Abstract
Life-history traits such as offspring size, number and sex ratio are affected by maternal feeding rates in many kinds of animals, but the consequences of variation in maternal diet quality (rather than quantity) are poorly understood. We manipulated dietary quality of reproducing female lizards (Amphibolurus muricatus; Agamidae), a species with temperature-dependent sex determination, to examine strategies of reproductive allocation. Females maintained on a poor-quality diet produced fewer clutches but massively (twofold) larger eggs with lower concentrations of yolk testosterone than did conspecific females given a high-quality diet. Although all eggs were incubated at the same temperature, and yolk steroid hormone levels were not correlated with offspring sex, the nutrient-deprived females produced highly male-biased sex ratios among their offspring. These responses to maternal nutrition generate a link between sex and offspring size, in a direction likely to enhance maternal fitness if large body size enhances reproductive success more in sons than in daughters (as seems plausible, given the mating system of this species). Overall, our results show that sex determination in these animals is more complex, and responsive to a wider range of environmental cues, than that suggested by the classification of ‘environmental sex determination’.
Keywords: Amphibolurus muricatus, dietary quality, maternal effects, sex ratio, temperature-dependent sex determination, yolk steroid hormones
1. Introduction
Life-history traits such as offspring size and number vary enormously both within and among species (Roff 2002). At an intraspecific level that variation is tightly linked to individual survival and reproductive success (and hence, paternal and especially maternal fitness) and is thus expected to be under strong selection (Sinervo et al. 1992; Kaplan 1998). However, such traits also are very flexible even within the lifetime of a single female; for example, shifts in maternal nutrition can massively alter the frequency of reproduction, total reproductive output and the ways in which that output is partitioned (e.g. into many small versus a few large offspring and relative numbers and sizes of sons versus daughters; Price 1998; Kudo & Nakahira 2005). Hence, rather than producing highly canalized life histories, selection generally fashions norms of reaction that link environmental variation to reproductive variation (Kaplan & Cooper 1984). Quantifying the form of such links is a major challenge for evolutionary ecologists (Doughty & Reznick 2004). Although correlational studies can be useful in this respect (e.g. Madsen & Shine 1996), experimental manipulation of resource levels provides the strongest evidence for causal links between maternal nutrition and reproductive output (Meijer & Langer 1995; Kudo & Nakahira 2005; Du 2006).
Most previous work on this topic has focused on the amounts of food available for reproducing females and documented strong maternal-allocation responses to experimental manipulation of resource quantity in ways likely to influence both maternal and offspring fitness (Meijer & Langer 1995; Selman & Houston 1996; Williams 1996; Rutstein et al. 2005). As well as the more obvious traits, such as offspring size and number, food availability also can influence patterns of sex allocation (relative investment into sons versus daughters; Rosenfeld & Roberts 2004; Robertson et al. 2006). Theoretical models predict that mothers will differentially allocate resources to the sexes when the fitness returns from producing sons differ from those gained by producing daughters (Trivers & Willard 1973). These relative fitness returns will often depend upon environmental situations, and accordingly, mothers in such situations tend to allocate more resources to the sex that benefits most from the present conditions (Komdeur 1996; Nager et al. 1999; Kalmbach et al. 2001). Such differential allocation of resources can be manifested in two different ways: mothers can either (i) produce biased offspring sex ratios (Clutton-Brock et al. 1984; Dittus 1998; Kalmbach et al. 2001) or (ii) they can produce balanced sex ratios, but invest more energy into individual offspring of one sex than the other (Velando 2002).
Although flexible resource-driven sex allocation strategies appear to be widespread (Appleby et al. 1997; Rosenfeld & Roberts 2004; Robertson et al. 2006), one important aspect of dietary variation largely has been neglected in previous studies. Natural environments vary through space and time not only in the quantity of food available to a reproducing female, but also in the quality of that food (Seigel & Fitch 1985). Does variation in nutritional quality, not simply in total food intake, drive corresponding variation in maternal reproductive tactics? This question is particularly interesting for a species in which offspring sex is labile, depending upon incubation conditions, rather than fixed by genetic inheritance; such labile systems might be more likely to exhibit adaptive sex-allocation shifts. Accordingly, we conducted experiments to explore the effects of dietary quality on maternal reproductive allocation in a lizard species with environmental sex determination. More specifically, we set out to (i) determine the effect of dietary quality (during the reproductive season) on maternal reproductive output, (ii) explore the ability of females to differentially allocate resources to male versus female offspring in response to variation in diet quality, (iii) evaluate the influence of maternal dietary quality on other phenotypic traits of offspring, and (iv) evaluate whether any differential sex allocation was mediated via adjustment of yolk hormone levels.
The jacky dragon (Amphibolurus muricatus) is a common agamid lizard found in coastal heathland habitat of southeastern Australia. This species provides an excellent model for addressing the above issues because (i) jacky dragons have a long reproductive season (October–February) and females produce 3–4 clutches of eggs within this period (Harlow & Taylor 2000). Hence, female jacky dragons likely are exposed to fluctuations in resource quality within as well as among seasons. (ii) Jacky dragons fuel reproduction with recently ingested food rather than stored energy reserves (Warner et al. unpublished data). Hence, manipulations to dietary quality during the reproductive season should have immediate effects. (iii) Jacky dragons have temperature-dependent sex determination (TSD), whereby the sex of the offspring is determined by egg incubation temperatures (Harlow & Taylor 2000; Warner & Shine 2005). Thus, if females modify sex allocation in response to diet, they could do so either by selecting nest sites with specific thermal regimes (St. Juliana et al. 2004) or by differentially allocating steroid hormones into egg yolks (Bowden et al. 2000; Lovern & Wade 2003) in order to overproduce the sex that provides the greatest fitness returns.
2. Material and methods
(a) Lizard housing and husbandry
We captured adult male and female jacky dragons in the Sydney region during the austral spring and summer of 2003/2004. These lizards were maintained in large (2 m long×2 m wide×1 m tall) outdoor enclosures for 1 year prior to our experimental manipulations. Enclosures contained sand substrate and several branches for perching and basking. Natural vegetation and cover boards provided the animals with shelter. Three lizards were housed per enclosure (one male with two females). Animals were fed roaches and crickets (dusted with vitamin/calcium mix) thrice a week.
Immediately after the overwinter period (Spring 2004), lizards were randomly assigned to either a low- or high-quality diet treatment group. The low-quality diet group (n=15 females) was maintained on a diet consisting of crickets that had been fed only on corn for one month prior to being offered to the jacky dragons. Lizards in the high-quality diet group (n=28) were maintained on a diet consisting of crickets and roaches that were primarily raised on cat food, but also were given apples, carrots and several types of leafy greens. Lizards were fed thrice a week and the overall quantity of food (in terms of number of insects) given to the lizards in the two treatments did not differ. Females were weighed and measured (snout–vent length, SVL and tail length, TL) at the beginning and end of the reproductive season (September 2004 and February 2005, respectively).
(b) Egg incubation and hatchling phenotypes
We monitored captive dragons regularly for signs of nesting, and collected and weighed all eggs within 24 h after oviposition. We then extracted a small sample of yolk from half of the eggs from each clutch using a sterile syringe with a 24 gauge needle. Yolk removal reduced egg mass by an average of 12.8% (s.d.=8.7). Yolk samples were then freeze-dried overnight and stored at −80°C until hormone analysis. All the eggs were placed individually in glass jars (125 ml) filled with vermiculite and incubated at standardized hydric (−200 kPa water potential) and thermal conditions (constant 28°C, a temperature that produces a 50 : 50 sex ratio; Harlow & Taylor 2000). Eggs were placed in one of three incubators, and rotated within and among the incubators thrice a week to minimize any potential effects of thermal variation among and within incubators. Yolk removal had no effect on hatching success (χ2=1.1, p=0.29; overall hatching success=91%, N=418 eggs from 82 clutches).
After eggs hatched, all hatchlings were measured (SVL and TL), weighed and sexed by hemipenis eversion. This method for sexing hatchling lizards has been verified by gonadal histology, laparoscopy and dissection (Harlow 1996). All hatchlings were individually marked by toe-clipping and then housed in large outdoor enclosures (1.3 m long×0.75 m wide×0.55 m deep) containing sand substrate with branches for perching and basking, and small tiles for shelter. Water was always available, and hatchlings were fed crickets and roaches (dusted in vitamin and mineral mix) thrice a week. No more than 15 hatchlings were housed together at a single time. Hatchlings were kept under these conditions for two weeks prior to being released in the field; at this point, the hatchlings were remeasured (SVL and TL) and weighed in order to calculate individual growth rates.
(c) Yolk hormone analyses
Concentrations of testosterone (T), 17β-oestradiol (E2) and corticosterone (CORT) in freeze-dried yolk samples were measured by radioimmunoassay (RIA) following extraction and chromatographic separation (Wingfield & Farner 1975; Schwabl 1993). Samples were mixed thoroughly prior to removing a 3–12 mg subsample (recorded to the nearest 0.5 mg for each sample) and reconstituted in 0.5 ml of ddH2O in 1.5 ml microcentrifuge tubes. We equilibrated samples overnight at 4°C with 1000 c.p.m. of 3H–T (NET-370, 70 Ci mmol−1), 3H–E2 (NET-317, 72 Ci mmol−1) and 1000 c.p.m. of 3H–CORT (NET-399, 71 Ci mmol−1) from Perkin Elmer Life Sciences, Inc., for individual recovery determinations.
For extraction, samples were transferred to 16×100 mm glass culture tubes; each microcentrifuge tube was rinsed with 0.5 ml of ddH2O and this was added to the culture tubes as well. We extracted the samples twice with 4 ml petroleum ether : diethyl ether (30 : 70 v/v), dried the extracts under nitrogen gas in a 37°C water bath and reconstituted them in 1 ml 90% ethanol. The samples were stored at −20°C overnight and centrifuged at 2000 r.p.m. at 0°C for 5 min. The supernatant was transferred to clean test tubes and dried under nitrogen gas in a 37°C water bath and reconstituted in 500 μl of 10% ethyl acetate in isooctane.
To remove neutral lipids and to isolate T, E2 and CORT, all samples were transferred to diatomaceous Earth (celite, Sigma) columns for chromatographic separation. Columns consisted of a celite : ethylene glycol : propylene glycol upper phase (4 : 1 : 1 m/v/v) and a celite : ddH2O (3 : 1 m/v) lower phase. Neutral lipids and dihydrotestosterone were eluted with isooctane and 10% ethyl acetate in isooctane, respectively, and discarded. T, E2 and CORT were eluted with 20, 40 and 52% ethyl acetate in isooctane, respectively, and saved. Samples were dried under nitrogen gas in a 37°C water bath, resuspended in phosphate buffer and placed overnight at 4°C.
Competitive binding RIAs were performed using the appropriate tritiated steroid tracer (see above) and antisera from Wien Laboratories for T (T-3003), Biogenesis for E2 (7010-2650) and Sigma–Aldrich for CORT (C8784). The standard curves ranged from 1.95 to 500 pg and were run in triplicate. Samples were run in duplicate, averaged and adjusted for individual recovery and initial sample mass. Average recoveries were 63, 60 and 46% for T, E2 and CORT, respectively. We randomized the samples across six assays. We stopped assaying E2 after three assays, as the majority of samples contained non-detectable amounts of this steroid. The average intra-assay CV was 10.0% for T and 9.7% for CORT, and the inter-assay CV was 7.4% for T and 13.6% for CORT.
(d) Data analyses
Statistical analyses were carried out with SAS software (v. 9.1, SAS Institute 1997). All variables were checked for normality and homogeneity of variances. When necessary, data were log- or square-root transformed to meet the assumptions of parametric analyses. If variables could not be normalized by transformation, we used non-parametric analyses.
To evaluate the effect of maternal diet on changes in maternal body condition and reproductive output, we used analysis of variance (ANOVA) and covariance (ANCOVA). Change in maternal body condition was calculated as the difference between body conditions (residual scores of regression of ln mass versus ln SVL) measured at the end versus beginning of the reproductive season. Reproductive output was defined in three ways: (i) the number of eggs produced per clutch, (ii) the number of clutches produced during the reproductive season, and (iii) the total number of eggs produced over the entire season. Maternal mass was used as a covariate when evaluating reproductive output. The effect of diet on egg mass was evaluated using clutch size as a covariate.
We used two factor mixed model ANOVAs and ANCOVAs to evaluate the effect of maternal diet, offspring sex and their interaction (fixed effects) on hatchling characteristics (dependent variables). For analyses of hatchling size (i.e. SVL and mass), egg mass was used as a covariate. For analyses of TL, SVL was a covariate. For analyses of hatchling condition, body mass was the dependent variable and SVL was the covariate. Hatchling growth rate was calculated as the change in body size (mass and SVL) over a two-week period divided by the number of days between measurements. For all analyses, maternal identity was defined as a random effect. These analyses were based upon mean trait values for each sex within each clutch to avoid psuedoreplication.
Since data on yolk hormone levels remained non-normally distributed even after transformation, the effect of diet on hormone levels was evaluated with Kruskal–Wallis tests; these analyses were based on mean trait values for each clutch to avoid pseudoreplication. Relationships between yolk hormone levels and offspring phenotypes were evaluated with correlation analyses, and sex differences in these relationships were evaluated with ANCOVA, using sex as the independent variable and hormone level as a covariate. Levels of oestradiol were extremely low or non-detectible in eggs from both treatments; thus, we did not consider oestradiol in any of our analyses.
Sex ratios were evaluated with generalized liner mixed models using the GLIMMIX procedure in SAS (Littell et al. 1996). Dependent variables included maternal diet, oviposition date, egg mass, testosterone levels, corticosterone levels and all higher order interactions in a single model. Clutch sex ratio, expressed as proportion sons, was the dependent variable. Maternal identity was defined as a random effect. The model contained a binomial error structure with a logit link function (Wilson & Hardy 2002). Analyses began with the full model including all interactions and we subsequently backwards eliminated factors, starting with higher order interactions, at p values of 0.25 (Quinn & Keough 2002). In the final model, significant effects were accepted at p≤0.05. Three clutches from the poor-quality diet treatment failed to hatch (one clutch desiccated and two were apparently not fertile), thus sex-ratio data were not obtained from these three clutches.
3. Results
(a) Effect of maternal diet on reproductive output
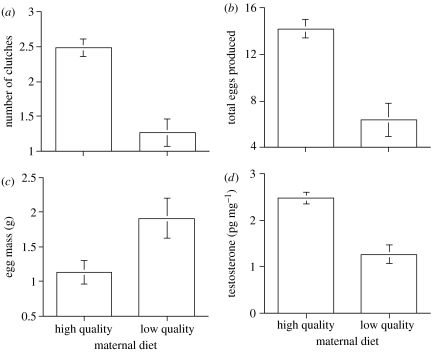
Dietary quality had a substantial effect on maternal body condition and reproductive output. The body condition of females fed the low-quality diet declined dramatically throughout the reproductive season (mean±s.e. change in maternal body condition =−2.35±0.82), whereas the condition of females on the high-quality diet increased (mean±s.e. change in maternal body condition =+0.74±0.64; F1,35=7.5, p=0.009). Females fed the high-quality diet produced more clutches over the reproductive season than did females on the low-quality diet (figure 1a), but the number of eggs per clutch (i.e. clutch size) did not differ between diet treatments (F1,33=3.6, p=0.067). Consequently, females given the high-quality diet produced 55% more eggs over the entire season than those on the low-quality diet (figure 1b). Although the poor-quality diet reduced reproductive output, females on this diet invested more energy per clutch. In other words, females on the low-quality diet produced heavier eggs (figure 1c) and an overall greater clutch mass (F1,33=3.3, p=0.005) than those fed the high-quality diet.
Figure 1.
Effect of maternal dietary quality on reproductive output, and egg characteristics, of jacky dragons. (a) Mean number of clutches produced by females (F1,25=22.3, p<0.001). (b) Mean total number of eggs produced by females (F1,33=23.3, p<0.001). (c) Mean egg size produced by females (F1,33=7.8, p=0.009). (d) Mean concentration of testosterone allocated toward egg yolk (χ2=5.7, p=0.020). Error bars represent 1 s.e.
Maternal diet also had a substantial effect on the timing of oviposition and hormone allocation into egg yolks. On average, females on the low-quality diet produced their first clutch much later than females on the high-quality diet (Kruskal–Wallis test: χ2=7.2, p=0.007). Consequently, the poor-quality diet reduced the reproductive season by an average of 25 days. Females fed the high-quality diet allocated more testosterone into their eggs than did females from the low-quality diet treatment (figure 1d), but the concentration of corticosterone in egg yolks did not differ between treatments (mean±s.e. for low- and high-quality diets =7.8±1.1 and 6.1±0.7 pg mg−1, respectively; Kruskal–Wallis test: χ2=1.6, p=0.20). Yolk hormone concentrations were not related to oviposition date (testosterone: r2=0.002, p=0.70; corticosterone r2=−0.005, p=0.53). Maternal diet did not affect egg survival (overall egg survival=91%; chi-square test: χ2=1.1, p=0.30).
(b) Effect of maternal diet on sex ratios
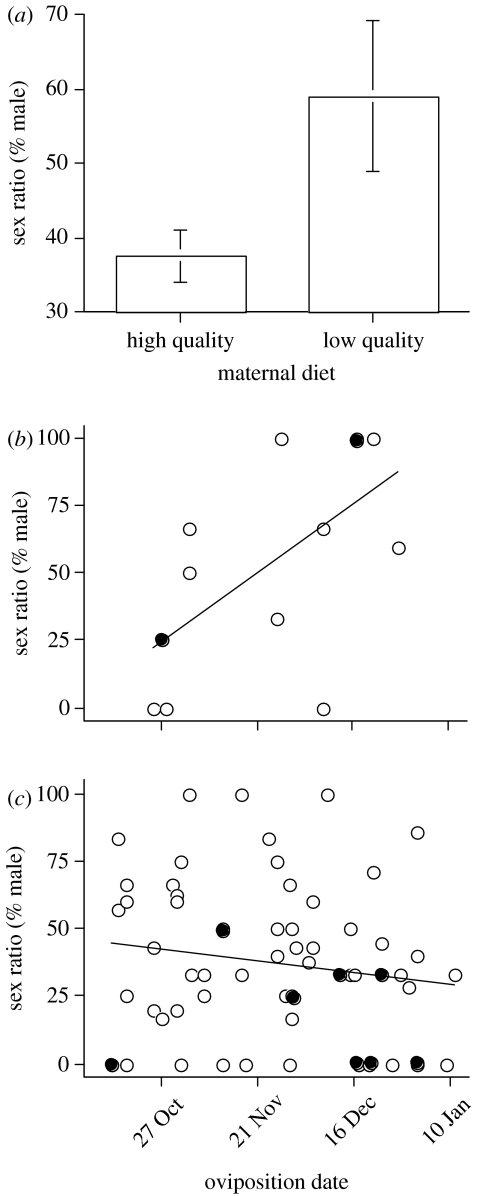
Offspring sex ratios were strongly affected by maternal diet, the date of oviposition and their interaction (table 1). In general, females fed the high-quality diet produced female-biased sex ratios and those on the low-quality diet produced male-biased sex ratios (figure 2a). Moreover, sex ratios shifted seasonally for females on the low-quality diet, with female-biased clutches early in the season, but male-biased clutches later in the season (figure 2b); clutches produced by females on the high-quality diet were slightly female-biased across the entire reproductive season (figure 2c). In support of these findings, the slopes of the relationship between sex ratio and oviposition dates differed between maternal diet treatments (ANCOVA: F1,75=10.8, p=0.002). Egg mass and yolk hormone concentrations were not associated with clutch sex ratios, although the significance of corticosterone levels was marginal (p=0.059; table 1); clutches with relatively high concentrations of corticosterone produced slightly more females.
Table 1.
Effects of diet, egg mass, oviposition date and yolk hormone levels on offspring sex ratios in the jacky dragon. (Analyses were carried out with a generalized linear mixed model with proportion males per clutch (i.e. clutch sex ratio) as the dependent variable. The fixed effects in the table represent the variables used in the final model after elimination of non-significant terms (p>0.25). See text for full description of the initial model. Asterisks denote statistical significance **p<0.01.)
| fixed effects in final model | statistics |
|---|---|
| diet | F1,33=9.91** |
| oviposition date | F1,33=8.27** |
| diet × oviposition date | F1,33=9.46** |
| egg mass | F1,33=2.48 |
| egg mass × diet | F1,33=1.67 |
| corticosterone | F1,33=3.82 |
| corticosterone × diet | F1,33=3.43 |
Figure 2.
Effect of diet and oviposition date on offspring sex ratios of jacky dragons. (a) Mean clutch sex ratios produced by females on the poor-quality diet (mean=59.1%male) and the high-quality diet (mean=37.6%male). Error bars represent 1 s.e. (b) Relationship between oviposition date and clutch sex ratios for females fed the low-quality diet (r2=0.394, p=0.016); 14 clutches are represented in this regression analysis. (c) Relationship between oviposition date and clutch sex ratios for females fed the high-quality diet (r2=−0.024, p=0.215); 65 clutches are represented in this regression analysis. Closed circles represent two overlapping data points. Additional statistics are reported in table 1.
(c) Effect of maternal diet and sex on offspring phenotypes
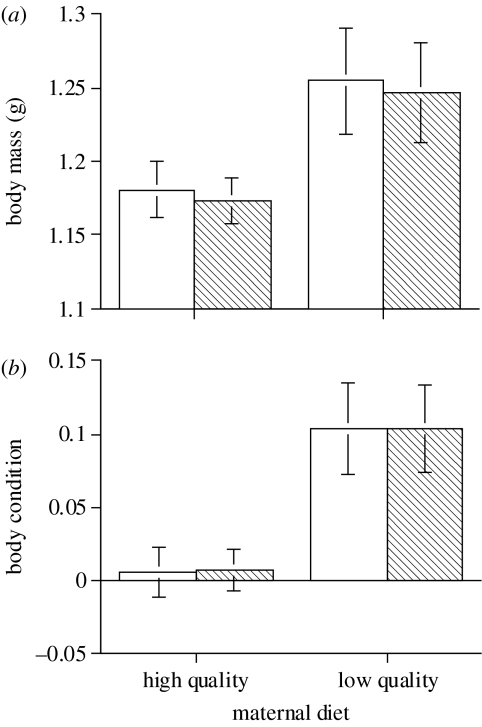
The only phenotypic traits significantly affected by maternal diet were hatchling size (SVL and mass) and body condition. These effects were statistically significant even when the data were corrected for egg size (table 2). Hatchlings produced by females on the low-quality diet were larger and in better body condition than those produced from females on the high-quality diet (figure 3). We found no significant sex differences in hatchling phenotypes, and maternal diet did not affect phenotypes of sons differently from those of daughters (as indicated by non-significant interaction terms; table 2). In addition, the levels of steroid hormones within egg yolks were not significantly associated with any phenotypic trait for either male or female offspring (all p>0.10). Only four hatchlings died during this study, thus offspring survival was not affected by maternal diet treatment (χ2=0.13, p=0.723) nor did survival differ between the sexes (χ2=0.69, p=0.406).
Table 2.
Effect of maternal diet, sex and their interaction on offspring phenotypes of the jacky dragon. (Analyses were carried out with mixed model analyses of variance or covariance using maternal identity as a random factor. SVL, snout–vent length. Asterisks denote statistical significance *p<0.05, **p<0.01.)
| phenotype | covariate | maternal diet effect | sex effect | interaction |
|---|---|---|---|---|
| incubation duration (days) | — | F1,91=0.1 | F1,91=1.1 | F1,91=0.4 |
| snout–vent length (mm) | egg mass (g) | F1,89=4.4* | F1,89=0.2 | F1,89=0.1 |
| tail length (mm) | SVL (mm) | F1,91=0.5 | F1,91=0.5 | F1,91=0.0 |
| body mass (g) | egg mass (g) | F1,89=10.3** | F1,89=0.0 | F1,89=0.8 |
| body mass (condition) | SVL (mm) | F1,91=7.9** | F1,91=0.0 | F1,91=0.9 |
| growth rate | ||||
| in SVL (Δ mm days−1) | — | F1,91=0.6 | F1,91=1.5 | F1,91=0.0 |
| in mass (Δ g days−1) | — | F1,91=3.6 | F1,91=0.1 | F1,91=0.4 |
Figure 3.
Effect of maternal dietary quality on mass (a) and body condition (b) of hatchling jacky dragons. Body condition is represented by the residual scores of the relationship between mass and snout–vent length. Error bars represent 1 s.e. Open bars represent male offspring and hatched bars represent female offspring. Statistics are reported in table 2.
4. Discussion
Several studies have demonstrated that diet quantity (i.e. maternal feeding rate) influences maternal reproductive output in reptiles (Ballinger 1977; Seigel & Fitch 1985; Du 2006), but the effects of dietary quality have been largely overlooked. Our experiment suggests that the quality of food eaten by reproducing females not only influences maternal reproductive output but also modifies the size and sex of offspring. Hence, the reaction norms that link maternal dietary quality to reproductive output may have important fitness implications.
The negative impacts of the low-quality diet on maternal condition and the number of clutches produced suggest that females in this treatment had little energy available for reproduction. Moreover, females on the low-quality diet did not begin reproduction until nearly one month after those on the high-quality diet had already produced their first clutch, probably because they needed more time to gather enough energy for clutch production in the face of low nutrient availability. However, although females on the poor diet produced fewer eggs overall, they produced much larger eggs than those maintained on the high-quality diet. Interestingly, this pattern remained significant even when analyses were adjusted for clutch size, indicating that investment per egg increased without a reduction in egg number. This pattern suggests that diet quality affects the way reproductive females allocate energy towards reproduction. In this respect, our results mirror those recently reported in insects (Fischer et al. 2006).
This lability suggests that strategies of maternal reproductive allocation can shift spatially or temporally depending upon the quality as well as quantity of available energy. For example, in years or habitats when diet quality is poor, females may invest more energy into individual clutches rather than producing multiple clutches. This strategy results in larger eggs and offspring, as indicated by our study. On the other hand, in years or habitats with an abundance of high-quality food, females will produce more clutches per season, but relatively small eggs. Thus, a female exposed to poor resources may make the best out of a suboptimal situation by allocating her energy towards producing fewer, better-quality offspring. Presumably, larger offspring size enhances survival prospects under poor resource conditions (Semlitsch & Gibbons 1990).
Maternal diet quality also influenced clutch sex ratios. Females maintained on the low-quality diet produced male-biased clutches, whereas those on the high-quality diet produced female-biased clutches. At first glance, these results appear to run counter to adaptive predictions of sex allocation theory, where ‘better quality’ females overproduce male offspring (i.e. the sex with the higher reproductive variance; Trivers & Willard 1973). However, if large hatchling body size (as produced by females on the poor diet) is more beneficial to sons than to daughters, then the patterns found in our study fit well with predictions from sex-allocation theory (Trivers & Willard 1973). Indeed, paternity analyses in jacky dragons show that large male size is associated with high reproductive success (D. A. Warner & R. Shine, unpublished work).
One fascinating complexity in the maternal responses involved seasonal shifts in offspring sex ratio: females on the low-quality diet produced female-biased clutches early in the season and male-biased clutches later in the season; no such pattern was evident in clutches produced by females maintained on the high-quality diet. The seasonal shift in sex ratio seen in offspring of our ‘low-diet-quality’ females may enhance maternal fitness, because previous work on this species suggests that early hatching benefits fitness of daughters more than that of sons (Warner & Shine 2005). Thus, the low reproductive output of ‘low-diet-quality’ females may be balanced by the relatively high fitness returns provided by their early hatched daughters. Why, then, did females on the high-quality diet not use the same strategy? We suggest that in nature, females in this latter situation can take advantage of their prolonged egg-laying period to manipulate offspring sex ratios via selection of thermally suitable nest-sites. This option is not available to the ‘low-diet-quality’ females because their relatively brief nesting period reduces the thermal diversity of nests available, potentially favouring some intrinsic (non-thermally driven) sex-ratio adjustment linked to season. Long-term field studies are needed to evaluate these ideas and especially to clarify how different patterns of sex-allocation affect maternal fitness.
Recent studies have shown that maternally derived steroid hormones in egg yolks can play a significant role in sex determination (Bowden et al. 2000; Lovern & Wade 2003; Love et al. 2005). However, sex allocation in response to maternal diet was not mediated by maternally derived steroid hormones in our jacky dragons. Although dietary quality affected the levels of testosterone that females allocated to their eggs, we found no direct association between testosterone concentrations in the egg yolks and clutch sex ratios. Similar effects of diet on yolk testosterone allocation have been described in the zebra finch (Taeniopygia guttata; Rutstein et al. 2005), but in contrast to our study, testosterone levels were associated with offspring sex in their study. However, corticosterone levels in the egg yolk of jacky dragons were marginally associated with offspring sex ratios (p=0.059)—a pattern shown in other reptiles (Sinervo & DeNardo 1996; D. Allsop, personal communication) and birds (Love et al. 2005; Pike & Petrie 2005). Clutches with relatively high concentrations of corticosterone produced slightly more females. In contrast to this pattern, however, previous studies on mammals suggest that stressed mothers overproduce sons, perhaps as a result of increased levels of circulating glucose, which has also been shown to affect sex ratios (Cameron 2004). Indeed, females in our poor-diet treatment appeared nutritionally stressed and overproduced sons.
Our results have important implications for the mechanisms involved in sex determination. Since jacky dragons have TSD, previous research predicts that clutch sex ratios shift over the season as a response to seasonal increases in ambient (and nest) temperatures (Harlow & Taylor 2000). The seasonal shifts in sex ratios predicted by these studies resemble the pattern produced by females fed the low-quality diet in our experiment. Interestingly, clutch sex ratios of females on the low-quality diet shifted seasonally despite all eggs being incubated at a constant temperature (28°C). In addition, we found no seasonal changes in the quantity of maternally derived hormones in the egg yolks, nor were steroid hormone levels associated with clutch sex ratios (albeit, corticosterone was marginal). Although past studies have emphasized the importance of sex steroid hormones in sex determination (Bowden et al. 2000; Lovern & Wade 2003), our work suggests that factors other than temperature and steroid hormones play important roles in sex determination in jacky dragons.
Current paradigms suggest a broad dichotomy of mechanisms that determine sex in reptiles. In other words, species have either genotypic sex determination (GSD) or temperature-dependent sex determination (TSD), and the presence of one mechanism excludes the other (Valenzuela et al. 2003). However, recent findings strongly challenge this paradigm, suggesting instead that an individual's sex is the outcome of complex interactions among genetic, physiological and environmental factors (Shine et al. 2002; Sarre et al. 2004). Indeed, multiple modes of sex determination have been discovered within single species of reptiles (Shine et al. 2002), fish (Conover & Heins 1987; Baroiller et al. 1995) and insects (Kozielska et al. 2006). The sex ratio patterns exhibited by the jacky dragons in our experiment fit well with this emerging paradigm shift, providing the first evidence that maternal diet can influence offspring sex in a reptile with TSD. The pathways by which diet affects sex determination remain obscure, but we predict that future research will reveal far greater complexity than embodied in current paradigms of sex determination in reptiles.
Acknowledgments
We thank D. Allsop, M. Elphick, H. Giragossyan, M. Hagman, T. Langkilde, R. Peters, B. Phillips, R. Radder, S. Ruggeri, T. Schwartz, J. Thomas, M. Thompson, D. Van Dyk and M. Wall for their assistance in the field and/or in the laboratory. D.A.W. was supported by an International Postgraduate Research Scholarship and by an International Postgraduate Award. Funding was provided by the Ecological Society of Australia, a James Kentley Memorial Scholarship (to D.A.W.) and the Australian Research Council (to R.S.). Lizards were collected under permit S10658 of the New South Wales National Parks Service. All protocols for this research were approved by the Animal Care and Ethics Committees at the University of Sydney (L04/12-2004/1/4018) and Macquarie University (2004/014).
References
- Appleby B.M, Petty S.J, Blakey J.K, Rainey P, MacDonald D.W. Does variation of sex ratio enhance reproductive success of offspring in tawny owls (Strix aluco)? Proc. R. Soc. B. 1997;264:1111–1116. doi:10.1098/rspb.1997.0153 [Google Scholar]
- Ballinger R.E. Reproductive strategies: food availability as a source of proximal variation in a lizard. Ecology. 1977;58:628–635. doi:10.2307/1939012 [Google Scholar]
- Baroiller F.J, Chourrout D, Fostier A, Jalabert B. Temperature and sex chromosomes govern sex ratios of the mouthbrooding cichlid fish Oreochromis niloticus. J. Exp. Zool. 1995;273:216–223. doi:10.1002/jez.1402730306 [Google Scholar]
- Bowden R.M, Ewert M.A, Nelson C.E. Environmental sex determination in a reptile varies seasonally and with yolk hormones. Proc. R. Soc. B. 2000;267:1745–1749. doi: 10.1098/rspb.2000.1205. doi:10.1098/rspb.2000.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron E.Z. Facultative adjustment of mammalian sex ratios in support of the Trivers–Willard hypothesis: evidence for a mechanism. Proc. R. Soc. B. 2004;271:1723–1728. doi: 10.1098/rspb.2004.2773. doi:10.1098/rspb.2004.2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T.H, Albon S.D, Guiness F.E. Maternal dominance, breeding success and birth sex ratios in red deer. Nature. 1984;308:358–360. doi:10.1038/308358a0 [Google Scholar]
- Conover D.O, Heins S.W. Adaptive variation in environmental and genetic sex determination in a fish. Nature. 1987;326:496–498. doi: 10.1038/326496a0. doi:10.1038/326496a0 [DOI] [PubMed] [Google Scholar]
- Dittus W.P.J. Birth sex ratios in toque macaques and other mammals: integrating the effects of maternal condition and competition. Behav. Ecol. Sociobiol. 1998;44:149–160. doi:10.1007/s002650050527 [Google Scholar]
- Doughty P, Reznick D.N. Patterns and analysis of adaptive phenotypic plasticity in animals. In: DeWitt T.J, Scheiner S.M, editors. Phenotypic plasticity: functional and conceptual approaches. Oxford University Press; Oxford, UK: 2004. pp. 126–150. [Google Scholar]
- Du W. Phenotypic plasticity in reproductive traits induced by food availability in a lacertid lizard, Takydromus sepentrionalis. Oikos. 2006;112:363–369. doi:10.1111/j.0030-1299.2006.13552.x [Google Scholar]
- Fischer K, Bot A.N.M, Brakefield P.M, Zwaan B.J. Do mothers producing large offspring have to sacrifice fecundity. J. Evol. Biol. 2006;19:380–391. doi: 10.1111/j.1420-9101.2005.01046.x. doi:10.1111/j.1420-9101.2005.01046.x [DOI] [PubMed] [Google Scholar]
- Harlow P.S. A harmless technique for sexing hatchling lizards. Herpetol. Rev. 1996;27:71–72. [Google Scholar]
- Harlow P.S, Taylor J.E. Reproductive ecology of the jacky dragon (Amphibolurus muricatus): an agamid lizard with temperature-dependent sex determination. Aust. Ecol. 2000;25:640–652. doi:10.1046/j.1442-9993.2000.01064.x [Google Scholar]
- Kalmbach R, Nager R.G, Griffiths R, Furness R.W. Increased reproductive effort results in male-biased offspring sex ratio: an experimental study in a species with reversed sexual size dimorphism. Proc. R. Soc. B. 2001;268:2175–2179. doi: 10.1098/rspb.2001.1793. doi:10.1098/rspb.2001.1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R.H. Maternal effects, developmental plasticity, and life history evolution: an amphibian model. In: Mousseau T.A, Fox C.W, editors. Maternal effects as adaptations. Oxford University Press; Oxford, UK: 1998. pp. 244–260. [Google Scholar]
- Kaplan R.H, Cooper W.S. The evolution of developmental plasticity in reproductive characteristics: an application of the “adaptive coin-flipping” principle. Am. Nat. 1984;123:393–410. doi:10.1086/284211 [Google Scholar]
- Komdeur J. Facultative sex ratio bias in the offspring of Seychelles warblers. Nature. 1996;263:661–666. [Google Scholar]
- Kozielska M, Pen I, Beukeboom L.W, Weissing F.J. Sex ratio selection and multi-factorial sex determination in the house fly: a dynamic model. J. Evol. Biol. 2006;19:879–888. doi: 10.1111/j.1420-9101.2005.01040.x. doi:10.1111/j.1420-9101.2005.01040.x [DOI] [PubMed] [Google Scholar]
- Kudo S, Nakahira T. Trophic-egg production in a subsocial bug: adaptive plasticity in response to resource conditions. Oikos. 2005;111:459–464. doi:10.1111/j.1600-0706.2005.14173.x [Google Scholar]
- Littell R.C, Milliken G.A, Stroup W.W, Wolfinger R.D. SAS Institute; Cary, NC: 1996. SAS system for mixed models. [Google Scholar]
- Love O.P, Chin E.H, Wynne-Edwards K.E, Williams T.D. Stress hormones: a link between maternal condition and sex-biased reproductive investment. Am. Nat. 2005;166:751–766. doi: 10.1086/497440. doi:10.1086/497440 [DOI] [PubMed] [Google Scholar]
- Lovern M.B, Wade J. Yolk testosterone varies with sex in eggs of the lizard, Anolis carolinensis. J. Exp. Zool. 2003;295A:206–210. doi: 10.1002/jez.a.10225. doi:10.1002/jez.a.10225 [DOI] [PubMed] [Google Scholar]
- Madsen T, Shine R. Determinants of reproductive output in female water pythons (Liasis fuscus: Pythonidae) Herpetologica. 1996;52:146–159. [Google Scholar]
- Meijer T, Langer U. Food availability and egg-laying of captive European starlings. Condor. 1995;97:718–728. [Google Scholar]
- Nager R.G, Monaghan P, Griffiths R, Houston D.C, Dawson R. Experimental demonstration that offspring sex ratio varies with maternal condition. Proc. Natl Acad. Sci. USA. 1999;96:570–573. doi: 10.1073/pnas.96.2.570. doi:10.1073/pnas.96.2.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike T.W, Petrie M. Offspring sex ratio is related to paternal train elaboration and yolk corticosterone in peafowl. Biol. Lett. 2005;1:204–207. doi: 10.1098/rsbl.2005.0295. doi:10.1098/rsbl.2005.0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price T. Maternal and paternal effects in birds: effects on offspring fitness. In: Mousseau T.A, Fox C.W, editors. Maternal effects as adaptations. Oxford University Press; Oxford, UK: 1998. pp. 202–226. [Google Scholar]
- Quinn G.P, Keough M.J. Cambridge University Press; Cambridge, UK: 2002. Experimental design and data analysis for biologists. [Google Scholar]
- Robertson B.C, Elliot G.P, Eason D.K, Clout M.N, Gemmel N.J. Sex allocation theory aids species conservation. Biol. Lett. 2006;2:229–231. doi: 10.1098/rsbl.2005.0430. doi:10.1098/rsbl.2005.0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff D.A. Sinauer Associates, Inc; Sunderland, MA: 2002. Life history evolution. [Google Scholar]
- Rosenfeld C.D, Roberts R.M. Maternal diet and other factors affecting offspring sex ratio: a review. Biol. Reprod. 2004;71:1063–1070. doi: 10.1095/biolreprod.104.030890. doi:10.1095/biolreprod.104.030890 [DOI] [PubMed] [Google Scholar]
- Rutstein A.N, Gilbert L, Slater P.J.B, Graves J.A. Sex-specific patterns of yolk androgen allocation depend on maternal diet in the zebra finch. Behav. Ecol. 2005;16:62–69. doi:10.1093/beheco/arh123 [Google Scholar]
- Sarre S.D, Georges A, Quinn A. The ends of continuum: genetic and temperature-dependent sex determination in reptiles. Bioessays. 2004;26:639–645. doi: 10.1002/bies.20050. doi:10.1002/bies.20050 [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS Institute, Inc; Cary, NC: 1997. SAS/STAT user's guide. [Google Scholar]
- Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc. Natl Acad. Sci. USA. 1993;90:11 446–11 450. doi: 10.1073/pnas.90.24.11446. doi:10.1073/pnas.90.24.11446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigel R.A, Fitch H.S. Annual variation in reproduction in snakes in a fluctuating environment. J. Anim. Ecol. 1985;54:497–505. doi:10.2307/4494 [Google Scholar]
- Selman R.G, Houston D.C. The effect of prebreeding diet on reproductive output in zebra finches. Proc. R. Soc. B. 1996;263:1585–1588. doi:10.1098/rspb.1996.0232 [Google Scholar]
- Semlitsch R.D, Gibbons J.W. Effects of egg size on success of larval salamanders in complex aquatic environments. Ecology. 1990;71:1789–1795. doi:10.2307/1937586 [Google Scholar]
- Shine R, Elphick M.J, Donnellan S. Co-occurrence of multiple, supposedly incompatible modes of sex determination in a lizard population. Ecol. Lett. 2002;5:486–489. doi:10.1046/j.1461-0248.2002.00351.x [Google Scholar]
- Sinervo B, DeNardo D.F. Costs of reproduction in the wild: path analysis of natural selection and experimental tests of causation. Evolution. 1996;50:1299–1313. doi: 10.1111/j.1558-5646.1996.tb02370.x. doi:10.2307/2410670 [DOI] [PubMed] [Google Scholar]
- Sinervo B, Doughty P, Huey R.B, Zamudio K. Allometric engineering: a causal analysis of natural selection on offspring size. Science. 1992;258:1927–1930. doi: 10.1126/science.258.5090.1927. doi:10.1126/science.258.5090.1927 [DOI] [PubMed] [Google Scholar]
- St. Juliana J.R, Bowden R.M, Janzen F.J. The impact of behavioural and physiological maternal effects on offspring sex ratio in the common snapping turtle, Chelydra serpentina. Behav. Ecol. Sociobiol. 2004;56:270–278. doi:10.1007/s00265-004-0772-y [Google Scholar]
- Trivers R.L, Willard D.E. Natural selection of parental ability of vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. doi:10.1126/science.179.4068.90 [DOI] [PubMed] [Google Scholar]
- Velando A. Experimental manipulation of maternal effort produces differential effects in sons and daughters: implications for adaptive sex ratios in the blue-footed booby. Behav. Ecol. 2002;13:443–449. doi:10.1093/beheco/13.4.443 [Google Scholar]
- Valenzuela N, Adams D.C, Janzen F.J. Pattern does not equal process: exactly when is sex environmentally determined? Am. Nat. 2003;161:676–683. doi: 10.1086/368292. doi:10.1086/368292 [DOI] [PubMed] [Google Scholar]
- Warner D.A, Shine R. The adaptive significance of temperature-dependent sex determination: experimental tests with a short-lived lizard. Evolution. 2005;59:2209–2221. doi:10.1554/05-085.1 [PubMed] [Google Scholar]
- Williams T.D. Variation in reproductive effort in female zebra finches (Taeniopygia guttata) in relation to dietary supplements during egg laying. Physiol. Zool. 1996;69:1255–1275. [Google Scholar]
- Wilson K, Hardy I.C.W. Statistical analysis of sex ratios: an introduction. In: Hardy I.C.W, editor. Sex ratios: concepts and research methods. Cambridge University Press; Cambridge, UK: 2002. pp. 48–92. [Google Scholar]
- Wingfield J.C, Farner D.S. The determination of five steroids in avian plasma by radioimmunoassay and competitive protein-binding. Steriods. 1975;26:311–327. doi: 10.1016/0039-128x(75)90077-x. doi:10.1016/0039-128X(75)90077-X [DOI] [PubMed] [Google Scholar]