Abstract
Immune mediation of aplastic anemia (AA) has been inferred from clinical responsiveness to immunosuppressive therapies and a large body of circumstantial laboratory evidence. However, neither the immune response nor the nature of the antigens recognized has been well characterized. We established a large number of CD4 and CD8 T cell clones from a patient with AA and analyzed their T cell receptor (TCR) usage. Most CD4 clones displayed BV5, whereas most CD8 clones displayed BV13. We found sequence identity for complementarity determining region 3 (CDR3) among a majority of CD4 clones; the same sequence was present in marrow lymphocytes from four other patients with AA but was not detected in controls. The dominant CD4 clone showed a Th1 secretion pattern, lysed autologous CD34 cells, and inhibited their hematopoietic colony formation. In three of four patients, successful immunosuppressive treatment led to marked decrease in clones bearing the dominant CDR3 BV5 sequence. These results suggest surprisingly limited heterogeneity of the T cell repertoire in an individual patient and similarity at the molecular level of the likely pathological lymphocyte response among multiple patients with AA, consistent with recognition of limited numbers of antigens shared by individuals with the same HLA type in this disease.
Introduction
In aplastic anemia (AA), severe pancytopenia occurs in the setting of an apparently empty bone marrow, the normal hematopoietic tissue being replaced by fat (1). AA’s many clinical associations (after heavy or chronic exposure to benzene; as an idiosyncratic reaction to different medical drugs; following hepatitis; or with pregnancy) have historically led to its consideration as a heterogeneous pathophysiological process resulting from diverse marrow insults. AA was first effectively treated by bone marrow transplantation to replace the absent hematopoietic stem cells. However, patients were occasionally observed to show improvement of blood counts, even after failure of donor marrow to engraft, suggesting benefit from the immunosuppressive conditioning treatment itself (2). With purposeful and systematic application of antilymphocyte globulins (ATG), cyclosporine (CsA), and high doses of corticosteroids and cyclophosphamide, the great majority of patients now show sufficient improvement in hematopoiesis.
Because of the low numbers of blood and marrow cells, AA is intrinsically difficult to study in the laboratory. Nevertheless, a large amount of data supports an immunological mechanism of hematopoietic failure (3). A role for T cells was first suggested by coculture and depletion experiments, in which inhibition of hematopoietic colony formation was associated with this lymphocyte population. Activated cytotoxic T cells can be measured by flow cytometry in patient blood and especially bone marrow. IFN-γ is a potent suppressor of hematopoiesis in vitro and induces Fas expression on CD34 target cells. A role in diseased individuals has been inferred from detection of excessive IFN-γ production by gene amplification of patient mRNA, as well as measurement of intracellular cytokines in blood and marrow lymphocytes. Marrow localization of pathophysiological T cells has been modeled in vitro (4, 5) and observed in vivo (6). These results support a view of AA as the culmination of cytotoxic lymphocyte type I–mediated (Tc1-mediated), highly specific attack on blood forming cells.
More detailed understanding of the immune process in AA, and especially of the nature of the responsible provoking or perpetuating antigens, has proved elusive. Recently, novel molecular methods have been developed to analyze the T cell repertoire using polymorphisms within the CDR3 region of the BV chain of the TCR (7). This approach is based on the prediction that antigen-driven T cell clonal expansion will result in molecular overrepresentation of the corresponding TCR idiotype. Skewing of the T cell BV spectrum has now been described for many animal models of immunologically mediated organ destruction (8–10), in human diseases (11–18), and during graft-versus-host disease (19).
Characterization, identification, and cloning of disease-specific T cells in AA would serve many purposes. First, isolation and quantification of these cells will elucidate the nature of the immune response. Second, comparison of overexpressed BV groups and their CD3 sequences at the molecular level will enable important inferences to be made as to the quality of antigen recognition in the disease. Third, T cell clones will facilitate the search for the antigens driving the immune destruction of bone marrow. In sum, such studies would demonstrate a distinction between two plausible models of immune-mediated marrow failure. In the first, every patient has confronted unique antigens with a highly individualized immune response. In the second, for patients who are defined by major histocompatability loci, the immune response is similar, suggesting either a common inciting antigen or shared secondary antigens present on hematopoietic cells.
Methods
Patients.
Patients were evaluated at the Hematology Branch of the National Heart, Lung and Blood Institute. The diagnosis of AA was established by bone marrow biopsy and peripheral blood counts as recommended by the International Study of Aplastic Anemia and Agranulocytosis (20); severity was classified by the criteria by Camitta et al. (21). Five patients with idiopathic severe AA were selected for our experiments; four were studied at initial presentation before immunosuppressive treatment, and in one further case, samples were obtained at relapse after an initial full hematological response to the combination of ATG and CsA (Table 1). Controls were ten healthy volunteers of defined HLA type (three HLA-DR2 [HLA-DRB1*15]). To obtain peripheral blood and bone marrow, informed consent was obtained according to protocols approved by the Institutional Review Board of the National Heart, Lung and Blood Institute.
Table 1.
Patients and their clinical features
TCR BV gene usage.
Bone marrow mononuclear cells (BMMCs) were separated by Ficoll-Hypaque sedimentation. Total RNA was extracted from BMMCs and T cell clones using TRIzol reagent (Life Technologies Inc., Bethesda, Maryland, USA), and then reverse-transcribed into cDNA in a reaction primed with oligo (dT)12-18 using the SuperScript II RT kit (Life Technologies Inc.). cDNA was amplified through 30 cycles with 22 different BV families (TCR BV10 and BV19 genes are now known to be pseudogenes and therefore were excluded from the analysis) and a BC primer (22).
CDR3 size distribution.
Details of the CDR3 size distribution assay have been reported (7, 23, 24). Briefly, 1 μl of each amplified product of 22 different BV subfamilies was mixed with 12.5 ml deionized formamide (Sigma Chemical Co., St. Louis, Missouri, USA) and 0.5-ml size standard at 95°C for 2 minutes, chilled on ice, applied to an ABI 310 sequencer, and analyzed using Genescan software (both from Perkin-Elmer Biosystems, Foster City, California, USA).
T cell culture.
Activated T cells (bearing CD2 and CD69 antigens) were obtained from BMMCs by flow cytometric sorting (EPICS; Coulter, Hialeah, Florida, USA). Cells fractions were resuspended in complete medium (RPMI 1640 supplemented with L-glutamine, minimal essential amino acids, sodium pyruvate [all from Life Technologies Inc., Grand Island, New York, USA], IL-2 (20 U/ml) plus 10% pooled AB serum [BioWhittaker, Walkersville, Maryland, USA]). Cells were cultured in round-bottom plastic plates for the 1st week and then in flat-bottom plates, in the presence of anti-CD28 (1 μg/ml), and anti-CD3 antibodies (10 μg/ml). After 3 weeks of culture, 1 × 106 T cells were inoculated with 105 tissue culture units of Herpesvirus saimiri (SHV-2) subgroup C strain 488 (American Type Culture Collection, Manassas, Virginia, USA) (25, 26). After 3–5 months, T cells were rechallenged with SHV-2.
T cell cloning.
Viable immortalized T cells were suspended in complete medium. Twenty microliters of cell suspension were plated in microtiter wells of Terasaki plates (Robbins Scientific, Sunnyvale, California, USA), at calculated final concentrations of three-tenths, one, and three cells per well and cultured; ten days to 2 weeks later, growing cells were transferred to 96-well flat-bottom plates and cultured, and finally to 48-well flat-bottom plates for further growth. T cell clones were maintained by regular supplementation with complete medium. The limiting dilution procedure was repeated two or three times to ensure monoclonality.
Isolation of BM CD34 cells.
CD34 cells were obtained from BMMCs using a commercial isolation kit (Miltenyi Biotec, Auburn, California, USA) according to the manufacturer’s instructions; the usual purity obtained was 85–98%.
Cytotoxicity assay.
For the microcytotoxicity assay a fluorescent technique was used. Briefly, 1 × 104 autologous (patient), allogeneic (patient’s sister), or control (normal donor) CD34 cells were incubated with Calcein-AM (Molecular Probes Inc., Eugene, Oregon, USA) at 37°C for 30 minutes and then washed. Viable target cells were counted, and the concentration was adjusted to obtain the desired effector to target (E/T) ratio for the T cell clones used; three replicative experiments were performed. As controls, each target cell concentration was plated without effectors to determine maximal fluorescence emission (max f); incubation with medium alone was used to determine background fluorescence (blank). Low-speed centrifugation was performed in order to maximize cell-to-cell contact, and effector and target cells were further incubated for 4 hours at 37°C. Differences in fluorescence emission were measured using an automated fluorescent reader (CytoFluor Multi-Well Plate Reader; Perspective Biosystems, Framingham, Massachusetts, USA). Percentage specific lysis was calculated for each E/T ratio by the following formula: % specific lysis =[1– (mean test-mean blank)/(mean max f-mean blank)] × 100. In some experiments, purified mAb’s were tested for their ability to block specific E/T interactions. Anti-DR, anti-DP, anti-DQ, anti-DM, and anti-ABC mAb (all from PharMingen, San Diego, California, USA) were added to the suspension of target cells at a concentration of 10 μg/ml an incubated for 30 minutes at 37°C before addition of effector cells.
Hematopoietic progenitor assays.
One thousand CD34 cells were cocultured in the presence or absence of T cells or T cell clones at an E/T ratio of 1:10 in Iscove’s modified DMEM (ICN Biochemicals, Aurora, Ohio, USA) containing 20% FCS. After low-speed centrifugation and coculture for 16 hours, methylcellulose medium containing colony-stimulating factors (stem cell factor [100 ng/ml], IL-3 [100 ng/ml], GM-CSF, 50 ng/ml, erythropoietin [EPO, 5 U/ml], and Flt3L [50 ng/ml]) was added to each culture tube to a final concentration of 1.2% methylcellulose. One milliliter of the cell mixture was plated in a 35-mm culture dish and incubated for 14–18 days.
Flow cytometry.
Immunophenotypic characterization was performed by flow cytometry. T cells were harvested in the logarithmic growth phase, washed, and incubated for 30 minutes with phycoerythrin- or FITC-conjugated CD4, CD8, CD28, CD45RA, CD45RO, CD86, CD11a, and CD95 mAb (all from Becton Dickinson Immunocytometry Systems, Mountain View, California, USA), at concentrations and conditions previously determined to be optimal. Intracellular staining assessed cytokine production: cells were washed, permeablized, and fixed using a kit (FIX&PERM; Caltag Laboratories Inc., Burlingame, California, USA); FITC-conjugated mAb to IL-2, -4, -10 and IFN-γ (all from PharMingen) were used according to the manufacturer’s instructions. Cells were analyzed by flow cytometry (EPICS).
ELISA.
Supernatants of each T cell clone (JZ1.1 and three other BV5 clones [JZ1.2, 1.3, and 1.4] with the same CDR3 sequence, as well as three clones [3.1, 3.2, and 3.3] of different BV families) were tested for IFN-γ and TNF-α production using ELISA kits (Endogen Inc., Woburn, Massachusetts, USA, and BioSource International, Camarillo, California, USA, respectively).
Southern hybridization.
BV5 PCR products from patients and normal controls were electrophoresed in 1.5% agarose, transferred to a nylon membrane, crosslinked, and hybridized with a biotinylated specific probe for CDR3. The membrane was washed and visualized by incubation with streptavidin and biotinylated alkaline phosphatase in a chemiluminescence detection system (KPL, Gaithersburg, Maryland, USA).
Cloning and sequencing of PCR-amplified cDNA.
Freshly generated PCR products of BV5 cDNA were directly ligated into a cloning vector using the TOPOTA cloning system (Invitrogen Corp., Carlsbad, California, USA). Sixteen to 21 colonies containing the insert fragment were randomly selected and sequenced using an ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction Kit and ABI 310 sequencer. The amino acid sequence of CDR3 was deduced using GCG-lite-DNA sequence analysis software (Genetics Computer Group, Madison, Wisconsin, USA). To avoid contamination, gene amplification and sequencing were performed in a secondary laboratory facility.
Statistical analysis.
Statistical analyses were performed using Medcalc software (Mariakerke, Belgium). Test of proportions was used to determine differences in frequency of clones exhibiting identical CDR3 sequence before and after clinical interventions. Other data were analyzed by the Student’s t test.
Results
Analysis of CDR3 size distribution of TCR BV subfamilies of patients.
We selected a group of AA patients who shared some important clinical characteristics (Table 1). All had experienced the acute onset of pancytopenia, at which time their bone marrows were extremely hypocellular. All showed evidence for activation of peripheral blood lymphocytes by standard flow cytometric testing, with abnormally elevated percentages of circulating CD8 cytotoxic lymphocytes bearing HLA-DR (27). In all but one patient, there was also present a small clone of cells globally lacking surface expression of glycosylphosphoinositol-linked proteins, indicative of the coexistence of paroxysmal nocturnal hemoglobinuria (PNH). All cases were similar in sharing the histocompatability antigen HLA-DRB1*15. Both the presence of PNH and this HLA-DR type antigen are associated with responsiveness to immunosuppressive therapy (28), and all five patients ultimately showed marked improvement in their peripheral blood counts after treatment with ATG and CsA.
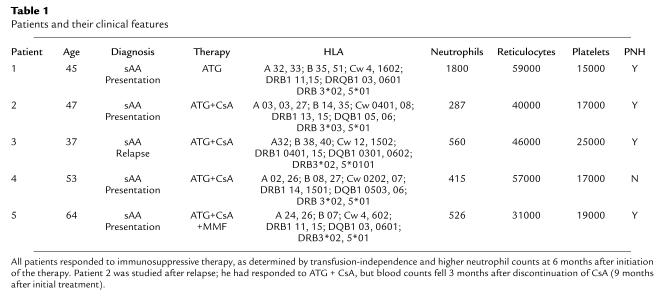
In a first set of experiments, we analyzed TCR CDR3 size distribution pattern in four patients (P1, P2, P3, and P4) to determine the presence of BV subfamily oligoclonal expansion. cDNA of all the BV subfamilies was amplified, using 22 BV primers and a fluorescent BC primer (in our hands, BV17 amplification was inconsistent). Most of the BV families of these patients showed an abnormal size distribution pattern, suggesting clonal proliferation of a limited number of T cells (Figure 1).
Figure 1.
Analysis of TCR BV CDR3 region size profiles. CDNA of 22 BV families from P1, P2, P3, and P4 were amplified using BV primer and a fluorescent BC primer, and the PCR products were analyzed for CDR3 size. x-axis, CDR3 size; y-axis, relative fluorescence units.
T cell inhibition of colony formation by CD34 cells.
For one patient (P1), activated T cells were sorted, cultured, and tested for their ability to inhibit hematopoietic colony formation in vitro: They decreased numbers of autologous erythroid (BFU-E; from 51 ± 2 to 3 ± 3; P < 0.05) and myeloid colonies (CFU – GM + CFU – G + CFU-M, from 53 ± 11 to 24 ± 5; P < 0.05).
Establishment of T cell clones and determination of TCR-BV gene usage.
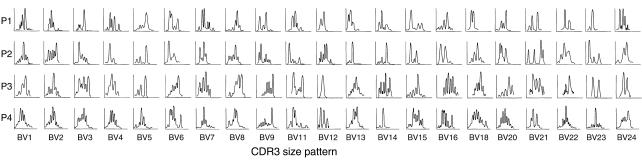
CDR3 size analysis suggested T cell clonal expansion, and activated T cells derived from P1 inhibited hematopoiesis in vitro. Therefore, we next designed experiments to isolate T cell clones responsible for this functional effect. Activated T cells were cultured and then immortalized by infection with SHV-2; individual clones were established by limiting dilution (Figure 2). A total of 112 CD4 T cell and 32 CD8 T cell clones were obtained. As all patients in this study expressed the HLA-DR2 haplotype, we chose to evaluate cells of helper phenotype, and a large number of CD4+ T cell clones were randomly selected for analysis of TCR-BV gene usage. Twenty-six of 32 CD4 T cell clones displayed BV5 TCR. Sequencing analysis revealed identical nucleotide sequence of the N-D-N region (CDR3) of BV5 in 20 of 26 of these CD4 T cell clones, with a predicted amino acid sequence of PGPGTSG (CCCGGGCCCGGGACTAGCGGA). The high frequency of clones bearing identical CDR3 sequence indicated overrepresentation of CD4 T cells with TCR BV5 PGPGTSG in this patient. One clone, designated JZ1.1, was selected for further study.
Figure 2.
Strategy to establish T cell clones. Activated T cells (bearing CD2 and CD69 antigens) were obtained from BMMCs by flow cytometric sorting. Cells fractions were resuspended in complete medium, cultured with IL-2 and CD28, and then immortalized with SHV-2, after limiting dilution, 112 CD4+ T cell clones were obtained. TCR BV usage was analyzed by PCR: BV5 was used in 26 of 32 T cell clones, and BV5 sequence analysis showed 20 of 26 have an identical CDR3 region.
Phenotype of the JZ1.1 T cell clone.
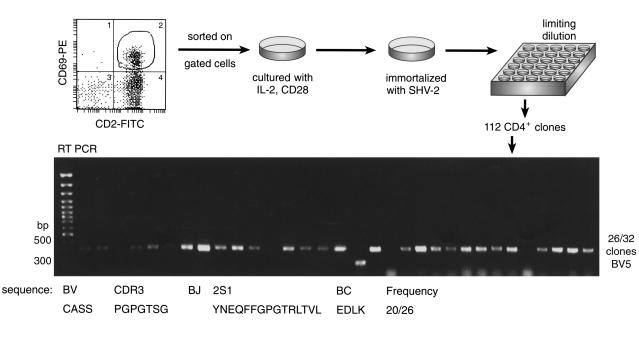
We next characterized the surface antigens and cytokine secretion profile of JZ1.1 T cell clone. As expected, the clone expressed surface CD3 and CD4 and not CD8; additionally, JZ1.1 showed expression of HLA-DR, TCRa/β, CD95, CD11a, CD86, and CD45RO but not CD45RA or CD28 (Figure 3a), indicative of a mature memory/effector phenotype (29). Intracellular cytokine staining was positive for IL-2 and IFN-γ but not for IL-4 or IL-10, consistent with a Th1 phenotype (30) (Figure 3b). JZ1.1 and three other clones (JZ1.2, JZ1.3, and JZ1.4) of identical TCR CDR3 sequence (and thus derived from the same cell in vivo) showed high levels of secretion into culture supernatant of IFN-γ (4,570 ± 504 pg/ml) and TNF-α (395 ± 138 pg/ml), whereas other isolated clones (JZ3.1, JZ3.2, JZ3.3) secreted low levels of these cytokines (IFN-γ, 1,358 ± 550 pg/ml; TNF-α, 65 ± 10 pg/ml; P = 0.001 and P = 0.017, respectively).
Figure 3.
Characterization of the JZ1.1 T cell clone. (a) Immunophenotype by flow cytometry. JZ1.1 was stained with mAb’s directed to CD3, CD4, CD8, CD45RA, CD45RO,CD28, HLA-DR, TCRa/β, CD95, CD86, CD11a (dashed lines) as described in Methods; subclass-matched IgG reagents served as controls (solid lines). (b) Cytokine secretion analysis by flow cytometry. JZ1.1cells were subjected to intracellular staining using fluorescent conjugated-mAb’s to IL-2, IL-4, IL-10, or IFN-γ. Data are representative of three independent experiments.
Functional analysis of JZ1.1.
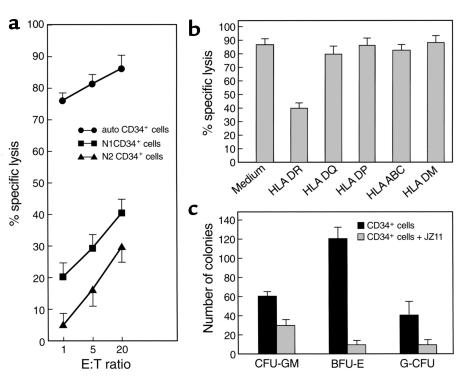
Pooled activated T cells from P1 were inhibitory of autologous hematopoietic progenitor cell proliferation. We tested a variety of immortalized T cell clones derived from this patient’s bone marrow for similar effects on colony formation and for cytotoxicity for hematopoietic cells. When autologous CD34 cells were used as targets, a large proportion of them was lysed by JZ1.1; the efficiency of lysis was indicated by high killing rates at low E/T ratios (Figure 4a). Normal CD34 cells matched for DR2 (N1) or DR mismatched (N2), when used as targets, showed much lower cytocidal activity, which was not distinguishable from killing of K562 cells (data not shown). JZ1.1, JZ3.1, and JZ3.3 showed no cytotoxicity against P1’s autologous CD34 cells obtained after the patient’s hematological recovery, to CD34 cells from P1’s HLA-matched healthy sister’s bone marrow, or against immortalized lymphoblastoid cell lines derived from the peripheral blood of the AA patients (data not shown). JZ1.1 cytotoxicity for autologous CD34 cells was blocked by anti-DR mAb (Figure 4b), but there was no effect when anti-DQ, anti-DP, anti-DM, or anti-HLA-ABC mAb’s were used.
Figure 4.
Functional analysis of JZ1.1. (a) Cytotoxicity for autologous CD34 cells, normal CD34+ cells (N1, HLA-matched for DR2; N2, DR-mismatched). CD34 cells were labeled with calcein-AM and incubated with differing numbers of JZ1.1 cells in a 4-hour cytotoxicity assay. (b) Blocking of cytotoxicity. Autologous CD34+ cells were incubated with JZ1.1 cells in medium alone or with addition of 10 μg/ml of the indicated mAb’s. Mean percentage of specific lysis was determined from triplicate cultures after 4 hours of incubation. (c) Effect of JZ1.1 on autologous hematopoietic progenitor cell growth. Ten thousand freshly isolated CD34 cells were incubated in medium alone or with 1 × 105 JZ1.1 cells for 16 hours and then mixed with methylcellulose medium containing hematopoietic growth factors; colonies were enumerated after 2–3 weeks of culture. Data are expressed as mean ± SD of replicate plates.
We further determined whether JZ1.1 showed inhibitory effects on hematopoietic progenitor cell growth in vitro (Figure 4c). Freshly isolated CD34 cells were preincubated with JZ1.1 T cells and plated in methylcellulose in the presence of hematopoietic growth factors. JZ1.1 markedly reduced the numbers of autologous erythroid colonies (BFU-E, from 120 ± 2 to 10 ± 4; P < 0.05) and myeloid colonies (CFU-GM, from 60 ± 7 to 30 ± 5; P < 0.05, and CFU-G, 40 ± 8 to 10 ± 7; P < 0.05), but not those derived from normal CD34 cells (data not shown). Thus, JZ1.1 was functionally active against autologous CD34 hematopoietic cell targets, similar to the behavior of pooled T cells from P1.
Presence of BV5 JZ1.1 TCR-bearing clones in AA patients.
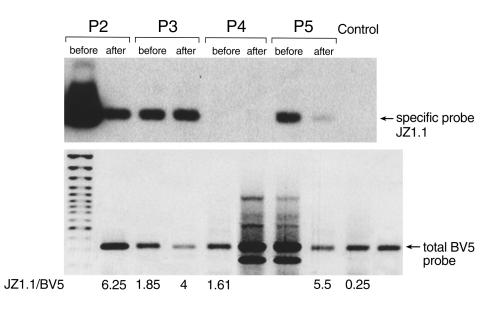
Previous experiments showed that the JZ1.1 T cell clone exerted HLA-DR-restricted cytotoxicity for hematopoietic cells. Therefore, we determined whether molecularly similar TCR bearing clones were also present in other AA patients of the same HLA-DR2 type, and whether clinical treatment affected the clone. After amplification of BV5 cDNA from BMMCs of P2, P3, P4, and P5, PCR products were subjected to Southern hybridization with a specific probe that encompassed the JZ1.1 BV5 N-D-N region sequence (Figure 5). The amplified products derived from three patients (P2, P3, and P5) exhibited strong bands before treatment, but the density of the specific band decreased after successful therapy. In contrast, the amplified products derived from P4 and a normal control failed to show any signal. Southern hybridization in another nine normal individuals also disclosed no specific band, although BV5 amplification products were always obtained (data not shown).
Figure 5.
TCR BV5 usage in AA patients in Southern blot analysis. BV5 PCR products from P2, P3, P4, P5 and normal individuals were electrophoresed on a 1.5% agarose gel (lower panel) and subjected to Southern hybridization using a biotinylated specific probe for the JZ1.1 N-D-N region sequence (upper panel). The numbers represent the calculated ratio between the signal intensities obtained with the specific BV5 /JZ1.1 compared with a universal BV5-probe. Note that the decrease in the BV5 JZ1.1 / BV5 ratio corresponds to the increase in the contribution of the specific clone to the total BV5 spectrum detected in a particular patient.
CDR3 size distribution of TCR BV5 cDNA in BMMCs.
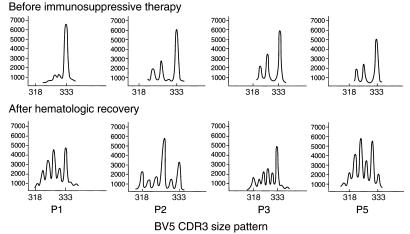
In parallel experiments, we also determined whether the presence of the JZ1.1 BV5 TCR was associated with changes in the CDR3 size distribution pattern and the effect of immunosuppressive therapy. cDNA of BV5 subfamilies was amplified, using a fluorescent BC primer and specific BV5 primer, and the CDR3 size distribution was compared before and after treatment. Specifically, the initial BV5 CDR3 size patterns of P1, P2, P3, and P5 were abnormal, showing very few peaks, whereas after hematological response, CDR3 BV5 size patterns showed more normal distributions (Figure 6). (The distinct 333-bp peak, which markedly decreased after treatment, may represent the size of the BV sequence of JZ1.1.)
Figure 6.
BV5 CDR3 size pattern and response to therapy. BV5 cDNA from P1, P2, P3, and P5 were amplified using a specific primer and a fluorescent BC primer; and the size of the PCR products was compared before and after treatment. x-axis, CDR3 size; y-axis, relative fluorescence units.
Deduced amino acid sequence of CDR3 of BV5 cDNA.
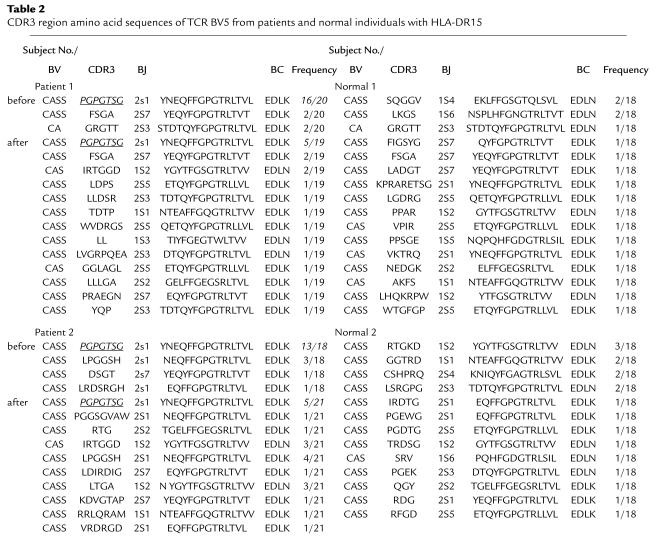
Both the results of Southern hybridization and the CDR3 size distribution indicated that oligoclonal expansion of a limited number of T cells might be a distinctive feature in AA patients of HLA-DR2 haplotype. To directly detect specific JZ1.1 BV5 sequence and to determine its relative overrepresentation within the TCR repertoire, BV5 cDNAs from BMMCs of P1 and P2 and two HLA-DRB1*15 normal were amplified and cloned, and the nucleotide sequence of the individual clones was determined (Table 2). From the deduced amino acid sequence of each clone, the most frequent amino acid (nucleotide) sequence of the N-D-N region of both of patients was PGPGTSG (CCCGGGCCCGGGACTAGC-GGA), identical to that of the JZ1.1 clone. Before treatment, the frequency of clones bearing this specific sequence was high (16 of 20 in P1; 13 of 18 in P2) and declined with successful therapy (P1, five of 19; P = 0.003 and five of 21 in P2; P = 0.007). In contrast, we did not detect the JZ1.1 BV sequence in any normal individual.
Table 2.
CDR3 region amino acid sequences of TCR BV5 from patients and normal individuals with HLA-DR15
Discussion
CDR3 of the BV chain of the TCR is a nongermline–encoded hypervariable region directly related to T cell recognition of specific peptides in the appropriate HLA context. CDR3 thus defines a unique clonotype. Somatic rearrangement leads to six to eight amino acid differences among CDR3 within each BV chain, detectable by a variety of molecular methods and apparent as deviation from the normal Gaussian size distribution. Murine studies have shown that the cellular immune response to even complex protein antigens is relatively restricted, to one or a few CDR3 size peaks, from which a few dominant specific T cell clones have been inferred (31). Both the normal immune response and autoimmune disease show CDR3 restriction, consistent with either epitope-driven or BV region–restricted responses, and both public and private TCR specificities have been identified in animal models.
Parallel investigations of patients with immune-mediated disease have been more difficult to interpret. First, peripheral blood cells may not reflect local and pathological T cell infiltration. Second, in vitro growth may artificially skew representation of BV. Third, many studies compare patients with diverse clinical manifestations and/or different histocompatability backgrounds. Fourth, the human immune response is both intrinsically more complex than the murine and evolves in an individual over many decades. Fifth, both young and old humans can show surprising degrees of BV skewing and nonpathological expansion of T cell clones (24, 32–34). Nevertheless, investigations of CDR3 in humans have generally validated animal observations, as applied to the immune response to viral infections (35, 36), immunity against tumors and during graft-versus-host disease, as part of graft-versus-tumor effects (37–39), and during recovery from stem cell transplant (24). TCR usage has been extensively applied to human autoimmune diseases, and although the confidence of identification of driving epitopes in these conditions varies, this analysis has often served as a surrogate for antigen specificity studies when the autoantigen is unknown (7, 40, 41). In many human autoimmune diseases, restricted CDR3 usage has been inferred from skewed size patterns, which may be particularly marked if locally infiltrating T cells are examined; the T cell response, as in animals, has been limited, and both private and shared public specificities have been described in detail when the antigens, such as myelin basic protein, were known. Indeed, these studies have been the basis for T cell vaccination strategies, in which the TCR itself is the antigen and the anti-idiotype response is therapeutically beneficial.
To maximize the likelihood of establishing this pattern in acquired AA, a disease in which there is still considerable debate as to underlying pathophysiology, the mechanism of action of effective therapies, and no suitable animal model, we chose first to examine the TCR only in lymphocytes that were activated and derived from the target organ, the marrow. In addition, our patients were similar in crucial but also common clinical characteristics, in showing evidence of T cell activation in blood, a good response to immunosuppressive therapy, the presence of an expanded PNH clone, and HLA-DR2 phenotype. To perform extensive functional and phenotypic characterization, we also immortalized the selected T cells; although artificial biasing of T cell representation as a result of viral infection is theoretically possible, SHV-2 has not been reported to produce TCR skewing in other published studies (25, 26, 42). Spectra typing did show the presence of multiple skewed BV families in patient bone marrow, whereas a single dominant clone emerged after cell culture and viral transformation; nevertheless, this dominant clone, and not other BV clones obtained from the same bone marrow, showed a cytokine profile suggestive of activation, suggesting that viral transformation does not result in obligatory alteration in T cell function. We obtained functionally active T cell clones that were specifically cytotoxic for autologous marrow CD34 cell targets from bone marrow obtained at disease presentation. That such a large number of both CD4 and CD8 clones in our first patient were identical suggests oligoclonal expansion in this individual. That the same TCR (for CD4) was detected in large proportions of activated lymphocyte populations from other patients indicated that public specificities were being detected (and also argued against artifact due to the viral infection protocol used to obtain the clones); as the CDR3 region from the AA cases was not detectable in normal individuals, their association with disease seems likely. The proportion of T cells bearing the abnormal TCR fell with effective treatment in three of four cases, consistent with a pathophysiological role. Our data thus provide strong direct evidence of an autoimmune pathophysiology of AA and describe similarity of the molecular genetic level between this form of bone marrow failure and other types of organ-specific, immune-mediated tissue damage and destruction.
In the current work, we present data derived from studies of CD4 cell clones, mainly because of the readier availability of other class II–matched patients and controls. Although CD4 cytotoxic lymphocytes are well described, our CD4 cell clones were particularly potent in this assay, perhaps reflecting their clonal origin from activated cells. Similar high activity has been described for other CD4 cell clones derived from AA patients (43, 44). In our study, both the CD4 and CD8 clones appeared to be Th1/Tc1 cells, but the immortalized CD8 cell clones await more intensive study utilizing class I matched target cells.
For AA, our results should be compared to a few previous studies of limited numbers of patients. When Swiss investigators performed spectratyping of unfractionated blood and marrow lymphocytes of severely affected patients, the expression pattern was broadly normal (45). Individual patients showed size skewing, indicative of selective CDR3 usage, but the BV subfamilies differed for each case, and there was no correlation of CDR3 size distribution and enhanced BV usage (both BV5 and BV13 were among a total of seven abnormal CDR3 patterns). Nakao and coworkers studied patients whose immunological disease could be inferred from the dependence of their blood counts on CsA administration; these patients showed a skewed CDR3 size pattern, in contrast to results with refractory or remitted cases (46). Furthermore, two patients showed homology in the amino acid sequence of their expanded CDR3 domains. Individual helper (43) and cytotoxic (44) T cell clones have been isolated and functionally characterized as responsive to or cytotoxic for target hematopoietic cells. CDR3 skewing was found in multiple BV families, and BV15 CDR3 sequencing showed dominant clones in these five patients. An abnormal T cell repertoire has also been found in PNH (47), which is often diagnosed concurrently with AA.
In our study, the CDR3 genotype shared among all five patients is probably the signature of a pathogenic T cell clone. If verified on examination of larger numbers of patients of similar clinical characteristics, CDR3 analysis may be a highly sensitive clinical assay for diagnosis and to evaluate the effectiveness of treatment. For example, we noted that our current therapeutic regimen reduced but did not eradicate evidence of this clone, consistent with the notoriously high rate of relapse after immunotherapy. CDR3 fine analysis may serve to distinguish subsets of marrow failure, such as post-hepatitis AA and immune-mediated pancytopenia in the myelodysplastic syndromes. Ultimately, the generation of immortalized T cell clones derived from cells clearly involved in the pathophysiology of the marrow disease offers a method for the identification of the elusive antigen(s) in AA, through screening of cell lines, subcellular fractions, peptide libraries, and randomly generated tetramers. Even at a comparatively early stage of investigation, however, we observed that T cell cytotoxicity was relatively specific for putative target antigens present only at disease presentation. Although the precise nature of the antigen must be determined, these data suggest that persistence of low levels of pathogenic T cell clones may be innocuous until antigen reappears to drive their expansion.
References
- 1.Young, N.S., and Aiter, B.P. 1994. Aplastic anemia, acquired and inherited. W.B. Saunders Co. Philadelphia, Pennsylvania, USA. 3–267.
- 2.Mathé G, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131–136. doi: 10.1136/bmj.2.5702.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young NS, Maciejewski J. The pathophysiology of acquired aplastic anemia. N Engl J Med. 1997;336:1365–1372. doi: 10.1056/NEJM199705083361906. [DOI] [PubMed] [Google Scholar]
- 4.Selleri C, Maciejewski J, Sato T, Young NS. Interferon-g constitutively expressed in the stromal microenvironment of human marrow cultures mediates potent hematopoietic inhibition. Blood. 1996;87:4149–4157. [PubMed] [Google Scholar]
- 5.Takashita E, et al. Destruction of hematopoietic microenvironment by cytotoxic T cells. Exp Hematol. 1997;25:1034–1041. [PubMed] [Google Scholar]
- 6.Melenhorst JJ, et al. T cells selectively infiltrate bone marrow areas with residual haemopoiesis of patients with acquired aplastic anaemia. Br J Haematol. 1997;99:517–519. doi: 10.1046/j.1365-2141.1997.4353245.x. [DOI] [PubMed] [Google Scholar]
- 7.Pannetier C, Even J, Kourilsky P. T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol Today. 1995;16:175–181. doi: 10.1016/0167-5699(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 8.Kim G, et al. CDR3 size spectratyping and sequencing of spectratype-derived TCR of spinal cord T cells in autoimmune encephalomyelitis. J Immunol. 1998;160:509–513. [PubMed] [Google Scholar]
- 9.Kang JA, Mohindru M, Kang BS, Park SH, Kim BS. Clonal expansion of infiltrating T cells in the spinal cords of SJL/J mice infected with Theiler’s virus. J Immunol. 2000;165:583–590. doi: 10.4049/jimmunol.165.1.583. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima M, Kong YM, Davies TF. The role of T cells expressing TCR Vβ13 in autoimmune thyroiditis induced by transfer of mouse thyroglobulin-activated lymphocytes: identification of two common CDR3 motifs. Clin Immunol Immunopathol. 1996;80:204–210. doi: 10.1006/clin.1996.0115. [DOI] [PubMed] [Google Scholar]
- 11.Hong J, et al. A common TCR V-D-J sequence in Vβ13.1 T cells recognizing an immunodominant peptide of myelin basic protein in multiple sclerosis. J Immunol. 1999;163:3530–3538. [PubMed] [Google Scholar]
- 12.Luppi P, et al. Restricted TCR V beta gene expression and enterovirus infection in type I diabetes: a pilot study. Diabetologia. 2000;43:1484–1497. doi: 10.1007/s001250051559. [DOI] [PubMed] [Google Scholar]
- 13.Dulphy N, et al. Common intra-articular T cell expansions in patients with reactive arthritis: identical beta-chain junctional sequences and cytotoxicity toward HLA-B27. J Immunol. 1999;162:3830–3839. [PubMed] [Google Scholar]
- 14.Goodall JC, Bledsoe P, Gaston JSH. Tracking antigen-specific human T lymphocytes in rheumatoid arthritis by T cell receptor analysis. Hum Immunol. 1999;60:798–805. doi: 10.1016/s0198-8859(99)00063-4. [DOI] [PubMed] [Google Scholar]
- 15.Mima T, et al. Dominant and shared T cell receptor chain variable regions of T cells inducing synovial hyperplasia in rheumatoid arthritis. Biochem Biophys Res Commun. 1999;263:172–180. doi: 10.1006/bbrc.1999.1128. [DOI] [PubMed] [Google Scholar]
- 16.Inada H, et al. T cell repertoire in the liver of patients with primary biliary cirrhosis. Hum Immunol. 2000;61:675–683. doi: 10.1016/s0198-8859(00)00129-4. [DOI] [PubMed] [Google Scholar]
- 17.Prinz JC, et al. Selection of conserved TCR VDJ rearrangements in chronic psoriatic plaques indicates a common antigen in psoriasis vulgaris. Eur J Immunol. 1999;29:3360–3368. doi: 10.1002/(SICI)1521-4141(199910)29:10<3360::AID-IMMU3360>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 18.Bour H, et al. T-cell repertoire analysis in chronic plaque psoriasis suggests an antigen-specific immune response. Hum Immunol. 1999;60:665–676. doi: 10.1016/s0198-8859(99)00027-0. [DOI] [PubMed] [Google Scholar]
- 19.Epperson, D.E., Margolis, D.A., McOlash, L., Janczak, T., and Barrett, A.J. 2000. In vitro T cell receptor Vβ repertoire analysis can identify which T cells mediate Graft-vs-Leukemia (GVL) and Graft-vs-Host (GVH) responses after HLA-matched sibling stem cell transplantation. Blood. In press. [DOI] [PubMed]
- 20.Kaufman DW, Kelly J, Levy M, Shapiro S. Drugs etiology in the aetiology of agranulocytosis and aplastic anemia. Eur J Haematol Suppl. 1991;60:23–30. doi: 10.1111/j.1600-0609.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 21.Camitta BM, et al. A prospective study of androgens and bone marrow transplantation for treatment of severe aplastic anemia. Blood. 1979;53:504–514. [PubMed] [Google Scholar]
- 22.Even J, et al. T-cell repertoires in healthy and diseased human tissues analysed by T-cell receptor beta-chain CDR3 size determination: evidence for oligoclonal expansions in tumours and inflammatory diseases. Res Immunol. 1995;146:65–80. doi: 10.1016/0923-2494(96)80240-9. [DOI] [PubMed] [Google Scholar]
- 23.Pannetier C, et al. The size of CDR3 hypervariable regions of the murine T-cell receptor beta chain vary as a function of the recombined germline segments. Proc Natl Acad Sci USA. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorski J, et al. Circulating T cell repertoire complexity in normal individuals and bone marrow recipients analyzed by CDR3 size spectratyping. J Immunol. 1994;152:5109–5119. [PubMed] [Google Scholar]
- 25.Meinl E, Hohlfeid R. T cell transformation with Herpesvirus Saimiri: a tool for neuroimmunological research. J Neuroimmunol. 2000;103:1–7. doi: 10.1016/s0165-5728(99)00217-9. [DOI] [PubMed] [Google Scholar]
- 26.Weber F, et al. Transformation of human T-cell clones by Herpesvirus saimiri: intact antigen recognition by autonomously growing myelin basic protein-specific T cells. Proc Natl Acad Sci USA. 1993;90:11049–11053. doi: 10.1073/pnas.90.23.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zoumbos N, Gascon P, Trost S, Djeu J, Young N. Circulating activated suppressor T lymphocytes in aplastic anemia. N Engl J Med. 1985;312:257–265. doi: 10.1056/NEJM198501313120501. [DOI] [PubMed] [Google Scholar]
- 28.Jaroslaw, P.M., et al. 2001. Increased frequency of HLA-DR2 in patients with paroxysmal nocturnal hemoglobinuria and PNH/aplastic anemia syndrome. Ann. Intern. Med. In press. [DOI] [PubMed]
- 29.Westermann J, Pabst R. How organ-specific is the migration of ‘naive’ and ‘memory’ T cells? Immunol Today. 1996;17:278–282. doi: 10.1016/0167-5699(96)80545-7. [DOI] [PubMed] [Google Scholar]
- 30.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 31.McHeyzer-Williams LJ, Panus JF, Mikszta JA, McHeyzer-Williams MG. Evolution of antigen-specific T cell receptors in vivo: preimmune and antigen-driven selection of preferred complementary-determining region 3 (CDR3) motifs. J Exp Med. 1999;189:1823–1838. doi: 10.1084/jem.189.11.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Beemd R, et al. Flow cytometric analysis of the Vβ repertoire in healthy controls. Cytometry. 2000;40:336–345. doi: 10.1002/1097-0320(20000801)40:4<336::aid-cyto9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 33.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen DF, Doukhan L, Kalams S, Delwart E. High-resolution analysis of T-cell receptor Vβ-chain repertoires using DNA heteroduplex tracking: generally stable, clonal CD8+ expansions in all healthy young adults. J Immunol Methods. 1998;215:113–121. doi: 10.1016/s0022-1759(98)00066-0. [DOI] [PubMed] [Google Scholar]
- 35.Lehner PJ, et al. Human HLA-A0201-restricted cytotoxic T lymphocyte recognition of influenza A is dominated by T-cells bearing the Vβ17 gene segment. J Exp Med. 1995;181:79–91. doi: 10.1084/jem.181.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Argaet VP, et al. Dominant selection of an invariant T cell antigen receptor in response to persistent infection by Epstein-Barr virus. J Exp Med. 1994;180:2335–2340. doi: 10.1084/jem.180.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dietrich PY, et al. In vivo T-cell clonal amplification at time of acute graft-versus-host disease. Blood. 1994;84:2815–2820. [PubMed] [Google Scholar]
- 38.Even J, et al. T-cell repertoires in healthy and diseased human tissues analysed by T-cell receptor b-chain CDR3 size determination: evidence for oligoclonal expansions in tumours and inflammatory diseases. Res Immunol. 1995;146:65–80. doi: 10.1016/0923-2494(96)80240-9. [DOI] [PubMed] [Google Scholar]
- 39.Orsini E, et al. Changes in T cell receptor repertoire associated with graft-versus-tumor effect and graft-versus-host disease in patients with relapsed multiple myeloma after donor lymphocyte infusion. Bone Marrow Transplant. 2000;25:623–632. doi: 10.1038/sj.bmt.1702187. [DOI] [PubMed] [Google Scholar]
- 40.Esch T, Clark L, Zhang XM, Goldman S, Heber-Katz E. Observations, legends, and conjectures concerning restricted T-cell receptor usage and autoimmune disease. Crit Rev Immunol. 1992;11:249–264. [PubMed] [Google Scholar]
- 41.Gold DP. TCR V gene usage in autoimmunity. Curr Opin Immunol. 1994;6:907–912. doi: 10.1016/0952-7915(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 42.Fickenscher H, Fleckenstein B. Herpesvirus saimiri. Philos Trans R Soc Lond B Biol Sci. 2001;356:545–567. doi: 10.1098/rstb.2000.0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakao S, et al. Establishment of a CD4+ T cell clone recognizing autologous hematopoietic progenitor cells from a patient with immune-mediated aplastic anemia. Exp Hematol. 1995;23:433–438. [PubMed] [Google Scholar]
- 44.Nakao S, et al. Isolation of a T-cell clone showing HLA-DRB1 0405-restricted cytotoxicity for hematopoietic cells in a patient with aplastic anemia. Blood. 1997;89:3691–3699. [PubMed] [Google Scholar]
- 45.Manz YZ, Dietrich PY, Schnuriger V, Nissen C, Wodnar-Filipowicz A. T-cell receptor b chain variability in bone marrow and peripheral blood in severe acquired aplastic anemia. Blood Cells Mol Dis. 1997;23:110–122. doi: 10.1006/bcmd.1997.0127. [DOI] [PubMed] [Google Scholar]
- 46.Zeng WH, et al. Characterization of T-cell repertoire of the bone marrow in immune-mediated aplastic anemia: evidence for the involvement of antigen-driven T-cell response in cyclosporine-dependent aplastic anemia. Blood. 1999;93:3008–3016. [PubMed] [Google Scholar]
- 47.Karadimitris A, et al. Abnormal T-cell repertoire is consistent with immune process underlying the pathogenesis of paroxysmal nocturnal hemoglobinuria. Blood. 2000;96:2613–2620. [PubMed] [Google Scholar]