Abstract
Objective
To investigate the hypothesis that plasma leptin may predict adiposity changes.
Design
A population-based cohort study.
Setting
Fleurbaix and Laventie, in the north of France.
Subjects
1175 subjects participated, of whom 946 completed measurements at baseline (1999) and follow-up (2001). After excluding 64 subjects obese at baseline, 882 subjects (478 adults, 404 children 8y and over) were included in the analysis.
Interventions
We measured plasma leptin concentrations at baseline and various adiposity parameters at baseline and follow-up. Partial correlation coefficients (rp) between baseline plasma leptin and each adiposity indicator at follow-up were calculated with adjustment for baseline age, pubertal stage, adiposity and familial correlations between siblings.
Results
Changes in body mass index and % body fat were not related to baseline plasma leptin. High baseline plasma leptin predicted an increase (rp (p value)) in the sum of the four skinfolds (0.18 (<0.0001)), the waist circumference (0.16 (0.0003)) and the waist-to-hip ratio (0.29 (<0.0001)) in adults only, and in the hip circumference in adults (0.20 (<0.0001)) and children (0.22 (<0.0001)). After adjustment for a set of 4 adiposity variables at baseline (% body fat, skinfolds, waist and hip circumferences), baseline plasma leptin predicted only changes in the sum of the four skinfolds in adults (0.15 (0.001)), with similar tendency although not significant in children (0.08 (0.13)).
Conclusions
A high leptin relative to baseline fat mass predicts fat mass gain over time, mainly in the subcutaneous location.
Sponsorship
Supported by Knoll, Centre d’Etudes et de Documentation du Sucre (CEDUS), Groupe Fournier, Lesieur, Nestlé France, Produits Roche, Le Centre d’Information Scientifique sur la Bière (CISB).
Descriptors
adipocytokins; insulin; adipose tissue; epidemiology
Keywords: Adult, Body Composition, Body Mass Index, Child, Cohort Studies, Female, Follow-Up Studies, France, Humans, Leptin, blood, Male, Obesity, blood, epidemiology, etiology, Predictive Value of Tests, Skinfold Thickness, Subcutaneous Fat, growth & development, metabolism, Waist-Hip Ratio, Weight Gain
Introduction
Leptin, the 16 kDa protein encoded by the ob gene, is recognized as an important factor in energy balance regulation. Leptin is mainly produced by white adipocytes, with higher secretion in subcutaneous than in omental adipose tissue in humans (van Harmelen et al, 2002). A strong positive correlation between plasma leptin concentrations and total body fat content is well documented in adults (Considine et al, 1996; Zimmet et al, 1996) and in children. (Blum et al, 1997; Arslanian et al, 1998). Experimental studies in rodents and data in leptin-deficient humans with mutations of the ob gene (Montague et al, 1997; Farooqi et al, 1999) support the concept of leptin as an anti-obesity hormone acting through a feedback loop between adipose tissue and the hypothalamus, resulting in decreased food intake. In children (Arslanian et al, 1998; Romon et al, 2004) and adults (Perusse et al, 1997; Gomez-Merino et al, 2002), there is also some evidence that circulating leptin levels are associated with energy expenditure related to physical activity, especially in females. Food intake stimulates leptin secretion (Dallongeville et al, 1998), perhaps through a direct action of insulin on the adipocyte (Saad et al, 1998).
An excess or default of circulating leptin for a given level of adiposity may reflect resistance or increased sensitivity to leptin, respectively, which may result in energy imbalance. Therefore, relative leptin levels may be hypothesized to predict adiposity changes over time. Three studies found a positive relationship between baseline leptin levels adjusted for the body mass index (BMI), and subsequent body weight or BMI changes in obese girls (Savoye et al, 2002), adult women (Lissner et al, 1999), and adults of both genders (Chessler et al, 1998). Using a different approach, Van Rossum et al. (van Rossum et al, 2003) compared baseline levels of plasma leptin between weight gainers and weight-stable subjects among 259 non-obese adults followed for 7 years. They found higher baseline leptin levels in weight gainers than in weight-stable subjects. To our knowledge, none of these studies referred specifically to subcutaneous adiposity, and none included both adults and children.
As plasma leptin concentration is strongly associated with body fat content, the relationship between baseline leptin levels and adiposity changes overtime may be confounded by inadequate adjustment for baseline fat content and distribution or by the statistical approach performed.
The aims of this study were to describe by two distinct statistical approaches, in non-obese children and adults, the relationships between plasma leptin concentrations and various adiposity markers, and to investigate whether leptin predicts 2 year-changes in adiposity.
Subjects and Methods
Participants
The subjects were participants in the Fleurbaix-Laventie Ville Santé (FLVS) II study, the purpose of which is to investigate genetic, metabolic and environmental determinants of adiposity development in children and adults. In 1992, 579 families of Fleurbaix and Laventie, two small cities in the North of France, participated in a 5-year nutritional education program at school: the “Fleurbaix Laventie Ville Santé” study (Lafay et al, 1997). In 1999, 393 families who had previously participated in the FLVS study, who were still living in Fleurbaix or Laventie and who could be contacted, were asked to participate in the FLVS II study. Among these 393 families, 294 (1175 subjects 8 years and over) agreed to participate. The entire assessment protocol of adiposity measurements and biological parameters was completed by 1081 subjects at inclusion in 1999, of whom 946 also completed adiposity measurements at follow-up in 2001.
Overweight and obesity were determined by the BMI, according to the standard definitions (respectively 25 and 30 kg.m−2) in adults and to the age and gender related cut-off points established by Cole et al. (Cole et al, 2000) in children and adolescents. We excluded from the analysis 64 subjects obese at baseline for which adiposity changes over time may be related to determinants distinct from those involved in non-obese subjects. Therefore, 882 subjects were eventually included in the analysis.
All participants gave written consent, the protocol was in accordance with the Helsinki declaration and approved by the ethics committee of Lille in July 1998 and the data files have been declared to the “Commission Nationale Informatique et Liberté”.
Measurements
At baseline and follow-up, clinical examinations were performed at home by trained physicians. Height (to the nearest 5 mm), weight (to the nearest 0.1 kg), and body composition were determined barefoot, in light clothes with a stadiometer and a Tanita® TBF 310 body fat analyser (Tanita Corp, France). The bicipital, tricipital, suprailiac and subscapular skinfolds were measured twice (to the nearest 0.1 mm) with a Harpenden caliper and averaged. The waist circumference (WC) was recorded to the nearest 5 mm at the midpoint between the iliac crest level and the lowest rib. The hip circumference (HC) was recorded to the nearest 5 mm at the largest circumference over the buttocks, and the waist-to-hip ratio was calculated. In a separate sample of 64 children, the correlation coefficients between BIA % fat and anthropometric variables were higher than 0.70 and in the same range in adults and children of both genders (Kettaneh et al, 2005). In subjects under 18 years, pubertal stage was recorded according to the Tanner classification (Tanner, 1952).
The dietary questionnaire used in the study was specifically developed for the FLVS subjects. It is a semi-quantitative self-administered food questionnaire, which covers intake during the previous 12 months, and enquires about 124 different foods or food groups. From the reported frequencies of foods and drinks and gender-specific portions, it was possible to estimate an average daily intake of foods, drinks, energy and major nutrients (de Lauzon et al, 2004). Ambulatory activity was measured by an electronic pedometer (yamax, Digiwalker, Yamax, Japan) worn at the waist from morning to evening during seven consecutive days (Bassett et al, 2000). To limit the effect of technical errors due to misuse of the device, we excluded measurements, in each gender and generation group, a value out of the range mean ±3 SD.
Leisure time physical activity (LTPA) was assessed with a French version (Vuillemin et al, 2000) of Kriskas’s Modifiable Activity Questionnaire (MAQ) validated in adults (Kriska et al, 1990) and teenagers (Aaron et al, 1995). In 1999, the teenager MAQ was administered by a trained interviewer to evaluate the past-weeks and past-year’s leisure time activity. The younger children were assisted by their parents to answer the MAQ. At follow-up, subjects < 18 years answered the teenager version and subjects over 18 years-old the adult version of the MAQ. Both versions include identical questions and measure the total time per week (h.wk−1) spent in leisure-time physical activity.
A 20 ml fasting venous blood sample was drawn for a measurement of plasma glucose, insulin (Bi-Insulin IRMA, Kit Sanofi Pasteur, France) and leptin (Human leptin RIA kit DSL-23100, Webster USA GMBH, Germany).
Statistical analysis
The analysis was conducted separately in four groups according to gender, and age (<18 and ≥ 18 y). In both generations, we compared baseline characteristics between genders with the Student t test.
In each group, a two step-procedure calculated partial correlation coefficients, adjusted for covariates, between baseline leptin and other adiposity and metabolic variables, first at baseline, then at follow-up. In the first step we constructed linear regression models with leptin or adiposity or metabolic measurements as dependent variables and adjustment factors as independent variables. Adjustment factors included baseline age in all models, baseline pubertal stage in children. All models at follow-up were also adjusted for the corresponding adiposity variable at baseline or a set of four adiposity variables at baseline (sum of 4 skinfolds (SSK), BIA % fat, WC, HC). All models including circumference measurements were also adjusted for height. Mixed linear models were used and included the nuclear family as a random effect. In the second step, we correlated the residuals of the regression models with leptin, with those of regression models with adiposity or metabolic variables, to obtain partial correlation coefficients between leptin and adiposity or metabolic variables, taking into account the adjustment factors. For leptin, the BMI, SSK, plasma insulin measurements, and the HOMA fasting index of insulin resistance (HOMAIR) (Matthews et al, 1985), a logarithmic transformation was performed because of a skewed distribution We also examined the effect, on the relationship between leptin and the SSK or variation, of adding into the model variables related to physical activity (LTPA and ambulatory activity), energy intake and alcohol consumption. We compared correlation coefficients between genders by a Z-test after Fisher’s Z′ transformation of correlation coefficients.
To describe subjects with high or low baseline leptin relative to the level of body fat content and to quantify adiposity changes related with baseline leptin concentration, subjects were divided into two groups according to the median of the residuals of a linear regression of baseline log-transformed leptin on a set of 5 variables (SSK, % BIA % fat, HC, WC, height). This regression model was performed separately in each gender and generation group, adjusted for age, and included a family variable as a random effect. In children only, there was an additional adjustment for pubertal stage. Baseline and changes in anthropometric and metabolic variables were compared between “high” and “low” leptin groups by the Student t test.
All analyses were performed with the SAS® statistical package (version 8.2, SAS, Cary, NC).
Results
Population characteristics (Table 1)
Table 1.
Population characteristics at baseline
| Adults | Children | |||||
|---|---|---|---|---|---|---|
| Men | Women | P-Value | Boys | Girls | P-Value | |
| N | 208 | 270 | 209 | 195 | ||
| Age (years) | 39.9 ± 10 | 38.8 ± 9.3 | 0.22 | 13.6 ± 2.4 | 13.3 ± 2.6 | 0.33 |
| Weight (kg) | 76.6 ± 9.7 | 61.7 ± 8.3 | <0.0001 | 49.0 ± 13.9 | 45.7 ± 11.7 | 0.009 |
| BMI (kg m-2) | 24.5 ± 2.9 | 23.0 ± 3.0 | <0.0001 | 18.5 ± 2.9 | 18.6 ± 2.8 | 0.73 |
| BIA % fat (%) | 20.3 ± 6.1 | 30.6 ± 6.3 | <0.0001 | 13.4 ± 6.1 | 22.3 ± 7.6 | <0.0001 |
| SSK (mm) | 52.4 ± 22.4 | 66.5 ± 25.2 | <0.0001 | 37.3 ± 21.7 | 48.2 ± 20.8 | <0.0001 |
| Wc (cm) | 87.7 ± 9.4 | 75.4 ± 8.2 | <0.0001 | 67.5 ± 8.1 | 634.4 ± 7.1 | <0.0001 |
| HC (cm) | 95.7 ± 7.4 | 94.3 ± 9.3 | 0.06 | 79.3 ± 10.8 | 79.5 ± 10.8 | 0.82 |
| WHR | 0.92 ± 0.07 | 0.80 ± 0.06 | <0.0001 | 0.86 ± 0.06 | 0.81 ± 0.07 | <0.0001 |
| Plasma fasting lepting (nmol l-1) | 0.34 ± 0.25 | 1.05 ± 0.61 | <0.0001 | 1.16 ± 0.35 | 0.77 ±0.52 | <0.0001 |
| Plasma fasting insulin (pmol l-1) | 26 ± 23 | 25 ± 16 | 0.67 | 34 ± 20 | 38 ± 22 | 0.04 |
| Plasma fasting glucose (mmol l-1) | 5.0 ± 0.56 | 4.80 ± 0.50 | <0.0001 | 4.80 ± 0.39 | 4.7 ± 0.39 | 0.003 |
| HOMAIR | 0.96 ± 0.70 | 0.90 ± 0.63 | 0.73 | 1.22 ± 0.82 | 1.36 ±0.82 | 0.09 |
| Energy intake (MJ day-1) | 13.3 ± 4.3 | 10.5 ± 3.3 | <0.0001 | 13.2 ± 5.7 | 10.3 ± 4.0 | <0.0001 |
| Alcohol intake (g day-1) | 20.7 ± 20.7 | 6.6 ± 9.6 | <0.0001 | |||
| Ambulatory activity (ksteps wk-1) | 56.9 ± 23.4 | 50.2 ± 19.2 | 0.001 | 63.9 ± 28.3 | 53.2 ± 20.2 | <0.0001 |
| LPTA (h wk-1) | 5.1 ± 6.0 | 3.4 ± 4.4 | 0.0007 | 7.0 ± 5.8 | 4.0 ± 3.5 | <0.0001 |
Abbreviations: BIA, bioelectrical impedance analysis; BMI, body mass index; HC, hip circumference; HOMA, homestatsis model assessment; LTPA, Leisure time physical activity; SSK, sum of four skinfolds; WC waist circumference; WHR, waist-to-hip ratio. Data shown as ± s.d. or %.
Baseline characteristics of adults and children show expected differences between genders for adiposity variables. The mean plasma leptin concentration was higher in females than in males in both children and adults (all p values <0.0001). Plasma fasting glucose was higher in males than in females (all p values <0.004). Plasma fasting insulin was higher in girls than in boys (p=0.04) but did not differ between men and women. The HOMAIR did not differ significantly between genders. Energy intake, alcohol consumption, ambulatory activity and LTPA were higher in males than in females (all p values <0.001).
Association between plasma leptin and adiposity, metabolic, and lifestyle variables at baseline (Table 2)
Table 2.
Pearson's correlation coefficients between plasma leptin and measurements at baseline
| Adults | Children | |||||
|---|---|---|---|---|---|---|
| Men | Women | P-Value | Boys | Girls | P-Value | |
| N | 208 | 270 | 209 | 195 | ||
| BMIa | 0.66 (<0.0001) | 0.62 (<0.0001) | 0.47 | 0.68 (<0.0001) | 0.62 (<0.0001) | 0.30 |
| BIA body fat % | 0.68 (<0.0001) | 0.67 (<0.0001) | 0.84 | 0.71 (<0.0001) | 0.65 (<0.0001) | 0.27 |
| SSKa | 0.70 (<0.0001) | 0.68 (<0.0001) | 0.68 | 0.80 (<0.0001) | 0.69 (<0.0001) | 0.01 |
| WCb | 0.64 (<0.0001) | 0.58 (<0.0001) | 0.30 | 0.62 (<0.0001) | 0.49 (<0.0001) | 0.06 |
| HCb | 0.49 (<0.0001) | 0.60 (<0.0001) | 0.09 | 0.53 (<0.0001) | 0.46 (<0.0001) | 0.36 |
| WHR | 0.39 (<0.0001) | 0.03 (0.62) | 0.0004 | 0.13 (0.06) | -0.03 (0.67) | 0.11 |
| Plasma fasting insulina | 0.59 (<0.0001) | 0.39 (<0.0001) | 0.004 | 0.47 (<0.0001) | 0.38 (<0.0001) | 0.27 |
| Plasma fasting glucose | 0.35 (<0.0001) | 0.03 (0.59) | 0.0003 | 0.08 (0.26) | 0.21 (0.003) | 0.19 |
| HOMAIRa | 0.61 (<0.0001) | 0.37 (<0.0001) | 0.0006 | 0.46 (<0.0001) | 0.39 (<0.0001) | 0.39 |
| Energy intake | -0.14 (0.04) | -0.02 (0.77) | 0.08 | 0.05 (0.50) | 0.02 (0.80) | 0.77 |
| Alcohol intake | 0.02 (0.80) | -0.02 (0.69) | 0.67 | |||
| Ambulatory activity | -0.19 (0.006) | -0.02 (0.81) | 0.06 | -0.05 (0.48) | -0.04 (0.62) | 0.92 |
| LTPAa | -0.01 (0.89) | -0.04 (0.53) | 0.75 | -0.17 (0.01) | -0.20 (0.005) | 0.76 |
Abbreviations: BIA, bioelectrical impedance analysis; BMI, body mass index; HC, hip circumference; HOMA, homestatsis model assessment; LTPA, Leisure time physical activity; SSK, sum of four skinfolds; WC waist circumference; WHR, waist-to-hip ratio.
Data shown as partial correlation coefficient (p value) adjusted for age, and pubertal stage in children.
Logtransformed.
Additional adjustment on height.
All models are also adjusted for a random effect nuclear family variable.
After adjustment for age and Tanner stage in children, there was a positive correlation in all gender generation groups between fasting plasma leptin and all adiposity markers (r range 0.46–0.80) except for the WHR in females (Table 2). The highest correlation coefficients were between leptin and the SSK, ranging from 0.64 to 0.80, with a relationship stronger in boys than in girls. Plasma fasting insulin and the HOMAIR were correlated to leptin in all groups. However these relationships were stronger in adult men than in women Ambulatory activity and energy intake were negatively correlated with plasma leptin in men only. Plasma leptin was negatively correlated to LTPA in children only.
The relationship between leptin and the SSK at baseline was not substantially modified after adjusting successively for ambulatory activity, LTPA, energy intake, and alcohol consumption (results not shown).
Adiposity at follow-up predicted by baseline leptin after adjusting for baseline corresponding adiposity (Table 3)
Table 3.
Pearson's correlation coefficients between plasma leptin at baseline and adiposity at follow-up
| Adults | Children | P-Value | |
|---|---|---|---|
| N | 478 | 404 | |
| BMIa | 0.02 (0.68) | 0.10 (0.06) | 0.24 |
| BIA body fat % | 0.07 (0.11) | 0.08 (0.11) | 0.99 |
| SSKa | 0.18 (<0.0001) | 0.08 (0.10) | 0.13 |
| WCb | 0.16 (0.0003) | 0.09 (0.07) | 0.37 |
| HCb | 0.20 (<0.0001) | 0.22 (<0.0001) | 0.76 |
| WHR | 0.29 (<0.0001) | 0.04 (0.41) | 0.0007 |
Abbreviations: BMI, body mass index; BIA, bioelectrical impedance analysis ; SSK, sum of four skinfolds; WC waist circumference; HC, hip circumference;WHR, waist-to-hip ratio.
Data shown as partial correlation coefficient (p value) adjusted for age, and pubertal stage in children.
Logtransformed.
Additional adjustment on height.
All models are also adjusted for a random effect nuclear family variable, for gender and interactions between gender and covariates (age and pubertal stage).
The strength of the relationship between baseline leptin and all adiposity markers at follow-up did not differ significantly between genders in adults and children. Therefore in the current analysis, we grouped genders in adults and children. In adults and children, BMI or bioimpedance % fat at follow-up were no associated with baseline leptin, after adjustment for the corresponding adiposity variable. High baseline plasma leptin predicted an increase in the hip circumference in adults (0.20 (<0.0001)) and children (0.22 (<0.0001)). In adults only, high baseline plasma leptin predicted an increase (rp (p value)) in the sum of the four skinfolds (0.18 (<0.0001)), the waist circumference (0.16 (0.0003)) and the waist-to-hip ratio (0.29 (<0.0001)).
After concomitant adjustment for baseline BIA Fat%, skinfolds waist and hip circumference, height, only SSK at follow-up was positively correlated with baseline leptin in adults (rp = 0.15; p = 0.001), with similar tendency although not significant in children (rp = 0.08; p = 0.13). The relationships between baseline leptin and SSK at follow-up were not substantially modified after an additional adjustment for baseline energy intake, alcohol consumption, ambulatory activity and LTPA (not shown).
Metabolic, anthropometric, and lifestyle characteristics of adiposity-related leptin profiles (Table 4)
Table 4.
Baseline characteristics in subjects with high or low leptin levels with reference to baseline level of adiposity
| Low leptin | High leptin | P | |
| Plasma fasting insulin (pmol l-1) | |||
| Adult men | 22 ± 16 | 29 ± 17 | 0.0005 |
| Adult women | 23 ± 14 | 27 ± 17 | 0.03 |
| Boys | 31 ± 17 | 38 ± 22 | 0.01 |
| Girls | 35 ± 19 | 42 ± 23 | 0.04 |
| HOMAIR | |||
| Adult men | 0.79 ± 0.66 | 1.12 ± 0.71 | <0.0001 |
| Adult women | 0.82 ± 0.58 | 0.96 ± 0.67 | 0.03 |
| Boys | 1.1 ± 0.68 | 1.4 ± 0.92 | 0.01 |
| Girls | 1.23 ± 0.69 | 1.5 ± 0.91 | 0.06 |
| Energy intake (MJ day-1) | |||
| Adult men | 13.2 ± 3.8 | 13.4 ± 4.8 | 0.84 |
| Adult women | 10.5 ± 3.3 | 10.4 ± 3.3 | 0.76 |
| Boys | 13.7 ± 5.9 | 12.8 ± 5.4 | 0.25 |
| Girls | 10.5 ± 4.2 | 10.2 ± 3.7 | 0.61 |
| Steps (10-3 wk-1) | |||
| Adult men | 61.7±23.4 | 52.4±2.5 | 0.005 |
| Adult women | 50.0±18.7 | 50.3±19.8 | 0.91 |
| Boys | 64.2± 31.8 | 63.7±25.1 | 0.89 |
| Girls | 54.7±21.4 | 51.6±18.9 | 0.30 |
| Leisure time physical activity (h wk-1) | |||
| Adult men | 4.6±4.3 | 5.4±7.2 | 0.34 |
| Adult women | 3.2±3.6 | 3.5±5.0 | 0.58 |
| Boys | 8.2±6.8 | 5.9±4.5 | 0.002 |
| Girls | 4.8±4.1 | 3.2±2.7 | 0.002 |
Low and high leptin levels were determined by a median split of the residuals from a linear model estimating mean baseline level of leptin according to the SSK, WC and HC, BIA % fat, age and pubertal stage at baseline. The model included a nuclear family variable as a random effect.
Data shown as ± s.d.
We divided subjects into two groups according to sex and age specific low and high baseline concentration of plasma leptin adjusted for a set of four baseline adiposity variables (BIA % fat, SSK, WC, HC). At baseline, as expected all anthropometric characteristics were similar in the low and high leptin groups. In adults as in children, plasma insulin and the HOMAIR were significantly higher in the high leptin group.
Leisure time physical activity (h.wk−1) was significantly higher in the low leptin group than in the high leptin group, in boys and in girls but not in adults. Ambulatory activity (steps.10−3.wk−1) was significantly higher in the low leptin group than in the high leptin group, in adult men only (61.7 ± 23.4 versus 52.4±22.5; p=0.005)
At follow-up, men (p<0.01) and boys (p<0.05) in the high leptin group increased significantly more their skinfolds thickness (Figure) while no difference was observed for the gain in other adiposity variables or weight. Although the corresponding relationships were not significant in women and girls, they did not differ significantly between men and women (p=0.20), or boys and girls (p=0.15). Biological measures were not available at follow-up to assess changes in these variables.
Figure.
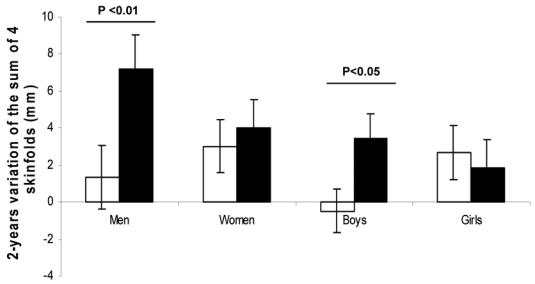
Variation of the sum of four skinfolds from baseline to follow-up, in groups of high (black bars) or low (white bars) leptin relative to adiposity at baseline. Results are the means ± standard error.
Discussion
In this study, high plasma leptin predicted adiposity increase over time in non-obese children and adults. This predictive effect was mainly observed for subcutaneous adiposity, although the same trend was seen with WC and BIA % fat in males. Two studies reported that high plasma leptin predicts in adults an increase in visceral adiposity, independently of subcutaneous abdominal fat accumulation (Tong et al, 2005), and a worsening of some of the metabolic syndrome features independently of the BMI (Franks et al, 2005). Although we have identified a positive relationship between WHR or WC changes and baseline leptin concentration, our results did not confirm these findings after taking into account concomitanly several markers of fat amount and distribution. They rather suggest that the relationship between plasma leptin and visceral adiposity changes may be confounded by the amount of peripheral subcutaneous fat. In agreement with our findings, Folsom et al. (Folsom et al, 1999) and Hodge et al. (Hodge et al, 1998) were unable to predict weight changes from baseline leptin level relative to baseline BMI in large adult population-based cohorts. Because BMI is a composite measurement, it has been criticized as not being a relevant indicator to assess changes in adiposity associated with leptin (Jenkins & Campbell, 2003). Indeed, in 85 children followed for at least 3 years, Johnson et al. (Johnson et al, 2001) demonstrated positive relationships between baseline leptin adjusted for baseline fat mass and changes in fat mass as assessed by dual X-ray absorptiometry. Chessler et al. (Chessler et al, 1998) showed similar results in 574 adults of both genders, using computed-tomography to assess changes in fat mass over 5 years. It is of note that only three studies, all conducted on small samples (12 to 36 subjects), found inverse relationships between leptin and adiposity changes (Ravussin et al, 1997; Byrnes et al, 1999; Ahmed et al, 2001). In addition, one of these studies (Ravussin et al, 1997) was performed in severely obese young subjects (mean BMI over 34 kg.m−2). After adjusting for baseline percent body fat, baseline leptin was significantly lower in weight gainers compared to weight-stable subjects in this study. Primary or secondary alteration in the regulation of the OB gene may explain these results in obese subjects. Jenkins et al. (Jenkins et al, 2001; Jenkins & Campbell, 2003) suggested that different associations among studies may be explained by differences in methods of adjustment for the variability in body composition. They argued that imperfect adjustments for baseline body fat are responsible for spurious positive association between baseline leptin and weight gain. They demonstrated that correct adjustment for body composition was achieved by incorporating height as well as fat mass in the models. In the last part of our analysis, we have simultaneously adjusted for baseline height and several total and regional adiposity markers and showed that high relative leptin was still predictive of subcutaneous fat gain. Therefore we do not believe that imperfect adjustment for initial body composition may have biased substantially our results which provide evidence that, in non-obese subjects, an excess of plasma leptin may predict adiposity gain over time, particularly in the subcutaneous location.
Animal models as well as rare cases of human obesity related with mutations in the OB gene, suggest that one of the characteristics of leptin may be to protect against excessive adiposity gain. The fact that leptin concentrations are raised in a majority of obese subjects has been interpreted as reflecting resistance to leptin (Considine et al, 1996). However it has also been argued that this relationship was not clear evidence of resistance to leptin but rather an adaptative attempt to oppose other forces promoting obesity (Arch et al, 1998). Excess leptin may provide indirect information about behavioral aspects related to adiposity gain such as a lack of physical activity, overfeeding (Kolaczynski et al, 1996) or alcohol consumption (Mantzoros et al, 1998). Our results show a negative association between plasma leptin and physical activity. However, the correlation between leptin and adiposity change was not substantially modified after adjustment for ambulatory activity, LTPA, alcohol consumption or energy intake. However the measure of physical activity, and assessment of energy intake by a questionnaire, indeed provide only an approximate and partial estimation of the energy balance.
Another hypothesis is that resistance to leptin may be present in some non-obese subjects in whom it would predict subsequent adiposity gain. According to Banks et al. (Banks, 2004) three mechanisms may incur central resistance to leptin: blood-brain barrier transport failure, leptin receptor/post-receptor failure and failure of down-stream neuronal circuitry. Our location specific results are not in favor of central leptin resistance as a potential explanation for our finding. Finally, one can also hypothesize a paracrine effect of leptin promoting the development of subcutaneous adipose tissue. Indeed, the stroma of this tissue includes vascular cells and resident macrophages, important targets for leptin which promotes recruitment of circulating monocytes and upregulates adhesion molecules in the endothelial cells (Curat et al, 2004). Angiogenesis is involved in the development of adipose tissue (Hausman & Richardson, 2004) and leptin has a proangiogenic activity (Artwohl et al, 2002) and a vasodilating effect (Nakagawa et al, 2002) in humans, and promotes vascular remodelling in mice (Schafer et al, 2004). However why such a paracrine effect would be seen predominantly in males remains unclear. In conclusion, we have shown that a high leptin relative to baseline fat mass predicts fat mass gain over time, mainly in the subcutaneous location. The clinical implications of these results cannot be inferred from our study. However further studies investigating the role of leptin in fat mass development and its metabolic consequences, should take into account subcutaneous fat mass as accurately as possible.
Acknowledgments
We thank the directors and teachers of the schools who made the study possible. MA Charles received grants from the French Speaking Association for the Study of Diabetes and Metabolism (ALFEDIAM) and from the Mutuelle Générale de PEducation Nationale (MGEN).
References
- 1.Aaron DJ, Kriska AM, Dearwater SR, Cauley JA, Metz KF, LaPorte RE. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am J Epidemiol. 1995;142:191–201. doi: 10.1093/oxfordjournals.aje.a117618. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed ML, Ong KK, Watts AP, Morrell DJ, Preece MA, Dunger DB. Elevated leptin levels are associated with excess gains in fat mass in girls, but not boys, with type 1 diabetes: longitudinal study during adolescence. J Clin Endocrinol Metab. 2001;86:1188–1193. doi: 10.1210/jcem.86.3.7320. [DOI] [PubMed] [Google Scholar]
- 3.Arch JR, Stock MJ, Trayhurn P. Leptin resistance in obese humans: does it exist and what does it mean? Int J Obes Relat Metab Disord. 1998;22:1159–1163. doi: 10.1038/sj.ijo.0800779. [DOI] [PubMed] [Google Scholar]
- 4.Arslanian S, Suprasongsin C, Kalhan SC, Drash AL, Brna R, Janosky JE. Plasma leptin in children: relationship to puberty, gender, body composition, insulin sensitivity, and energy expenditure. Metabolism. 1998;47:309–312. doi: 10.1016/s0026-0495(98)90262-1. [DOI] [PubMed] [Google Scholar]
- 5.Artwohl M, Roden M, Holzenbein T, Freudenthaler A, Waldhausl W, Baumgartner-Parzer SM. Modulation by leptin of proliferation and apoptosis in vascular endothelial cells. Int J Obes Relat Metab Disord. 2002;26:577–580. doi: 10.1038/sj.ijo.0801947. [DOI] [PubMed] [Google Scholar]
- 6.Banks WA. The many lives of leptin. Peptides. 2004;25:331–338. doi: 10.1016/j.peptides.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Bassett DR, Jr, Ainsworth BE, Swartz AM, Strath SJ, O’Brien WL, King GA. Validity of four motion sensors in measuring moderate intensity physical activity. Med Sci Sports Exerc. 2000;32:S471–480. doi: 10.1097/00005768-200009001-00006. [DOI] [PubMed] [Google Scholar]
- 8.Blum WF, Englaro P, Hanitsch S, Juul A, Hertel NT, Muller J, et al. Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. J Clin Endocrinol Metab. 1997;82:2904–2910. doi: 10.1210/jcem.82.9.4251. [DOI] [PubMed] [Google Scholar]
- 9.Byrnes SE, Baur LA, Bermingham M, Brock K, Steinbeck K. Leptin and total cholesterol are predictors of weight gain in pre-pubertal children. Int J Obes Relat Metab Disord. 1999;23:146–150. doi: 10.1038/sj.ijo.0800783. [DOI] [PubMed] [Google Scholar]
- 10.Chessler SD, Fujimoto WY, Shofer JB, Boyko EJ, Weigle DS. Increased plasma leptin levels are associated with fat accumulation in Japanese Americans. Diabetes. 1998;47:239–243. doi: 10.2337/diab.47.2.239. [DOI] [PubMed] [Google Scholar]
- 11.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. Bmj. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 13.Curat CA, Miranville A, Sengenes C, Diehl M, Tonus C, Busse R, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–1292. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- 14.Dallongeville J, Hecquet B, Lebel P, Edme JL, Le Fur C, Fruchart JC, et al. Short term response of circulating leptin to feeding and fasting in man: influence of circadian cycle. Int J Obes Relat Metab Disord. 1998;22:728–733. doi: 10.1038/sj.ijo.0800648. [DOI] [PubMed] [Google Scholar]
- 15.de Lauzon B, Romon M, Deschamps V, Lafay L, Borys JM, Karlsson J, et al. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J Nutr. 2004;134:2372–2380. doi: 10.1093/jn/134.9.2372. [DOI] [PubMed] [Google Scholar]
- 16.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 17.Folsom AR, Jensen MD, Jacobs DR, Jr, Hilner JE, Tsai AW, Schreiner PJ. Serum leptin and weight gain over 8 years in African American and Caucasian young adults. Obes Res. 1999;7:1–8. doi: 10.1002/j.1550-8528.1999.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 18.Franks PW, Brage S, Luan J, Ekelund U, Rahman M, Farooqi IS, et al. Leptin predicts a worsening of the features of the metabolic syndrome independently of obesity. Obes Res. 2005;13:1476–1484. doi: 10.1038/oby.2005.178. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Merino D, Chennaoui M, Drogou C, Bonneau D, Guezennec CY. Decrease in serum leptin after prolonged physical activity in men. Med Sci Sports Exerc. 2002;34:1594–1599. doi: 10.1097/00005768-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925–934. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 21.Hodge AM, de Courten MP, Dowse GK, Zimmet PZ, Collier GR, Gareeboo H, et al. Do leptin levels predict weight gain?--A 5-year follow-up study in Mauritius. Mauritius Non-communicable Disease Study Group. Obes Res. 1998;6:319–325. doi: 10.1002/j.1550-8528.1998.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins AB, Campbell LV. Does relative leptinemia predict weight gain in humans? Obes Res. 2003;11:373–374. doi: 10.1038/oby.2003.49. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins AB, Samaras K, Gordon MA, Snieder H, Spector T, Campbell LV. Lack of heritability of circulating leptin concentration in humans after adjustment for body size and adiposity using a physiological approach. Int J Obes Relat Metab Disord. 2001;25:1625–1632. doi: 10.1038/sj.ijo.0801802. [DOI] [PubMed] [Google Scholar]
- 24.Johnson MS, Huang TT, Figueroa-Colon R, Dwyer JH, Goran MI. Influence of leptin on changes in body fat during growth in African American and white children. Obes Res. 2001;9:593–598. doi: 10.1038/oby.2001.78. [DOI] [PubMed] [Google Scholar]
- 25.Kettaneh A, Heude B, Lommez A, Borys JM, Ducimetiére P, Charles MA. Reliability of bioimpedance analysis compared with other adiposity measurements in children: The FLVS II Study. Diabetes Metab. 2005;31:534–541. doi: 10.1016/s1262-3636(07)70228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolaczynski JW, Ohannesian JP, Considine RV, Marco CC, Caro JF. Response of leptin to short-term and prolonged overfeeding in humans. J Clin Endocrinol Metab. 1996;81:4162–4165. doi: 10.1210/jcem.81.11.8923877. [DOI] [PubMed] [Google Scholar]
- 27.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 28.Lafay L, Basdevant A, Charles MA, Vray M, Balkau B, Borys JM, et al. Determinants and nature of dietary underreporting in a free-living population: the Fleurbaix Laventie Ville Sante (FLVS) Study. Int J Obes Relat Metab Disord. 1997;21:567–573. doi: 10.1038/sj.ijo.0800443. [DOI] [PubMed] [Google Scholar]
- 29.Lissner L, Karlsson C, Lindroos AK, Sjostrom L, Carlsson B, Carlsson L, et al. Birth weight, adulthood BMI, and subsequent weight gain in relation to leptin levels in Swedish women. Obes Res. 1999;7:150–154. doi: 10.1002/j.1550-8528.1999.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 30.Mantzoros CS, Liolios AD, Tritos NA, Kaklamani VG, Doulgerakis DE, Griveas I, et al. Circulating insulin concentrations, smoking, and alcohol intake are important independent predictors of leptin in young healthy men. Obes Res. 1998;6:179–186. doi: 10.1002/j.1550-8528.1998.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa K, Higashi Y, Sasaki S, Oshima T, Matsuura H, Chayama K. Leptin causes vasodilation in humans. Hypertens Res. 2002;25:161–165. doi: 10.1291/hypres.25.161. [DOI] [PubMed] [Google Scholar]
- 34.Perusse L, Collier G, Gagnon J, Leon AS, Rao DC, Skinner JS, et al. Acute and chronic effects of exercise on leptin levels in humans. J Appl Physiol. 1997;83:5–10. doi: 10.1152/jappl.1997.83.1.5. [DOI] [PubMed] [Google Scholar]
- 35.Ravussin E, Pratley RE, Maffei M, Wang H, Friedman JM, Bennett PH, et al. Relatively low plasma leptin concentrations precede weight gain in Pima Indians. Nat Med. 1997;3:238–240. doi: 10.1038/nm0297-238. [DOI] [PubMed] [Google Scholar]
- 36.Romon M, Lafay L, Bresson JL, Oppert JM, Borys JM, Kettaneh A, et al. Relationships between physical activity and plasma leptin levels in healthy children: the Fleurbaix-Laventie Ville Sante II Study. Int J Obes Relat Metab Disord. 2004 doi: 10.1038/sj.ijo.0802725. [DOI] [PubMed] [Google Scholar]
- 37.Saad MF, Riad-Gabriel MG, Khan A, Sharma A, Michael R, Jinagouda SD, et al. Diurnal and ultradian rhythmicity of plasma leptin: effects of gender and adiposity. J Clin Endocrinol Metab. 1998;83:453–459. doi: 10.1210/jcem.83.2.4532. [DOI] [PubMed] [Google Scholar]
- 38.Savoye M, Dziura J, Castle J, DiPietro L, Tamborlane WV, Caprio S. Importance of plasma leptin in predicting future weight gain in obese children: a two-and-a-half-year longitudinal study. Int J Obes Relat Metab Disord. 2002;26:942–946. doi: 10.1038/sj.ijo.0802018. [DOI] [PubMed] [Google Scholar]
- 39.Schafer K, Halle M, Goeschen C, Dellas C, Pynn M, Loskutoff DJ, et al. Leptin promotes vascular remodeling and neointimal growth in mice. Arterioscler Thromb Vase Biol. 2004;24:112–117. doi: 10.1161/01.ATV.0000105904.02142.e7. [DOI] [PubMed] [Google Scholar]
- 40.Tanner JM. The assessment of growth and development in children. Arch Dis Child. 1952;27:10–33. doi: 10.1136/adc.27.131.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong J, Fujimoto WY, Kahn SE, Weigle DS, McNeely MJ, Leonetti DL, et al. Insulin, C-peptide, and leptin concentrations predict increased visceral adiposity at 5- and 10-year follow-ups in nondiabetic Japanese Americans. Diabetes. 2005;54:985–990. doi: 10.2337/diabetes.54.4.985. [DOI] [PubMed] [Google Scholar]
- 42.van Harmelen V, Dicker A, Ryden M, Hauner H, Lonnqvist F, Naslund E, et al. Increased lipolysis and decreased leptin production by human omental as compared with subcutaneous preadipocytes. Diabetes. 2002;51:2029–2036. doi: 10.2337/diabetes.51.7.2029. [DOI] [PubMed] [Google Scholar]
- 43.van Rossum CT, Hoebee B, van Baak MA, Mars M, Saris WH, Seidell JC. Genetic variation in the leptin receptor gene, leptin, and weight gain in young Dutch adults. Obes Res. 2003;11:377–386. doi: 10.1038/oby.2003.51. [DOI] [PubMed] [Google Scholar]
- 44.Vuillemin A, Oppert JM, Guillemin F, Essermeant L, Fontvieille AM, Galan P, et al. Self-administered questionnaire compared with interview to assess past-year physical activity. Med Sci Sports Exerc. 2000;32:1119–1124. doi: 10.1097/00005768-200006000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Zimmet P, Hodge A, Nicolson M, Staten M, de Courten M, Moore J, et al. Serum leptin concentration, obesity, and insulin resistance in Western Samoans: cross sectional study. Bmj. 1996;313:965–969. doi: 10.1136/bmj.313.7063.965. [DOI] [PMC free article] [PubMed] [Google Scholar]


