells.
Our present findings suggest that 5-HT released from the EC cells in the ductal epithelium may directly regulate fluid secretion from neighboring duct cells in a paracrine fashion (Figure 9). Bülbring (4) originally proposed that the EC cells in the gut are pressure-responsive sensory receptors that secrete 5-HT and regulate intestinal peristalsis and secretion. Similarly, the EC cells in the pancreatic duct may function as intraductal pressure sensors and regulate ductal fluid secretion. When the intraluminal pressure of pancreatic ducts increases, 5-HT is released into the interstitium from the ductal EC cells, and the released 5-HT binds to 5-HT3 receptors on the basolateral membrane of duct cells and inhibits fluid secretion.
Figure 9.
A hypothetical function of 5-HT: a local feedback control of pancreatic duct fluid secretion by the luminal pressure. EC cell–like cells in the duct epithelium are pressure-responsive sensory receptors. The increase in the luminal pressure stimulates the release of 5-HT into interstitium. 5-HT binds to its receptors on the basolateral membrane of the duct cells and inhibits fluid secretion.
To test this hypothesis, we examined the effect of the intraductal pressure on secretin-stimulated fluid secretion in vivo. In isolated segments of guinea pig ileum, peristalsis was induced when the intraluminal pressure was elevated to 0.3–2.0 cmH2O (36), which may be mediated by the release of 5-HT (2). In the present study, the elevation of the intraductal pressure by +3.0 cmH2O induced a reversible reduction of fluid secretion by 26%, which was significantly attenuated (51%) by a 5-HT3 receptor antagonist (Figure 8). This observation is consistent with a hypothesis deduced from our observations in vitro that ductal fluid secretion is controlled by 5-HT released on elevation of the intraductal pressure. This local feedback mechanism would be beneficial for preventing damage to the exocrine system when the luminal pressure was elevated by the ductal stricture or protein plug that disturbs the flow of pancreatic juice.
In summary, we have shown that 5-HT–containing cells are present in the ductal epithelium of the guinea pig pancreas and that 5-HT strongly inhibits secretin- and ACh-stimulated fluid secretion as well as basal secretion in interlobular duct segments via the basolateral 5-HT3 receptor on duct cells. The inhibition is probably due to the reduced uptake of HCO3– via Na+-HCO3– cotransport across the basolateral membrane. A small elevation of the intraductal pressure in vivo reduced secretin-stimulated fluid secretion, which was attenuated by granisetron, a 5-HT3 receptor antagonist. Thus the ductal EC cells may function as intraductal pressure sensors and regulate fluid secretion of neighboring duct cells in a paracrine fashion.
Acknowledgments
This study was supported by grants from Japan Society for the Promotion of Science and Uehara Memorial Foundation. We thank S. Kobayashi, M. C. Steward, V. Wray, and Y. Sohma for helpful discussions.
References
- 1.Sjolund K, Sanden G, Hakanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–1130. [PubMed] [Google Scholar]
- 2.Gershon, M.D., Kirchgessner, A.L., and Wade, P.R. 1994. Functional anatomy of the enteric nervous system. In Physiology of the gastrointestinal tract. L.R. Johnson, editor. Raven Press. New York, New York, USA. 381–422.
- 3.Nilsson O, Ahlman H, Geffard M, Dahlström A, Ericson LE. Bipolarity of duodenal enterochromaffin cells in the rat. Cell Tissue Res. 1987;248:49–54. doi: 10.1007/BF01239961. [DOI] [PubMed] [Google Scholar]
- 4.Bülbring E, Crema A. The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. J Physiol. 1959;146:18–28. doi: 10.1113/jphysiol.1959.sp006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubel KA, Renquist KS, Varley G. Secretory reflexes in ileum and jejunum: absence of remote effects. J Auton Nerv Syst. 1991;35:53–62. doi: 10.1016/0165-1838(91)90038-5. [DOI] [PubMed] [Google Scholar]
- 6.Gale, J.D., and Bunce, K.T. 1996. Pharmacological characterization of 5-hydroxytryptamine receptors in the gastrointestinal tract. In Serotonin and gastrointestinal function. T.S. Gaginella and J.J. Galligan, editors. CRC Press. Boca Raton, Florida, USA. 33–52.
- 7.Ekholm R, Ericson LE, Lundquist I. Monoamines in the pancreatic islets of the mouse: subcellular localization of 5-hydroxytryptamine by electron microscopic autoradiography. Diabetologia. 1971;7:339–348. doi: 10.1007/BF01219468. [DOI] [PubMed] [Google Scholar]
- 8.Cetin Y. Biogenic amines in the guinea pig endocrine pancreas. Life Sci. 1992;50:1343–1350. doi: 10.1016/0024-3205(92)90285-w. [DOI] [PubMed] [Google Scholar]
- 9.Kirchgessner AL, Gershon MD. Innervation of the pancreas by neurons in the gut. J Neurosci. 1990;10:1626–1642. doi: 10.1523/JNEUROSCI.10-05-01626.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishiguro H, et al. Fluid secretion in interlobular ducts isolated from guinea-pig pancreas. J Physiol. 1998;511:407–422. doi: 10.1111/j.1469-7793.1998.407bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ieda H, et al. Coexistence of proguanylin (1–15) and somatostatin in the gastrointestinal tract. J Gastroenterol Hepatol. 1998;13:1255–1233. [PubMed] [Google Scholar]
- 12.Ishiguro H, Steward MC, Lindsay ARG, Case RM. Accumulation of intracellular HCO3- by Na+- HCO3- cotransport in interlobular ducts from guinea-pig pancreas. J Physiol. 1996;495:169–178. doi: 10.1113/jphysiol.1996.sp021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steward, M.C., Lang, T.F., San-Román, J.I., and Case, R.M. 1998. Measurement of secretory rate in isolated pancreatic duct segments by digital videomicroscopy. J. Physiol. 509:3P. (Abstr.)
- 14.Ko SBH, et al. Arginine vasopressin inhibits fluid secretion in guinea pig pancreatic duct cells. Am J Physiol. 1999;277:G48–G54. doi: 10.1152/ajpgi.1999.277.1.G48. [DOI] [PubMed] [Google Scholar]
- 15.Gylfe E. Association between 5-hydroxytryptamine release and insulin secretion. J Endocrinol. 1978;78:239–248. doi: 10.1677/joe.0.0780239. [DOI] [PubMed] [Google Scholar]
- 16.Ohtani O. Microcirculation of the pancreas: a correlative study of intravital microscopy with scanning electron microscopy of vascular corrosion casts. Arch Histol Jpn. 1983;46:315–325. [PubMed] [Google Scholar]
- 17.Kirchgessner AL, Gershon MD. Presynaptic inhibition by serotonin of nerve-mediated secretion of pancreatic amylase. Am J Physiol. 1995;268:G339–G345. doi: 10.1152/ajpgi.1995.268.2.G339. [DOI] [PubMed] [Google Scholar]
- 18.Masuda M, Miyasaka K, Funakoshi A. Involvement of 5-hydroxytryptamine (5-HT)3 receptor mechanisms in regulation of basal pancreatic secretion in conscious rats. J Auton Nerv Syst. 1997;62:58–62. doi: 10.1016/s0165-1838(96)00109-9. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Hao Y, Zhu J, Owyang C. Serotonin released from intestinal enterochromaffin cells mediates luminal non-cholecystokinin-stimulated pancreatic secretion in rats. Gastroenterology. 2000;118:1197–1207. doi: 10.1016/s0016-5085(00)70373-8. [DOI] [PubMed] [Google Scholar]
- 20.Case, R.M., and Argent, B.E. 1993. Pancreatic duct cell secretion: control and mechanisms of transport. In The pancreas: biology, pathobiology and disease. V.L.W. Go, et al, editors. Raven Press. New York, New York, USA. 301–350.
- 21.Cooke HJ, Wang Y-Z, Frieling T, Wood JD. Neural 5-hydroxytryptamine receptors regulate chloride secretion in guinea pig distal colon. Am J Physiol. 1991;261:G833–G840. doi: 10.1152/ajpgi.1991.261.5.G833. [DOI] [PubMed] [Google Scholar]
- 22.Kuwahara A, Kuramoto H, Kadowaki M. 5-HT activates nitric oxide-generating neurons to stimulate chloride secretion in guinea pig distal colon. Am J Physiol. 1998;275:G829–G834. doi: 10.1152/ajpgi.1998.275.4.G829. [DOI] [PubMed] [Google Scholar]
- 23.Garner C, Feniuk W, Brown PD. Serotonin activates Cl- channels in the apical membrane of rat choroid plexus epithelial cells. Eur J Pharmacol. 1993;239:31–37. doi: 10.1016/0014-2999(93)90972-k. [DOI] [PubMed] [Google Scholar]
- 24.Jung JS, Oh SO, Kim MG, Kang DS, Lee SH. Cl- secretion induced by 5-hydroxytryptamine and calcitonin gene-related peptide in rat tracheal epithelia. Pflügers Arch. 1997;435:20–27. doi: 10.1007/s004240050479. [DOI] [PubMed] [Google Scholar]
- 25.Leung GPH, Dun SL, Dun NJ, Wong PYD. Serotonin via 5-HT1B and 5-HT2B receptors stimulates anion secretion in the rat epididymal epithelium. J Physiol. 1999;519:657–667. doi: 10.1111/j.1469-7793.1999.0657n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashton N, Argent BE, Green R. Effect of vasoactive intestinal peptide, bombesin and substance P on fluid secretion by isolated rat pancreatic duct. J Physiol. 1990;427:471–482. doi: 10.1113/jphysiol.1990.sp018182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishiguro H, et al. Luminal ATP stimulates fluid and HCO3- secretion in the guinea-pig pancreatic duct. J Physiol. 1999;519:551–558. doi: 10.1111/j.1469-7793.1999.0551m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derkach V, Surprenant A, North RA. 5-HT3 receptors are membrane ion channels. Nature. 1989;339:706–709. doi: 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- 29.Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- 30.Hoyer D, et al. VII. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmac Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 31.Pinkus LM, Sarbin NS, Barefoot DS, Gordon JC. Association of [3H]zacopride with 5-HT3 binding sites. Eur J Pharmacal. 1989;168:355–362. doi: 10.1016/0014-2999(89)90797-8. [DOI] [PubMed] [Google Scholar]
- 32.Sakurai-Yamashita Y, Yamashita K, Yoshimura M, Taniyama K. Differential localization of 5-hydroxytryptamine3 and 5-hydroxytryptamine4 receptors in the human rectum. Life Sci. 2000;66:31–34. doi: 10.1016/s0024-3205(99)00558-5. [DOI] [PubMed] [Google Scholar]
- 33.Doucet E, Hamon M, Emerit MB. Immunolabelling of the rat intestinal tract with antibodies specific to the long form of the 5-hydroxytryptamine3 receptor. Neuroscience. 1998;87:691–707. doi: 10.1016/s0306-4522(98)00184-5. [DOI] [PubMed] [Google Scholar]
- 34.Ishiguro H, Steward MC, Wilson RW, Case RM. Bicarbonate secretion in interlobular ducts from guinea-pig pancreas. J Physiol. 1996;495:179–191. doi: 10.1113/jphysiol.1996.sp021583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray MA, Argent BE. Non-selective cation channel on pancreatic duct cells. Biochim Biophys Acta. 1990;1029:33–42. doi: 10.1016/0005-2736(90)90433-o. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji S, Anglade P, Ozaki T, Sazi T, Yokoyama S. Peristaltic movement evoked in intestinal tube devoid of mucosa and submucosa. Jpn J Physiol. 1992;42:363–375. doi: 10.2170/jjphysiol.42.363. [DOI] [PubMed] [Google Scholar]