Abstract
Herbivore attack is widely known to reduce food quality and to increase chemical defenses and other traits responsible for herbivore resistance. Inducible defenses are commonly thought to allow plants to forgo the costs of defense when not needed; however, neither their defensive function (increasing a plant’s fitness) nor their cost-savings function have been demonstrated in nature. The root-produced toxin nicotine increases after herbivore attack in the native, postfire annual Nicotiana attenuata and is internally activated by the wound hormone, jasmonic acid. I treated the roots of plants with the methyl ester of this hormone (MeJA) to elicit a response in one member of each of 745 matched pairs of plants growing in native populations with different probabilities of attack from herbivores, and measured the lifetime production of viable seed. In populations with intermediate rates of attack, induced plants were attacked less often by herbivores and survived to produce more seed than did their uninduced counterparts. Previous induction did not significantly increase the fitness of plants suffering high rates of attack. However, if plants had not been attacked, induced plants produced less seed than did their uninduced counterparts. Jasmonate-induced responses function as defenses but are costly, and inducibility allows this species to forgo these costs when the defenses are unnecessary.
Keywords: Nicotiana attenuata/nicotine/jasmonic acid/physiological cost of defense/phenotypic plasticity
All plants use chemical defenses to protect themselves from attack by herbivores and pathogens, and most of these chemical defenses are deployed inducibly in some species (1), that is, their production increases dramatically after attack. Inducible defenses are inherently inferior to constitutively deployed defenses, because of the time lag between the first attack and the activation of the defense. As a result of this delay, a plant could remain vulnerable for hours or even days while waiting for the defense to be activated. Why then is this mode of defense deployment so common, having been demonstrated in more than 110 plant-herbivore associations (1)? The commonly held explanation is that although chemical defenses are beneficial and increase a plant’s fitness when it is under attack, they are costly when not needed, using resources that could be used instead for growth or reproduction (2), or by other means (3, 4) decrease the fitness of well-defended plants when grown in competition with less-defended plants in environments lacking herbivores. In short, inducible defenses are thought to have evolved as a cost-savings measure, allowing plants to time the production of a chemical defense with the prevailing environmental conditions and forgo the payment of defense costs when they are not needed.
Despite the general belief that inducible responses are adaptive, but costly, responses (but see ref. 5), two critical assumptions remain untested. Surprisingly, no one has yet demonstrated that an inducible defense increases a plant’s fitness in natural populations. The vast majority of the ecological research in this area examines how these responses influence herbivore performance (1). Diminished herbivore performance may increase plant fitness, but the complexities of ecological interactions in nature frequently wreak havoc with the best defense strategies. For example, plant chemical defenses frequently are sequestered by specialist herbivores for their own defense (6), thereby turning a plant’s defense against itself (7). Many inducible chemical responses, such as protease inhibitors (8), slow herbivore growth by reducing their digestive efficiency. Some of these function only as defenses in nature when expressed in concert with other responses, such as the induced “alarm” calls of plants (9) that increase the vulnerability of the herbivore to its own predators. Without the use of the third trophic level, a plant may lose more leaf area and have a lower fitness as a result of slowing the growth rates of its herbivores (10). These considerations underscore the importance of examining the benefits of induced defenses in natural populations.
A second, untested assumption is that the induced, well-defended phenotype has a lower fitness than the uninduced, poorly defended phenotype in environments without herbivores. Although genetical analysis of constitutively deployed chemical defenses supports the view that chemical defense can be costly (4, 11), the phenotypic costs of an induced defense, that is, the fitness difference between the induced and uninduced members of the same genotype, remains to be critically evaluated, because of, in large part, the experimental difficulties of activating defenses without wounding or controlling for the fitness consequences of tissue lost during induction (1). If induced defenses are costly because of either the resource demands of their production or other ecological costs, then these costs can best be evaluated in the environments in which these responses presumably evolved.
Nicotiana attenuata, like its congener N. sylvestris, synthesizes the toxic alkaloid nicotine in its roots and dramatically increases its rate of nicotine synthesis after leaf wounding or herbivory, which in turn, results in a systemic increase in nicotine concentrations in above-ground vegetative and reproductive tissues (12–15). In laboratory feeding trials, induced levels of nicotine protect plants against nicotine-tolerant herbivores (16), but these herbivores may suffer lower rates of parasitism when feeding on plants with high nicotine concentrations (17), indicating that this induced defense may have both ecological benefits and costs. Moreover, because 6% of the total nitrogen content of an induced N. attenuata plant resides in this toxin alone (13, 18), this nitrogen is unavailable for other activities such as seed production (18, 19), suggesting that inducing nicotine production may incur large resource-based costs. Hence, it is reasonable to suppose that the fitness consequences of producing this toxin will vary greatly depending on a plant’s habitat.
The discovery of the endogenous wound signals that plants use to activate induced responses has provided researchers with valuable tools for activating defenses independently of herbivore attack (1, 12). Jasmonic acid (JA), a ubiquitous wound hormone known to increase the synthesis of diverse defense-related metabolites (1, 20, 21), is strongly implicated as a long-distance endogenous wound signal, activating nicotine synthesis in the roots after leaf wounding (22, 23). Leaf wounding dramatically increases endogenous JA levels (5–500 ng per plant) within 90 min after wounding in proportion to the amount of wounding (23–25), and these endogenous levels of JA are strongly correlated with the whole-plant nicotine response 5 days later (23, 24). Inhibition of the wound-induced JA response in the leaves with lypoxygenase and cyclooxidase inhibitors inhibits the nicotine response (23, 26); the addition of JA and its methyl ester, MeJA, to the roots of both hydroponically and soil-grown plants increases de novo nicotine synthesis and whole-plant nicotine accumulations just as leaf damage does (12). Moreover, with the use of 14C-labeled jasmonate, we have established that the exogenous application of JA in microgram quantities per plant is required to effect changes in endogenous JA pools in nanogram quantities per plant (27). The treatment of roots with jasmonate thus will stimulate responses comparable to those elicited by leaf wounding. The quantities required to elicit such a response will vary with the edaphic characteristics of the plant’s growing environment and need to be determined empirically.
N. attenuata has life history characteristics that make it particularly useful to test the cost-benefit model for induced defenses. It is an ephemeral member of the annual community in burned sagebrush, blackbrush, and pinyon–juniper forests of the Great Basin desert, and synchronizes its growth with the postfire environment by producing dormant seeds that germinate in response to cellulose combustion product(s) found in wood smoke (28). Such synchronization allows this species to exploit the ephemeral, but nutrient-rich, herbivore- and competition-poor environments that commonly exist after fires (29–31). This temporal window of growth opportunity is quite short, for as postfire succession proceeds, herbivores and competitors quickly recolonize the burned habitats. As a result, plants are progressively smaller and attacked more frequently by herbivores each successive growing season after a fire. N. attenuata can be the most abundant species during the first growing season after a fire, but its abundance rarely persists for more than 3 years (references in ref. 28). Hence, by studying plants growing in different-aged burns, one can study plants with different probabilities of being attacked by herbivores.
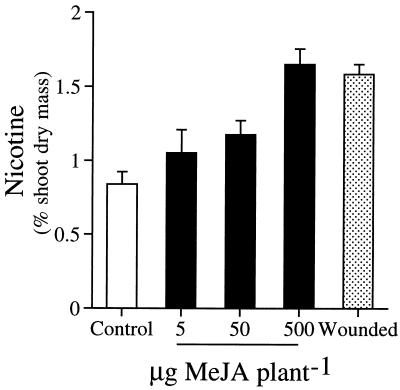
To test the cost-benefit model, I established 745 matched pairs of naturally occurring plants in four natural populations in southwestern Utah: two in 1-year-old burns (IA and IB) where herbivory is typically low (350 plant pairs) and two in 2-year-old burns (IIA and IIB) where herbivory is typically much higher (345 pairs). A fifth artificial population of 50 pairs was created in a nearby field plantation where plants were protected from herbivores with fencing and pyrethrin insecticide applications. I determined the amount of MeJA required to add to the roots of plants to elicit an induced nicotine response comparable to that elicited by a standardized leaf-wounding protocol (Fig. 1) and treated one member of each pair twice during early rosette stage growth (May-June). I measured the consequences of this jasmonate induction by comparing nicotine concentrations, herbivory, survival, and lifetime viable seed production between members of each pair. In additional plant pairs the fitness consequences of jasmonate induction were compared with those of leaf removal.
Figure 1.
Mean (±1 SEM) shoot nicotine concentrations of N. attenuata plants (5/treatment) growing in a 2-year-old burn (population IIA) that had their roots treated with MeJA suspended in 10 ml of water by vigorous shaking or four of their leaves wounded with two rolls from a fabric tracing wheel (which produces a line of 4.5 punctures per cm of leaf lamina) 6 days before analysis. The amount of MeJA required to induce plants growing in the soil was approximately 10 times that required to induce plants growing in water culture (12). Controls were treated with 10 ml of water.
MATERIALS AND METHODS
Populations.
Plants were growing in juniper habitats burned by fires begun by lightning strikes on July 1, 1995 (BLM fire R213–1163 hectares burned: population IA), August 8, 1995 (fire R256–168 hectares burned; population IB), July 2, 1994 (fire R332–809 hectares burned; population IIA), and August 12, 1994 (fire R398–67 hectares burned; population IIB) in southeastern Nevada and southwestern Utah. Each population contained more than 1,000 plants growing among the charred stumps of Juniperus spp. When the pairs were labeled and treated (April 19–23 and May 13, 1996 for the populations in 2-year-old burns and May 22–28, 1996 for the populations in 1-year-old burns), plants in a pair grew within 40–120 cm of each other and were undamaged and of the same rosette diameter (±0.5 cm). At the time of treatment, all plants were in the rosette stage of growth with diameters that ranged from 5 to 22 cm. In addition, a 16 × 8-m plantation was established on April 6–9, 1996, at Brigham Young University’s field station near Santa Clara, UT (Lytle Preserve) with seedlings transplanted from population IIA. The plantation was surrounded by plastic fencing to exclude mammalian herbivores, and plants were inspected daily for lepidopteran and orthopteran herbivores (which were removed by hand) and sprayed with a 0.1% aqueous pyrethrin solution after May 18 when seed-feeding negro bugs (Corimelaena spp.) arrived on the plants. The pyrethrin spray does not affect nicotine levels in N. attenuata (unpublished results).
Treatments.
I determined the amount of MeJA [which was obtained from Bedoukian Research, Danbury, CT, and close to its thermodynamic equilibrium (90.1% 1R,2R-MeJA and 8.3% 1R,2S-MeJA] for the two natural epimers, as determined by GC-MS, that was required to add to the soil at the base of a plant to elicit an induced nicotine response comparable to that elicited by a standardized leaf-wounding protocol (Fig. 1). One member of each of the 745 matched pairs of plants was treated twice during the growing season with 500 μg of MeJA suspended, by vigorous shaking, in 10 ml of water to elicit an induced response (the second application was 12–18 days after the first). The applications were made with a syringe inserted below the soil surface at the base of the rosette with approximately 5 ml applied to each side of each plant. The control member of each pair was treated with 10 ml of water during each MeJA application. Also, for 50 additional pairs of plants in population IB, one member of each pair had half of its leaf area removed (by pinching every other leaf off at the petiole) at the same time that the other pairs in the same population were first treated with MeJA. These pairs were used to compare the fitness consequences of MeJA induction and leaf loss.
Nicotine Analysis.
To assess the effect of MeJA application on chemical defense levels, nicotine was measured in the shoots of 14 randomly selected pairs of plants harvested from the herbivore-free plantation population 9 days after the second MeJA treatment. In addition, nicotine was analyzed in one leaf from the basal rosette of 205 pairs of plants from the four native populations, 10 days after the second MeJA treatment. All analyzed leaves were undamaged and from the same node on each member of the pair. All pairs of plants selected for analysis were unattacked (with less than 5% leaf area lost to herbivores at the time of sampling) except those from population IIB, all of whose pairs had been attacked, so the least-attacked pairs were selected for analysis. Leaves were air-dried with a battery-powered fruit drier or in a convection oven at temperatures below 90°C, sealed in plastic bags, and shipped to the laboratory for nicotine analysis by HPLC with an external standard quantification technique (15, 24). Nicotine loss during drying and shipping was quantified by including approximately 0.5 g of freeze-dried N. attenuata leaves of known nicotine concentration with each batch of samples. Nicotine losses varied from 2% to 8% among batches, and all values were corrected for these losses. All values were expressed as a percentage of leaf dry mass. Leaves used in the grasshopper feeding trial were dried for nicotine analysis immediately after the trial.
Resistance Estimate.
To determine whether treatments had altered the resistance of plant tissues at the time of nicotine analysis, an undamaged, same-sized leaf from the same node from each member of 24 haphazardly selected pairs of plants from population IA was tested in a feeding bioassay with Trimerotropis pallidipennis nymphs. Grasshopper nymphs were collected between 6 and 8 a.m. from population IA and placed individually in 1-liter white plastic containers with mesh lids. One matched leaf from each plant pair was placed on opposite sides of the 24 containers at 6–7 p.m., and after 12 h, the area eaten was quantified (by tracing on graph paper), and the remaining leaf material was dried for nicotine analysis.
Damage and Fitness Estimates.
The percentage of each plant’s leaf area removed by browsing mammals [principally rock and ground squirrels (Spermophilus and Ammospermophilus spp.), black-tailed jack and desert cottontail rabbits (Lepus californicus and Sylvilagus audbonii, respectively)], orthopteran nymphs and adults (mostly Trimerotropis pallidipennis), and the number of hornworm larvae (Manduca quinquemaculata, M. sexta) on each plant was estimated by visual comparisons with two leaf templates (ovate-elliptical for basal leaves and linear-lanceolate for leaves on stems) twice during the growing season for each plant. The proportion of leaf area lost to herbivores from basal and stem leaves was estimated by the sum of the number of leaves times the (visually estimated) proportional area removed from each leaf type divided by the number of leaves of each type. Whole-plant losses were estimated by assigning four times the leaf area to each basal leaf and x amount of leaf area to each stem leaf (an empirically determined value).
N. attenuata is an annual plant that survives to the next growing season only by producing seed; hence, lifetime viable seed production should represent an accurate correlate of a plant’s fitness. Lifetime viable seed production was estimated for all plants that survived to produce seed by counting the number of capsules matured at the end of the growing season and collecting two mature capsules that had yet to dehisce mature seeds from each plant. The capsules were collected from floral stalk positions 1–5, positions known to be uniform in their seed production characteristics (32). These two mature capsules were returned to the laboratory and air-dried at 27–29°C. All seeds from each capsule were weighed to the nearest microgram on a Mettler MT5 microbalance and counted by digital image analysis to determine an average mass per seed and an average number of seeds per capsule for each plant. Seed viability was determined by germinating three replicate samples of 20 seeds from each plant with a 21-day germination protocol, which provided the optimum conditions (including smoke extracts and nitrate) for germinating all viable seeds from this species (28). Lifetime viable seed production was estimated from the product of capsules per plant × seeds per capsule × proportion of seeds that were viable.
RESULTS AND DISCUSSION
MeJA applications significantly increased nicotine concentrations in all populations regardless of which tissues were analyzed. In the herbivore-free plantation population where all above-ground tissues were harvested from 14 pairs of plants 9 days after the second treatment, MeJA treatment increased nicotine concentration 54% (Table 1). For the individual leaves from the basal rosette of the 205 pairs of plants harvested 10 days after the second MeJA treatment, MeJA treatments increased the nicotine concentrations by 22% (population IA), 30% (IB), and 25% (IIA). It is interesting to note that in population IIB, all of whose plants had lost more than 10% of their leaf area to herbivores at the time of analysis, nicotine concentrations of leaves from control plants were comparable to those of the unattacked but induced plants from the other populations. However the effect of MeJA treatment was retained, because induced plants had nicotine concentrations that were 51% higher than their uninduced counterparts (Table 1). Clearly, the MeJA treatment had not saturated the plants’ nicotine responses.
Table 1.
Consequences of MeJA treatment for nicotine and fitness measures
| Population | MeJA induced | Control | Difference | P |
|---|---|---|---|---|
| Nicotine concentration | ||||
| Plantation | 1.09 ± 0.04 | 0.71 ± 0.03 | 0.38 | 0.0006 |
| 1-yr burn (IB) | ||||
| (MeJA treatment) | 1.17 ± 0.07 | 0.91 ± 0.06 | 0.27 | 0.007 |
| (Leaf removal) | 1.35 ± 0.13 | 1.02 ± 0.08 | 0.33 | 0.009 |
| 1-yr burn (IA) | 0.95 ± 0.05 | 0.78 ± 0.04 | 0.17 | 0.0001 |
| 2-yr burn (IIA) | 0.95 ± 0.07 | 0.77 ± 0.09 | 0.19 | 0.012 |
| 2-yr burn (IIB) | 1.95 ± 0.13 | 1.29 ± 0.08 | 0.66 | <0.0001 |
| Capsules per plant | ||||
| Plantation | 36.1 ± 4.5 | 46.1 ± 5.5 | −9.9 | 0.0061 |
| 1-yr burn (IB) | ||||
| (MeJA treatment) | 113.6 ± 22.8 | 140.0 ± 26.7 | −24.2 | 0.04 |
| (Leaf removal) | 62.6 ± 7.7 | 85.6 ± 10.7 | −22.9 | 0.0001 |
| 1-yr burn (IA) | 62.3 ± 2.7 | 56.1 ± 2.7 | +6.2 | 0.0009 |
| 2-yr burn (IIA) | 8.0 ± 1.3 | 5.7 ± 1.0 | +2.8 | 0.15 |
| 2-yr burn (IIB) | 0 ± 0 | 0 ± 0 | 0 | 1.0 |
| Lifetime viable seeds per plant | ||||
| Plantation 100% | 4,494 ± 603 | 6,047 ± 634 | −1,550 | 0.0049 |
| 1-yr burn (IB) | ||||
| (MeJA treatment) 100% | ND | ND | ND | ND |
| (Leaf removal) 100% | 5,314 ± 796 | 7,335 ± 893 | −2,026 | 0.0005 |
| 1-yr burn (AI) 83% | 4,860 ± 208 | 4,505 ± 240 | 349 | 0.033 |
| 2-yr burn (IIA) 7.6% | 841 ± 131 | 538 ± 88 | 371 | 0.073 |
| 2-yr burn (IIB) 0% | 0 ± 0 | 0 ± 0 | 0 | 1.0 |
Mean (±1 SEM) nicotine concentrations, lifetime capsule, and viable seed production of MeJA-induced and control members of matched pairs of N. attenuata plants growing in a plantation population and four natural populations (listed in order of decreasing probability of survival to seed production) with the mean of differences between matched pairs (where + values indicate benefit and − values indicate cost of induction) and the P values for the differences as determined by paired t tests. By the lifetime viable seed data headings, the percentage of pairs that survived to produce seed are given. Nicotine concentrations were determined by HPLC analysis 9–10 days after the second MeJA treatment. Plantation values are of all above-ground parts, and all other values are of individual leaves from the same nodes from each member of the pair. Lifetime viable seeds were calculated as the product of each plant’s lifetime capsules per plant × average seeds per capsule × proportion of seeds that were viable. For the MeJA treatment of population IB, seeds per capsule data were available only for 56% of the pairs of plants that survived to produce seed, and lifetime viable seed production was not determined (ND) for this population. Percentage viable seed data were not determined for any of surviving plants of population IIA, and hence values presented are the lifetime seeds per plant. Percentage viable seed data were available for 201 of the 241 pairs of plants that survived to produce seed from population IA; the population mean (Table 2) was used for the 40 plant pairs with missing values to calculate the lifetime viable seed production values presented in Table 1 and Fig. 2.
The MeJA treatments increased the resistance of leaves to a locally abundant native grasshopper herbivore. After 12 hr, the T. pallidipennis nymphs had removed 10 times more leaf area from the leaves of control plants (2.73 ± 0.52 cm2) than from those of MeJA-treated plants (0.25 ± 0.08 cm2). The nicotine concentrations of leaves used in this trial from treated plants (0.97 ± 0.06% dry mass) were significantly (t23 = 4.8; P = 0.001) higher than those from control plants (0.74 ± 0.05% dry mass) and very similar to those of unattacked plants from the same population (Table 1). Thus MeJA treatments significantly increased both nicotine levels and the resistance of leaves to an herbivore. How do these changes affect plant fitness as estimated by their lifetime viable seed production? The answer depends on the plant’s environment, specifically, the likelihood of herbivore attack. The rates of attack differed as expected among the populations and mirror the survival of plants to seed production among populations (Table 1). Most differences in viable seed production are caused by differences in capsule production, because seeds per capsule and % viable seeds did not differ between treatments in a population (Table 2).
Table 2.
Average reproductive performance
| Population | Seeds per capsule | Mass per seed | % viable seed |
|---|---|---|---|
| Plantation | 171.5 ± 5.5 | 125.9 ± 10.3 | 74.5 ± 13.2 |
| 1-yr burn (IB) | |||
| (MeJA treatment) | 128.8 ± 6.9 | 129.8 ± 9.0 | 80.8 ± 4.9 |
| (Leaf removal) | 130.6 ± 9.6 | 115.2 ± 8.0 | 65.3 ± 13.8 |
| 1-yr burn (IA) | 142.8 ± 3.7 | 158.5 ± 7.3 | 54.3 ± 4.2 |
| 2-yr burn (IIA) | 92.9 ± 12.6 | 56.0 ± 10.8 | ND |
| 2-yr burn (IIB) | 0 ± 0 | 0 ± 0 | 0 ± 0 |
Mean (±1 SEM) seeds per capsule, mass per seed, and % viable seed from all N. attenuata plants that survived to produce seed from a plantation population and four natural populations (listed in order of decreasing probability of survival to seed production) with the exceptions described in Table 1. None of the values were significantly different (as determined by paired t tests) between induced and control plants of any populations (all P > 0.18). ND, not determined.
For plants growing completely protected from herbivores (i.e., in the plantation), MeJA treatment significantly reduced lifetime viable seed production by 26% (9.9 capsules per plant or 1,550 viable seeds; Table 1). A similar cost was observed between induced and uninduced members of pairs growing in population IB, which largely had escaped herbivory and lost less than 5% of its leaf area to herbivores by the end of the growing season. MeJA treatment reduced the lifetime capsule production by 17% (24 capsules per plant; lifetime viable seed production was not determined for this population; Table 1). This reduction is comparable to those found in glasshouse experiments with N. attenuata plants growing in pots containing burned soil (13) in which MeJA treatment reduced lifetime seed production by 43%. These reductions are similar to the reduction associated with removing half of a plant’s leaf area at the rosette stage of growth: 27% reduction equivalent to 23 fewer capsules per plant or 2,026 viable seeds (Table 1). In summary, when plants are protected or escape from herbivore attack, plants treated with MeJA produced significantly fewer viable seeds at the time of senescence than did untreated control plants. This cost, however, becomes a net fitness benefit when plants are attacked by herbivores. For the 1,580 plants of this study, mammalian herbivores accounted for more than 75% of the leaf area removed by all herbivores in all populations.
For plants in environments with modest herbivory (population IA), 241 pairs (80%) survived to produce seed; of these surviving pairs, 358 individual plants (74%) had lost 20% or more of their leaf area to herbivores by the first week of July. The number of mature capsules and viable seeds produced at the end of the growing season differed significantly within pairs, and the magnitude and sign of these differences depended on whether or not the plants had been attacked by herbivores (Table 1; Fig. 2). Overall, MeJA-treated plants matured on average 11% more viable seed (six capsules or a 349 viable seeds per plant benefit of MeJA treatment). Of these 241 surviving pairs, both members of 160 pairs lost 20% or more of their leaf area to herbivores; in this subset of attacked plant pairs, MeJA-treated plants matured on average eight capsules and 537 viable seeds more (an 16–18% benefit) than their control counterparts. In 13 pairs, only the MeJA-treated member of the pair was similarly attacked, and in these, viable seed production did not differ significantly. In 25 pairs, only the control member of the pair was attacked, and in these pairs, the MeJA-treated plants matured on average 35 capsules and 2,848 viable seeds more than controls (a 58–60% benefit). To summarize, in populations with an intermediate level of attack, MeJA-induced plants had a higher fitness than control plants. Interestingly, the cost of MeJA induction was also detectable in the 43 pairs in which both members escaped herbivore attack (Fig. 2); MeJA-treated plants produced on average 20% less viable seed than did controls (13 capsules and 1,476 viable seeds per plant less).
Figure 2.
Lifetime capsule and viable seed production in matched pairs of N. attenuata plants growing in a 1-year-old burn (population IA) in which members of a pair were treated twice during the growing season with either methyl jasmonate (500 μg of MeJA suspended in water) or with 10 ml of water (see Fig. 1). (Left) The frequency distribution of differences in capsules produced per plant between treated and control plants in each pair. Seeds per capsule, mass per seed, and % viability of seeds did not differ significantly between plants in a pair (Table 2). Negative values reflect a cost of MeJA treatment (control member producing more capsules than its treated counterpart) and positive values reflect a benefit. (Middle) The mean (±1 SEM) capsules per plant for each treatment group (MeJA treatments, filled bars) and the statistical analysis of the differences between pairs. (Right) The mean (±1 SEM) lifetime viable seed production (calculated as described in Table 1) for each treatment group and the statistical analysis of the differences between pairs. Of the original 300 pairs, 241 survived to produce seed; of these survivors, both treated and control members of 160 pairs were attacked (losing 20% or more leaf area to herbivores by July). In 43 pairs both members escaped attack; in 13 pairs only the treated member was attacked; in 25 pairs only the control member of the pair was attacked.
For plants in environments with high herbivory (the 2-year-old burns), the benefit of MeJA treatments were readily seen. For example, in population IIA, 10 days after the first MeJA treatment, 33% of the control plants had lost more than 40% of their leaf area to herbivores, compared with 1% for MeJA-treated plants. However, this protective effect of jasmonate treatment did not translate into a fitness benefit for the plants in this population in which 81% of plants did not survive to produce any viable seed (Table 1). Although survival to seed production was higher for MeJA-treated plants (66 plants) as compared with control plants (48 plants), lifetime viable seed production did not differ among surviving plants. Of the 300 pairs of plants monitored in this population, both members survive to produce seed in only 23 pairs, and lifetime seed production of these survivors was low and not significantly different between MeJA-treated and control members (Table 1). No pairs survived to produce seed in the second 2-year-old burn (IIB; Table 1).
The defensive function of jasmonate-induced responses has been convincingly demonstrated in the laboratory with mutants deficient in the jasmonate cascade in both tomato (33) and Arabidopsis (34). Although my field study lacks the elegance and experimental control that characterize research on mutants, it demonstrates that the responses elicited by jasmonates increase plant fitness among the genotypes growing in natural populations. Similarly, this study offers proof of a fitness cost in nature when plants are induced but escape attack. Although the experiment demonstrates fitness costs and benefits of jasmonate induction, it does not identify which jasmonate-inducible response(s) is responsible for the fitness differences; we know only that nicotine induction is a component of the response.
The ability to detect benefits is potentially more limited than the ability to detect costs in this experiment, for in populations with high rates of attack, untreated members of each pair will rapidly attain the induced phenotype when they are attacked. Although nicotine concentrations differed significantly between treatments in a population suffering a high rate of attack early in the growing season (IIB: Table 1), whether these differences were maintained throughout the growing season and whether herbivores responded to this difference remains unclear. Because the induced response in attacked plants could not be suppressed, the fitness differences between plants in a pair in which both members are attacked will reflect the fitness consequences of an inducibly deployed defense with that of a constitutively deployed defense.
The fitness costs of jasmonate induction observed in this experiment mirror the costs of wound induction observed in a plantation experiment with the sibling species, N. sylvestris (35). In this experiment, plants were wounded with a standardized mechanical damage technique and had their wound-induced nicotine response suppressed with auxin applications to the wound site—a procedure that inhibits wound-induced jasmonate production in this species (23). The lifetime seed production of wounded plants that exhibited the normal wound-induced nicotine response was 32% less than that of similarly wounded plants that had their wound-induced nicotine suppressed with auxin. The similarity between the two estimates of fitness costs supports the contention that the MeJA treatment simulated the responses to wounding.
This study provides strong support for the contention that jasmonate-inducible responses exemplify adaptive phenotypic plasticity (36). By timing their germination and growth with the postfire environment, N. attenuata plants exploit a small phenological window of low herbivore pressure and high resource availability. However, within this window of opportunity, the probability of herbivore attack is highly variable. Inducible defenses allow plants to cope with this variability by altering their defensive phenotype. The resistance traits elicited by jasmonates are costly and can reduce lifetime seed production to a degree comparable to the reduction resulting from the loss of half a plant’s canopy early in the growing season. However, these costs are offset by their defensive benefit when plants are attacked. Hence, jasmonate-induced responses are adaptive, allowing plants to produce different, relatively fit phenotypes that track shifting biotic selection regimes. The changes in defensive phenotype in this study were directional (jasmonate treatment always increases nicotine production and resistance), which refutes a key prediction of the Moving Target Model (1, 5) for the evolution and maintenance of inducible resistance, a model that does not invoke the costs and benefits of the different phenotypes.
Whether or not the phenotypic costs and benefits of jasmonate-induced responses measured in this study answer the evolutionary question of whether genetic tradeoffs between resistance and life history traits exist (4) depends on the degree of overlap between the suite of traits that are altered by the jasmonate cascade (21) and those responsible for constitutive resistance. The costs of constitutive resistance are likely to be lower than those observed here, simply because jasmonates probably induce a large reconfiguration in both “civilian” and defensive traits, whereas selection on specific resistance traits is likely to be more precisely focused. However, across the genotypes of N. attenuata that germinated from the long-lived seed banks (estimated to be more than one century for population IIB; ref. 37) of this study, seed production was not well buffered from the resource demands of jasmonate-induced responses.
The fitness costs of producing the “wrong” phenotype (a defended phenotype in an herbivore-free environment), although large, should be compared with the costs of the organism’s other plastic responses. For example, these costs are small (20–40% reduction) compared with the consequences of germinating from the long-lived seed bank into the wrong habitat; N. attenuata plants germinating in burned environments (as compared with unburned) realize a 2- to 12-fold increase in lifetime seed production (13, 37). An understanding of adaptive phenotype plasticity requires both an understanding of the reliability of the environmental signals used by organisms to alter their phenotypes and the factors constraining their plastic responses (36). Characterizing the genotypic differences in the sensitivity of and the responses elicited by the ubiquitous jasmonate cascade will help ground the adage “a jack of all trades is a master of none” in mechanistic terms.
Acknowledgments
I thank B. McNulty for expert help with data analysis and figure preparation; G. Lynds, H. Madrigal, T. Ohnmeiss, and C. Preston for their unflagging assistance with field work; B. McNulty and C. Lewandowski for help with seed germination trials; Dr. Z.-P. Zhang, C. Lewandowski, and T. Ohnmeiss for their help with nicotine analysis; Dr. R. Karban for identifying T. pallidipennis; A. Dubrasky for determining fire locations and histories; E. Wheeler and E. Claußen for editorial assistance; and Drs. M. R. Berenbaum, M. Bisson, H. Lasker, J. Gershenzon, N. van Dam, and M. Webster for insightful comments on earlier drafts of this manuscript. This work was supported by the National Science Foundation (DEB-9505950), A.W. Mellon Foundation, and the Max-Planck-Gesellschaft.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: JA, jasmonic acid; MeJA, jasmonic acid methyl ester.
References
- 1.Karban R, Baldwin I T. Induced Responses to Herbivory. Chicago: Univ. of Chicago Press; 1997. [Google Scholar]
- 2.Givnish T J. In: Economics of Biotic Interaction. Givnish T I, editor. Cambridge, U.K.: Cambridge Univ. Press; 1986. pp. 667–679. [Google Scholar]
- 3.Simms E. In: Plant Resistance to Herbivores and Pathogens. Fritz R S, Simms E L, editors. Chicago: Univ. of Chicago Press; 1992. pp. 392–425. [Google Scholar]
- 4.Rausher M D. Trends Genet. 1996;12:212–217. doi: 10.1016/0168-9525(96)10020-2. [DOI] [PubMed] [Google Scholar]
- 5.Adler F R, Karban R. Am Nat. 1994;144:813–832. [Google Scholar]
- 6.Duffey S S. Annu Rev Entomol. 1980;25:447–477. [Google Scholar]
- 7.Smiley J T, Horn J M, Rank N E. Science. 1985;229:649–651. doi: 10.1126/science.229.4714.649. [DOI] [PubMed] [Google Scholar]
- 8.Koiwa H, Bressaw R A, Hasegawa P M. Trends Plant Sci. 1997;10:379–384. [Google Scholar]
- 9.Turlings T C J, Tumlinson J H, Lewis W J. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- 10.Moran N, Hamilton W D. J Theor Biol. 1980;86:247–254. [Google Scholar]
- 11.Bergelson J, Purrington C B. Am Nat. 1996;148:536–558. [Google Scholar]
- 12.Baldwin I T. Entomol Exp Appl. 1996;80:213–220. [Google Scholar]
- 13.Baldwin, I. T., Gorham, D., Schmelz, E. A., Lewandowski, C. A. & Lynd G. Y. (1998) Oecologia, in press. [DOI] [PubMed]
- 14.Baldwin I T, Karb M J. J Chem Ecol. 1995;21:897–909. doi: 10.1007/BF02033797. [DOI] [PubMed] [Google Scholar]
- 15.Baldwin I T, Ohnmeiss T E. J Chem Ecol. 1993;19:1143–1153. doi: 10.1007/BF00987376. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin I T. Oecologia. 1988;75:367–370. doi: 10.1007/BF00376939. [DOI] [PubMed] [Google Scholar]
- 17.Barbosa P, Saunders J A, Kemper J, Trumbule R, Olechno J, Martinat P. J Chem Ecol. 1986;12:1319–1328. doi: 10.1007/BF01012351. [DOI] [PubMed] [Google Scholar]
- 18.Baldwin I T, Karb M J, Ohnmeiss T E. Ecology. 1994;75:1703–1713. [Google Scholar]
- 19.Baldwin I T, Ohnmeiss T E. Oecologia. 1994;98:385–392. doi: 10.1007/BF00324228. [DOI] [PubMed] [Google Scholar]
- 20.Gundlach H, Müller M J, Kutchan T M, Zenk M H. Proc Natl Acad Sci USA. 1992;89:2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wasternack C, Parthier B. Trends Plant Sci. 1997;2:302–307. [Google Scholar]
- 22.Baldwin I T, Schmelz E A, Ohnmeiss T E. J Chem Ecol. 1994;20:2139–2157. doi: 10.1007/BF02066250. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin I T, Zhang Z-P, Diab N, Ohnmeiss T E, McCloud E S, Lynds G Y, Schmelz E A. Planta. 1997;201:397–404. [Google Scholar]
- 24.Ohnmeiss T, McCloud E S, Lynds G Y, Baldwin I T. New Phytol. 1997;137:441–452. doi: 10.1046/j.1469-8137.1997.00845.x. [DOI] [PubMed] [Google Scholar]
- 25.McCloud E S, Baldwin I T. Planta. 1997;203:436–441. [Google Scholar]
- 26.Baldwin I T, Schmelz E A, Zhang Z-P. J Chem Ecol. 1996;22:61–73. doi: 10.1007/BF02040200. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z-P, Baldwin I T. Planta. 1997;203:436–441. [Google Scholar]
- 28.Baldwin I T, Staszak-Kozinski L, Davidson R. J Chem Ecol. 1994;20:2345–2369. doi: 10.1007/BF02033207. [DOI] [PubMed] [Google Scholar]
- 29.Baldwin I T, Morse L. J Chem Ecol. 1994;20:2373–2391. doi: 10.1007/BF02033208. [DOI] [PubMed] [Google Scholar]
- 30.Wright H A, Bailey A W. Fire Ecology. New York: Wiley; 1982. [Google Scholar]
- 31.Whelan R J. The Ecology of Fire. Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 32.Baldwin I T, Preston C A, Euler M A, Gorham D. J Chem Ecol. 1997;23:2327–2344. [Google Scholar]
- 33.Howe G A, Lightner J, Browse J, Ryan C A. Plant Cell. 1996;8:2067–2077. doi: 10.1105/tpc.8.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McConn M, Creelman R A, Bell E, Mullet J E, Browse J. Proc Natl Acad Sci USA. 1997;94:5473–5477. doi: 10.1073/pnas.94.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldwin I T, Sims C L, Kean S E. Ecology. 1990;70:252–262. [Google Scholar]
- 36.De Witt T J, Sih A, Wilson D S. Trends Ecol Evol. 1998;13:77–81. doi: 10.1016/s0169-5347(97)01274-3. [DOI] [PubMed] [Google Scholar]
- 37.Preston, C. A. & Baldwin, I. T. (1998) Ecology, in press.