Abstract
Comparative immunology has been revitalized by the integration of genomics approaches, which allow a foothold into addressing problems that previously had been difficult to study. One such problem had been the enigmatic finding of overt immune anatomical structures in the lamprey, yet its apparent lack of bona fide immunoglobulin or T cell receptor molecules. The genomic characterization of a novel extended locus that undergoes rearrangements to generate receptor diversity and the subsequent implementation of this diversity in the immune system of lampreys have generated considerable interest as well as new avenues for investigation. Here, we review the anatomical structures of the lamprey that exhibit lympho-hematopoietic characteristics, with the ultimate goal of reconciling these data with contemporary molecular findings. By integrating these datasets we seek to better understand how an alternative adaptive immune system could have evolved.
Introduction
The lampreys are modern representatives of the jawless vertebrates, which, together with the hagfishes, are thought to have appeared around 450–500 million years ago. These species are considered keys to understanding the emergence of adaptive immunity because of their unique position at the interface between the jawless and jawed vertebrates, that is, the transition from jawless to jawed vertebrates bore not only the appearance of jaws but also other important physiological innovations, including an adaptive immune system. Comparative immunologists had struggled for years to try to understand the molecular underpinnings of the lamprey adaptive immune system, which lacks the hallmark components necessary for adaptive immunity in higher vertebrates, namely immunoglobulins (Ig) and T cell receptors (TCR) [1]. Recent findings in the lamprey, however, have revealed that it possesses an alternative immune receptor system that utilizes a Rag-independent strategy to recognize and facilitate elimination of pathogens [2••,3••]. This system undergoes de novo genomic rearrangements of leucine-rich repeat modules of the variable lymphocyte receptor (VLR) locus in order to generate immune receptor diversity that may rival that of the immunoglobulin system of mammals [2••]. It is very important to note, however, that conventional histological investigations on the lamprey had previously demonstrated the clear possession of tissues that closely resembled lympho-hematopoietic structures of higher vertebrates, and these, together with simple immune challenge experiments, predicted the existence of an anticipatory immune system [3••,4,5]. Thus, the discovery of the VLRs, which are considered to be a parallel system to the immunoglobulins, has resurrected interest in these immune structures in the lampreys, and how they are involved in the ontogeny and immune regulation of the VLR system. In this paper, we (i) summarize what is known about the major lympho-hematopoietic anatomical structures in lampreys; (ii) briefly review key aspects of the lamprey adaptive immune system with reference to the VLRs; and (iii) provide a backdrop for future investigations that seek to reconcile the anatomy and ontogeny of the lamprey immune system with molecular investigations on its adaptive immune system.
Anatomy and ontogeny of immune structures in the lamprey
The lympho-hematopoietic sites of lampreys change throughout the life cycle but occupy niches that are histologically similar
Lampreys undergo numerous transformations throughout their life that affect the anatomy and physiology of most tissues, including those concerned with blood cell formation and immune responses [6]. After the larval (ammocoete) period, lampreys undergo a profound metamorphosis that results in the formation of the oral sucker and eyes, and important modifications of gills, endostyle, gut, and circulatory system as well as the degeneration of larval opisthonephros and the appearance of the definitive adult opistonephros. As a consequence the hematopoietic sites must concomitantly change throughout these transformations, occupying sequentially those organs whose microenvironments provide the adequate conditions for the housing and differentiation of the hematopoietic stem cells (HSCs). Insofar as the earliest detectable blood-forming tissue that appears during the lamprey life cycle, both typhlosole (an invagination of the intestinal epithelium) and the nephric fold (including the larval opisthonephros and the associated adipose tissue) constitute the principal hematopoietic organs at this stage of development (ammocoete).
The typhlosole emanates from a lamina of mesenchymal cells located between the dorsal aorta and the upper intestine. These mesenchymal cells together with yolk-containing cells give rise to the blood islands that house the first hematopoietic progenitors arising from either primitive cells that lose their yolk, mesenchymal cells, or stromal reticular cells [7–9]. Accordingly, classical studies recognized these blood islands to be the first (primitive) hematopoietic site in embryonic or larval lampreys [10,11]. The typhlosole primordium is later embedded in the dorsal wall of intestine growing toward the cloaca (Fig. 1a). In 20-mm larvae, blood cells appear between the fat cells of the dorsal part of nephric fold and, in higher numbers, in the intertubular spaces of larval opisthonephros (Fig. 1b) [10,12]. The supraneural body (also known as the protovertebral arch or dorsal fat body) is organized initially from adipose progenitors coming from the fibroblasts of the dorsal connective tissue sheath that surrounds the spinal cord and the meningeal tissue. At the beginning of metamorphosis this loose connective tissue is colonized by immature blood cells that form small clusters between the adipose cells, though after metamorphosis (to free-swimming juveniles) the supraneural body, while still capable of hematopoietic activity, no longer serves as the main hematopoietic organ of adult lampreys (Fig. 1c).
Figure 1.
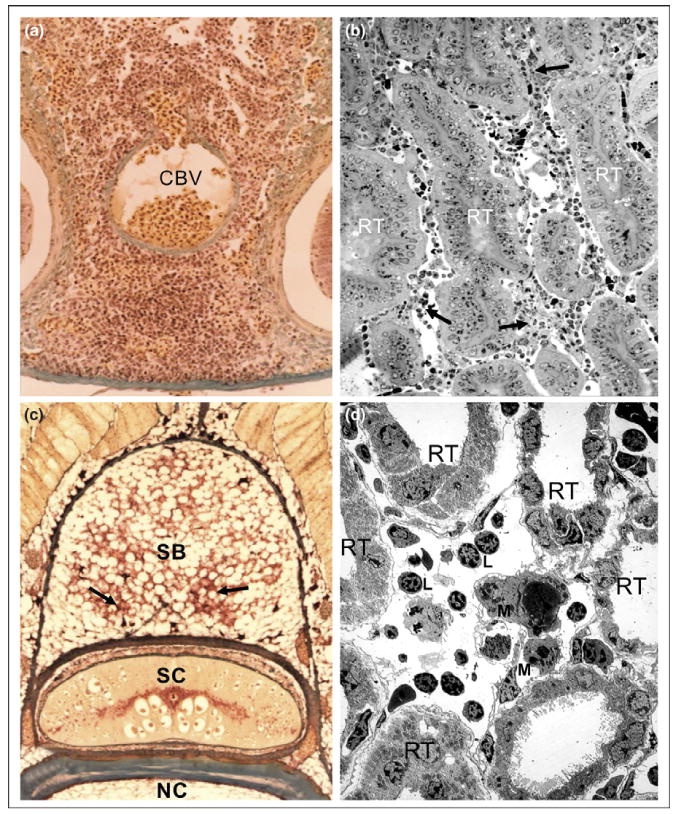
Immune structures in the sea lamprey, Petromyzon marinus. (a) Lympho-hematopoietic tissue in the typhlosole of an ammocoete. Note the masses of developing blood cells around the central blood vessel, CBV that constitutes the axis of typhlosole. (b) Hemato-lymphoid aggregations between the renal tubules of the larval opisthonephros (semi-thin section). Three renal tubules, RT, are denoted; a few hemato-lymphoid aggregations are indicated by arrows. (c) Groups of hematopoietic cells (arrows) in the adipose tissue of the supraneural body of a post-metamorphic sea lamprey. SB, supraneural body; SC, spinal column; NC, notochord. (d) A thin section of larval opisthonephros observed via transmission electron microscopy (TEM). Different developing and mature blood cells, including macrophages (M) and lymphocyte-like (L) cells, occur between renal tubules, RT.
Several years ago one of us (AZ) studied the ultrastructure of the lympho-hematopoietic organs of lampreys with special emphasis on the relevance of microenvironments for determining the presence or absence of hematopoiesis in the respective organs. These studies demonstrated striking similarities between the stromal organization of the distinct hematopoietic organs that occur in the larval, pre-metamorphic, metamorphic, post-metamorphic, and adult lampreys. In the typhlosole, as well as the nephric fold and the supraneural body, the lympho-hematopoietic tissue occupies cell cords arranged among blood sinusoids. In the nephric fold and the supraneural body the blood-forming tissue also appears between the renal tubules and the adipose cells [13,14]. In the cell cords of all these organs there are numerous developing blood cells belonging to different cell lineages, including erythroid, myeloid, and lymphoid cells, arranged in a network of reticular cells and blood sinusoids (Fig. 1d). Monocytes, macrophages, lymphocytes, and plasma cells, all morphologically similar to those of higher vertebrates, occur in both the cell cords and the blood sinusoids [14]. The supraneural body from hematopoietically stimulated lampreys also appears to be highly similar to ‘bone marrow’ in higher vertebrates since all types of blood cells in all stages of maturity, as well as their precursors, are present in this tissue [11]. This histological organization and cell content are also similar to that found in the hematopoietic nets occurring in the intestinal submucosa of the plexiform veins of hagfishes [15] and in numerous organs in cartilaginous and bony fishes that are considered to be morphological and functional equivalents of the bone marrow [16]. It is important to note that the inferences regarding hematopoietic development in the lamprey are based almost exclusively on histological and ultrastructural examination and by broad comparisons with other vertebrate species; that is, stem cell reconstitution and lineage tracing experiments have not as yet been attempted in this species for the hematopoietic lineage.
Lampreys have no histologically identifiable thymus
Classical studies by light microscopy had suggested that lymphoid accumulations occurring under the pharyngeal epithelium of ammocoetes were thymus equivalents [5,17]. We [18] and others [19] analyzed the ultrastructure of these pharyngeal accumulations and demonstrated that they are indeed blood sinuses within the pharyngeal lamina propria that contain numerous lymphocytes and macrophages (Fig. 2c). These structures appear to be involved in the uptake of foreign materials (particulates) from the pharyngeal cavity [19], but on the basis of our observations these clearly were neither a thymus nor a primordial precursor of such an organ. The search for a thymus in the lamprey was based on a legitimate premise that all higher vertebrates possess and require this structural innovation for immune function. While the lamprey clearly lacks a thymus, the most parsimonious explanation for its absence is that it is an invention within the lineage that led to the jawed vertebrates, thus raising healthy speculation as to the factors that allowed its emergence and extreme specialization within the jawed vertebrates.
Figure 2.
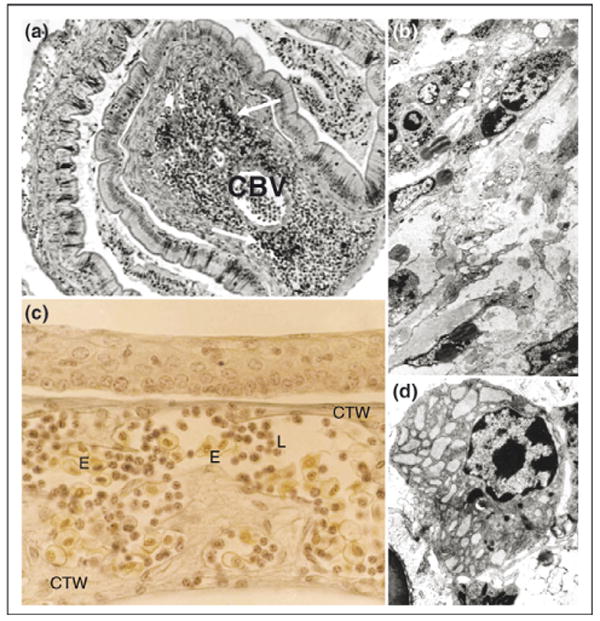
Immune structures in the sea lamprey, Petromyzon marinus. (a) Typhlosole in regression of a metamorphosing lamprey. A few, small groups of lympho-hematopoietic cells occur in lamina propria (arrows) that accumulate an increasing amount of dense connective tissue. CBV, central blood vessel. (b) Thin section of a typhlosole of metamorphosing lamprey (TEM). Fibrocytes and large masses of collagenous fibers have supplanted the loose connective tissue of the larval typhlosole resulting in the disappearance of lympho-hematopoietic tissue. (c) Larval pharyngeal epithelium (gill region). A labyrinth of dense connective tissue walls (CTW) and sinusoidal blood vessels containing numerous circulating blood cells occur closely associated with the pharyngeal epithelium of ammocoetes. L, lymphocyte-like cells; E, erythrocytes. (d) TEM of a mature plasma cell in the sea lamprey. Note the abundant, enlarged cisternae of rough endoplasmic reticulum and the lateral disposition of the nucleus in this cell type.
Lymphocyte-like cells are present in lampreys
We and many other authors have reported cells morphologically similar to vertebrate lymphocytes in histological sections or cytospin preparations of different lamprey and hagfish tissues [5,20,21•]. These findings have been confirmed by electron microscopy [12,14,18,22,23]. Lamprey lymphocytes have variable size (6–10 µm) with a very electron dense nucleus and distinct amount of cytoplasm that contains numerous ribosomes but a scarcity of membranous organelles. The functional identity of these cells is not clearly defined because of the inability to culture these cells in vitro; however, cell sorting experiments on buffy coats and subsequent EST analysis of the sorted population largely confirm a lymphocyte identity [24•,25•]. Perhaps even more remarkable is the presence of morphologically identifiable plasma-like cells in the lamprey lympho-hematopoietic organs [12,26]. These cells contain numerous enlarged cisternae of rough endoplasmic reticulum showing a moderate electron dense content, a typical feature of the plasma cells of ectothermic vertebrates (Fig. 2d).
The lamprey adaptive immune system
Early hints of the generation of antibody-like agglutinins in the lamprey
Serum from the arctic lamprey Lampetra reissneri had been shown to contain natural agglutinins that react, to different degrees, with the heterogeneous antigens of erythrocytes of different species; however, lampreys that received sheep red blood cells (SRBC) for six weeks, showed a dramatic increase in specific hemagglutination titers [27]. The induced agglutinins were heat stable and displayed a high degree of specificity to SRBC antigens. Similarly, the sea lamprey Petromyzon marinus possesses low titers of agglutinins to the H surface antigen of human ‘O’ cells, but subsequent immunization resulted in an increase in titer of these agglutinins [28]. Adult sea lampreys were also shown to produce agglutinins that were antigen specific in response to weekly injections of human O-type erythrocytes (‘O’-RBC) [4,5,29], those agglutinin-producing cells being mainly localized to the supraneural body [5,30]. Fujii [23] demonstrated that the specific agglutinin-producing cells present in the lamprey typhlosole corresponded morphologically to plasma cells.
Lamprey genes related to those found in the mammalian adaptive immune system
Relatives of genes of Spi-B, Ikaros, Ebf, Gata, Pax-2/5/8, and Bach2 transcription factors, which are involved in mammalian lymphocyte development, can be found in the lamprey [31], though they are probably not strictly orthologous to their mammalian counterparts. Genes like CD45 transmembrane protein tyrosine phosphatase, SYK protein tyrosine kinase, Src family members, and the HS-1 adaptor molecule, which are known mammalian lymphocyte activators, are also expressed in lamprey lymphocytes [24•,25•]. Lamprey lymphocyte-like cells also express BCAP (B-cell adaptor for phosphoinositide 3-kinase), CAST (CD3ε-associated signal transducer), RACK 1 (receptor for activated PKC), CD98 (SLC 3A2) and CD9, relatives of the chemokines and chemokine receptor, IL-17 receptor [24•,25•], and a single copy of a TCR-like gene having divergent V and J type sequences [32]. A VpreB-like gene expressed by lymphocyte-like cells was also identified in lampreys [33], and a family of paired Ig-like receptor genes encoding transmembrane proteins with activating and inhibitory potential has been reported in hagfish [34]. Although the identification of these genes in lamprey might infer that its lymphocyte-like cells are potentially capable of mediating adaptive immune responses, the numbers of gene homologs are comparatively low relative to that of jawed vertebrates. The significance of these differences in the context of the development and regulation of adaptive immunity per se is as yet unclear.
The VLR system
A subtractive EST survey from lymphoid cells of immunized ammocoetes led to the identification of sequences encoding a particularly complex set of leucine-rich repeat (LRR)-containing molecules, named variable lymphocyte receptors (VLRs) [3]. The basic composition of lamprey ‘mature’ VLR includes a conserved signal peptide, an N-terminal LRR (LRRNT), followed by up to nine variable and highly diverse LRRs, a connecting peptide, a C-terminal LRR (LRRCT), and a conserved C terminus composed of a glycosyl-phosphatidyl-inositol (GPI)-anchor site and a hydrophobic tail (reviewed in reference [2]). Sequences encoding the LRRs are present in germline cassettes residing upstream or downstream of the incomplete VLR locus. The VLR gene is subsequently assembled through an entirely novel genomic mechanism in which large banks of LRR cassettes are used to build the ‘diversity’ region of the receptor molecules4 [37,38].
The mechanism of rearrangement of the VLR locus is the subject of intense investigation. One recent study suggested that the assembly of the VLR gene probably involves the use of short regions of nucleotide homology that prime the copying of donor LRR-encoding sequences into the recipient locus through a recombination process called ‘copy choice’ [36••]. The genomic organization of the VLR locus permits copying from any LRR-encoding module for generating various hybrid LRRs (see Fig. 1 of Litman et al., this issue). The diversity of VLRs is thus generated by copying LRR segments in diverse combinations and the use of multiple sites in an LRR module for priming. In another study, the mode in which the rearrangements are inferred to occur invokes a stepwise and reiterative gene-conversion mechanism [2••], perhaps utilizing AID-APOBEC type molecules that have been cloned out of the lamprey and shown to exhibit in vitro mutagenesis and recombination activities in surrogate cellular assays [35••]. Both of the mechanisms may be valid in that empirical sequencing data of the VLR transcriptomes are not necessarily incompatible with one or the other model.
Investigation into the immune mechanisms utilized by the VLR system is not as advanced as that for genomic rearrangement, though the problems are equally compelling. Ammocoetes immunized with anthrax spores responded with the production of soluble antigen-specific VLRs, indicating that they use their VLRs for specific recognition of particulate and soluble protein antigens in a humoral response [2••]. On the basis of its known sequence diversity, its inferred three-dimensional structure, and by implication to other known LRR-containing proteins (such as Toll-like receptors) the ectodomains of the VLRs are assumed to be capable of binding a wide range of pathogen specificities. Plasma samples from lamprey ammocoetes immunized with human or mouse erythrocytes specifically agglutinate erythrocytes from the donor species [2••]. Moreover, the agglutinins are removed by adsorption with a monoclonal antibody against the VLR stalk region, indicating that lymphocytes release into the circulation VLR proteins that recognize carbohydrate and protein antigens. While immunohistochemistry using the VLR monoclonal antibody on unimmunized ammocoetes has shown broad expression within the typhlosole regions (unpublished data), the extent to which the expression patterns change after immunization is as yet undetermined. Given that immunization of lampreys results in marked increase in hematopoietic cellularity, one would predict to see a concomitant increase in VLR expression. The VLR-based immune system also raises several related questions, including those regarding the nature of immune activation, binding specificity, tolerance mechanisms, allelic exclusion, and monoclonality, and whether the VLR response is analogous to a T-independent response (as in mammalian B1 cells, for example).
Ontogeny and development of the VLR system
The finding that the VLRs are presumably used in lieu of immunoglobulins in the lampreys raises many questions with regard to its development and transcriptional regulation within the lympho-hematopoietic tissues described above. RT-PCR has been used to show that the VLRs are rearranged and expressed in various lympho-hematopoietic tissues of ammocoetes as well as adults (e.g. larval typhlosole, opistonephros, supraneural body, blood) [3••]; however, a systematic study of the expression of VLRs in non-lymphoid tissue types or during earlier developmental stages has not been done. The latter is particularly important in terms of defining the earliest rearrangements of the VLR genes and understanding whether these rearrangements are purely stochastic or deterministic, as in the Ig locus. The former is important as we do not know whether the VLR is strictly an immune invention or whether it is utilized in a more general sense.
We have begun to address these questions by focusing on the early ontogeny of the VLR system. Whole mount in situ hybridization and histology is very informative for gaining a broad perspective on the global expression patterns of the VLR genes. A somewhat surprising finding to emerge from these studies is that VLR is highly expressed in the pharyngeal regions of larval (and embryonic) lampreys (Fig. 3). The predominant expression is in the oral tentacles and the gill filaments (Fig. 3b and c). The lamprey pharyngeal arches and gills are certainly complex structures that contain many cell types, including lymphoid aggregations [39–42]. While some of the stained structures probably represent lymphoid cells as would be predicted, there is also staining in what appears to be structural elements per se in the pharyngeal arches as well as epithelioid structures. The identity of these structures and the role that VLRs play in their development await further investigation. The implication from these findings, however, is that the VLRs could be involved in some level of developmental patterning in addition to their role in the immune system. Or, perhaps, the VLRs are utilized in these other cell types for recognition of particulate antigens in some sort of innate immune defense mode, though its protein structure does not reveal an overt cytoplasmic signaling tail.
Figure 3.
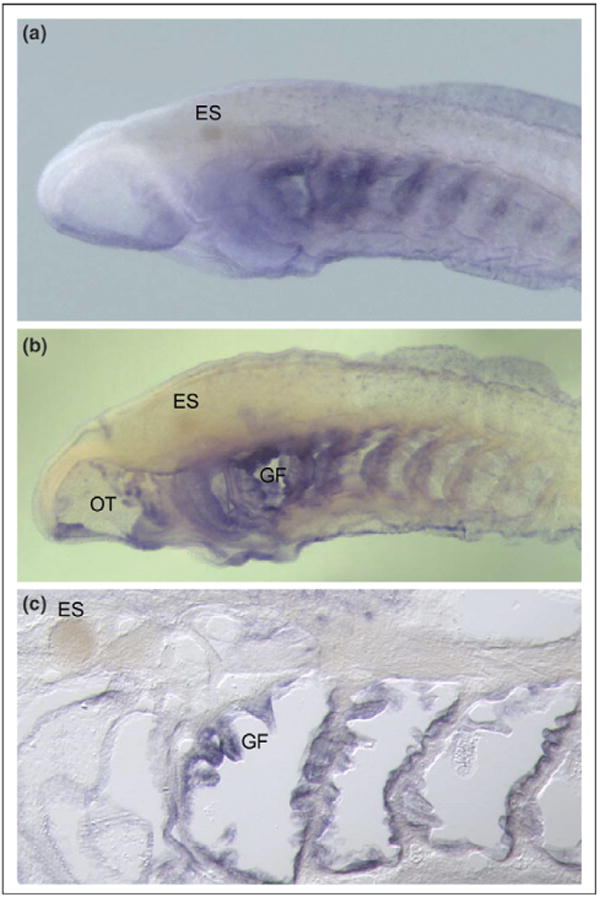
Expression of VLR in a sea lamprey larva. An antisense VLR riboprobe was used for whole mount in situ hybridization to a 45-day ammocoete. (a) Intact specimen (anterior half) is shown. The blue staining represents hybridization of the VLR probe (which was designed to the highly conserved and non-repetitive portion of the VLR-B sequence). Staining is seen particularly in the pharyngeal region. The eye spot is denoted by ES. (b) Same specimen as in (a) but after partially sectioning the specimen sagitally using a cryostat in order to remove the overlying skin and muscle. The cutaway view allows better visualization of the gill filaments, GF, and oral tentacles, OT, both structures being specifically hybridized with the VLR probe. (c) A 10 µM sagittal cryosection of the same specimen as in (a and b) showing individual gill filaments that were hybridized by the VLR probe. The identity of the stained structures is under investigation.
Concluding remarks
The presence of an adaptive immune system in the lampreys had been posited back in the 1960s and 1970s on the basis of anatomical structures that resembled lympho-hematopoietic tissues as well as simple immune challenge experiments. Identification of the VLRs as an alternative immune recognition strategy in the lampreys has rekindled interest in this area, and also fostered completely new avenues for immunological and genomic investigation. In the broadest sense, it is necessary to reconcile the historical findings on the immune anatomy of the lamprey with the contemporary molecular findings in order to better understand how this alternative form of adaptive immunity arose and evolved.
Acknowledgments
This work has been funded, partly, by grants from the National Institutes of Health (GM079492) and the National Science Foundation (IOS-0321461 and IR/D), and by institutional funds from the Benaroya Research Institute at Virginia Mason.
Footnotes
It should be noted that both lampreys and hagfish have two different VLR loci (VLRA and VLRB); however, most of the experiments have been performed with the VLRB genes [2••,3••,35••,36••].
Publisher's Disclaimer: This article was published in an Elsevier journal. The attached copy is furnished to the author for non-commercial research and education use, including for instruction at the author's institution, sharing with colleagues and providing to institution administration.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Du Pasquier L, Flajnik M. Origin and evolution of the vertebrate immune system. In: Paul WE, editor. Fundamental Immunology. 3. Raven Press, Ltd; 1998. pp. 199–233. [Google Scholar]
- 2••.Alder MN, Rogozin IB, Iyer LM, Glazko GV, Cooper MD, Pancer Z. Diversity and function of adaptive immune receptors in a jawless vertebrate. Science. 2005;310:1970–1973. doi: 10.1126/science.1119420. [DOI] [PubMed] [Google Scholar]; This is the second lamprey VLR paper to be published. The study made extensive use of rearrangement ‘intermediates’ for inferring that the locus may undergo rearrangement via some sort of gene-conversion mechanism. They also used computational analysis of sequences of existing VLR components, mature VLRs, and the transcriptome, to infer a staggering level of diversity of VLRs that could be generated. This study also showed that the lamprey can use their VLRs for specific recognition of particulate and soluble protein antigens in a humoral response.
- 3••.Pancer Z, Amemiya CT, Ehrhardt GRA, Ceitlin J, Gartland GL, Cooper MD. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–180. [Google Scholar]; This is the original lamprey VLR paper. A series of cDNAs had been identified that contained variable numbers of different LRR cassettes but whose 5′ and 3′ regions were absolutely conserved. It was inferred that this molecule, named variable lymphocyte receptor, could be a surrogate immune recognition molecule analogous to Ig. The researchers used long-range sequencing of the VLR locus to show that the locus is not complete and that the components of complete form of the VLR gene were outside of the locus so that genomic rearrangement was necessary to make an intact gene. They could show that rearrangement occurred at the genomic level and specifically in lymphoid cells, not erythrocytes. In addition, the authors performed single cell PCR to show that VLRs are generated in a monoclonal fashion.
- 4.Pollara B, Litman GW, Finstad J, Howell J, Good RA. The evolution of the immune response. VII. Antibody to human “O” cells and properties of the immunoglobulin in lamprey. J Immunol. 1970;105:738–745. [PubMed] [Google Scholar]
- 5.Good RA, Finstad J, Litman GW. Immunology. In: Hardesty MW, Potter IC, editors. The Biology of Lampreys. London: Academic Press; 1972. pp. 405–432. [Google Scholar]
- 6.Richardson MK, Wright GM. Developmental transformations in a normal series of embryos of the sea lamprey Petromyzon marinus (Linnaeus) J Morphol. 2003;257:348–363. doi: 10.1002/jmor.10119. [DOI] [PubMed] [Google Scholar]
- 7.Raunich L. Sullo svillupo degli isolotti sanguini e delle venue vitelline negli embrioni de lampreda di ruscello Petromyzon planeri. Monitore Zool Ital. 1946;55:115. [Google Scholar]
- 8.Jordan HE. The evolution of blood forming tissues. Q Rev Biol. 1933;8:58. [Google Scholar]
- 9.Torrey TW. The absorption of colloidal carbon from the body cavity of ammocoetes. A study of the structure and function of the larval kidneys and blood forming tissues. J Morphol. 1938;63:163. [Google Scholar]
- 10.Percy Lord R, Potter IC. Blood cell formation in the river lamprey, Lampetra fluviatilis. J Zool. 1976;178:319–340. [Google Scholar]
- 11.Piavis GW, Hiatt JL. Blood cell lineage in the sea lamprey, Petromyzon marinus (Pisces: Petromyzontidae) Copeia. 1971;1971:722–728. [Google Scholar]
- 12.Kilarski W, Plytycz B. The presence of plasma cells in the lamprey (Agnatha) Dev Comp Immunol. 1981;5:361–366. doi: 10.1016/0145-305x(81)90045-8. [DOI] [PubMed] [Google Scholar]
- 13.Ardavin CF, Gomariz RP, Barrutia MG, Fonfria J, Zapata AG. The lympho-hemopoietic organs of the anadromous sea lamprey, Petromyzon marinus. A comparative study throughout its life cycle. Acta Zool Stockh. 1984;65:1–15. [Google Scholar]
- 14.Ardavin CF, Zapata A. Ultrastructure and changes during metamorphosis of the lympho-hemopoietic tissue of the larval anadromous sea lamprey Petromyzon marinus. Dev Comp Immunol. 1987;11:79–93. doi: 10.1016/0145-305x(87)90010-3. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka Y, Saito Y, Gotoh H. Vascular architecture and intestinal hematopoietic nests of two cyclostomes, Eptatretus burgeri and ammoncoetes of Entosphenus reissneri: a comparative morphological study. J Morphol. 1981;170:71–93. doi: 10.1002/jmor.1051700106. [DOI] [PubMed] [Google Scholar]
- 16.Zapata AG, Torroba M, Vicente A, Varas A, Sacedon R, Jimenez E. The relevance of cell microenvironments for the appearance of lympho-haemopoietic tissues in primitive vertebrates. Histol Histopathol. 1995;10:761–778. [PubMed] [Google Scholar]
- 17.Gerard P. Sur le systeme athrophagocytaire chez l'ammocece de la Lampetra planeri (Bloch.) Archs Biol Paris. 1933;44:327. [Google Scholar]
- 18.Ardavin CF, Zapata A. The pharyngeal lymphoid tissue of lampreys. A morpho-functional equivalent of the vertebrate thymus? Thymus. 1988;11:59–65. [PubMed] [Google Scholar]
- 19.Page M, Rowley AT. A morphological study of pharyngeal lymphoid accumulations in larval lampreys. Dev Comp Immunol. 1982;2(Suppl):35. [Google Scholar]
- 20.Zapata AG, Amemiya CT. Phylogeny of lower vertebrates and their immunological structures. Curr Top Microbiol Immunol. 2000;248:67–110. doi: 10.1007/978-3-642-59674-2_5. [DOI] [PubMed] [Google Scholar]
- 21•.Zapata AG, Cooper EL. The Immune System: Comparative Histophysiology. Chichester: John Wiley & Sons; 1990. [Google Scholar]; This is a major treatise on the comparative anatomy of the immune system.
- 22.Betz UA, Mayer WE, Klein J. Major histocompatibility complex class I genes of the coelacanth Latimeria chalumnae. Proc Natl Acad Sci USA. 1994;91:11065–11069. doi: 10.1073/pnas.91.23.11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii T. Electron microscopy of the leucocytes of the typhlosole in ammocoetes, with special attention to the antibody-producing cells. J Morphol. 1982;173:87–100. doi: 10.1002/jmor.1051730108. [DOI] [PubMed] [Google Scholar]
- 24•.Mayer WE, Uinuk-Ool T, Tichy H, Gartland LA, Klein J, Cooper MD. Isolation and characterization of lymphocyte-like cells from a lamprey. Proc Natl Acad Sci USA. 2002;99:14350–14355. doi: 10.1073/pnas.212527499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Uinuk-Ool T, Mayer WE, Sato A, Dongak R, Cooper MD, Klein J. Lamprey lymphocyte-like cells express homologs of genes involved in immunologically relevant activities of mammalian lymphocytes. Proc Natl Acad Sci USA. 2002;99:14356–14361. doi: 10.1073/pnas.212527699. [DOI] [PMC free article] [PubMed] [Google Scholar]; The above two papers are important because they described how they isolated lamprey lymphocytes via flow sorting using forward-scatter and side-scatter properties of the lymphocytes, and subsequently how they analyzed the transcriptomes of these cells. The impetus of these experiments was to look for evidence of receptors of the Ig and TCR types. While many transcripts were identified of molecules involved in immunity, no obvious Ig or TCR transcripts were identified.
- 26.Zapata A, Ardavin CF, Gomariz RP, Leceta J. Plasma cells in the ammocoete of Petromyzon marinus. Cell Tissue Res. 1981;221:203–208. doi: 10.1007/BF00216582. [DOI] [PubMed] [Google Scholar]
- 27.Fujii T, Nakagawa H, Murakawa S. Immunity in lamprey. I. Production of haemolytic and haemagglutinating antibody to sheep red blood cells in Japanese lampreys. Dev Comp Immunol. 1979;3:441–451. doi: 10.1016/s0145-305x(79)80040-3. [DOI] [PubMed] [Google Scholar]
- 28.Boffa GA, Fine JM, Drilhon A, Amouch P. Immunoglobulins and transferrin in marine lamprey sera. Nature. 1967;214:700–702. doi: 10.1038/214700b0. [DOI] [PubMed] [Google Scholar]
- 29.Hagen M, Filosa MF, Youson JH. The immune response in adult sea lamprey (Petromyzon marinus L.): the effect of temperature. Comp Biochem Physiol A. 1985;82:207–210. doi: 10.1016/0300-9629(85)90727-3. [DOI] [PubMed] [Google Scholar]
- 30.Hagen M, Filosa MF, Youson JH. Immunocytochemical localization of antibody-producing cells in adult lamprey. Immunol Lett. 1983;6:87–92. doi: 10.1016/0165-2478(83)90086-x. [DOI] [PubMed] [Google Scholar]
- 31.Rothenberg EV, Pant R. Origins of lymphocyte developmental programs: transcription factor evidence. Semin Immunol. 2004;16:227–238. doi: 10.1016/j.smim.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Pancer Z, Mayer WE, Klein J, Cooper MD. Prototypic T cell receptor and CD4-like coreceptor are expressed by lymphocytes in the agnathan sea lamprey. Proc Natl Acad Sci USA. 2004;101:13273–13278. doi: 10.1073/pnas.0405529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cannon JP, Haire RN, Pancer Z, Mueller MG, Skapura D, Cooper MD, Litman GW. Variable domains and a VpreB-like molecule are present in a jawless vertebrate. Immunogenetics. 2005;56:924–929. doi: 10.1007/s00251-004-0766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki T, Shin I, Fujiyama A, Kohara Y, Kasahara M. Hagfish leukocytes express a paired receptor family with a variable domain resembling those of antigen receptors. J Immunol. 2005;174:2885–2891. doi: 10.4049/jimmunol.174.5.2885. [DOI] [PubMed] [Google Scholar]
- Rogozin IB, Iyer LM, Liang L, Glazko GV, Liston VG, Pavlov YI, Aravind L, Pancer Z. Evolution and diversification of lamprey antigen receptors: evidence for involvement of an AID-APOBEC family cytosine deaminase. Nat Immunol. 2007;8:647–656. doi: 10.1038/ni1463. [DOI] [PubMed] [Google Scholar]; In this report, two putative deaminases genes of the AID-APOBEC family were identified and the implied role of these deaminase genes in VLR diversification was discussed through in vitro mutagenesis and recombination activity experiments in a surrogate system. The finding of the VLRA gene was also reported (second type of VLR gene that was found originally in the hagfish). The authors expand on their model of stepwise gene conversion.
- 36••.Nagawa F, Kishishita N, Shimizu K, Hirose S, Miyoshi M, Nezu J, Nishimura T, Nishizumi H, Takahashi Y, Hashimoto S, et al. Antigen-receptor genes of the agnathan lamprey are assembled by a process involving copy choice. Nat Immunol. 2007;8:206–213. doi: 10.1038/ni1419. [DOI] [PubMed] [Google Scholar]; This paper described a different model for the generation of diversity at the VLR locus. They used a fosmid library of a single specimen of Japanese lamprey and extensive rearrangement analyses to show that the stepwise rearrangement at the VLR locus could be one of ‘copy-choice’ recombination. This mechanism involves a replication-coupled template switching event where short regions of nucleotide homology initiate priming of donor LRR-encoding sequences into the recipient gene. They also corroborated the idea that VLR expression is monoclonal.
- 37.Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–822. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Pancer Z, Cooper MD. The evolution of adaptive immunity. Annu Rev Immunol. 2006;24:497–518. doi: 10.1146/annurev.immunol.24.021605.090542. [DOI] [PubMed] [Google Scholar]
- 39.McCauley DW, Bronner-Fraser M. Importance of SoxE in neural crest development and the evolution of the pharynx. Nature. 2006;441:750–752. doi: 10.1038/nature04691. [DOI] [PubMed] [Google Scholar]
- 40.Sauka-Spengler T, Baratte B, Lepage M, Mazan S. Characterization of Brachyury genes in the dogfish S. canicula and the lamprey L. fluviatilis. Insights into gastrulation in a chondrichthyan. Dev Biol. 2003;263:296–307. doi: 10.1016/j.ydbio.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Sauka-Spengler T, Le Mentec C, Lepage M, Mazan S. Embryonic expression of Tbx1, a DiGeorge syndrome candidate gene, in the lamprey Lampetra fluviatilis. Gene Expr Patterns. 2002;2:99–103. doi: 10.1016/s0925-4773(02)00301-5. [DOI] [PubMed] [Google Scholar]
- 42.Neidert AH, Virupannavar V, Hooker GW, Langeland JA. Lamprey Dlx genes and early vertebrate evolution. Proc Natl Acad Sci USA. 2001;98:1665–1670. doi: 10.1073/pnas.98.4.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]


