Abstract
Resource competition is thought to play a major role in driving evolutionary diversification. For instance, in ecological character displacement, coexisting species evolve to use different resources, reducing the effects of interspecific competition. It is thought that a similar diversifying effect might occur in response to competition among members of a single species. Individuals may mitigate the effects of intraspecific competition by switching to use alternative resources not used by conspecific competitors. This diversification is the driving force in some models of sympatric speciation, but has not been demonstrated in natural populations. Here, we present experimental evidence confirming that competition drives ecological diversification within natural populations. We manipulated population density of three-spine sticklebacks (Gasterosteus aculeatus) in enclosures in a natural lake. Increased population density led to reduced prey availability, causing individuals to add alternative prey types to their diet. Since phenotypically different individuals added different alternative prey, diet variation among individuals increased relative to low-density control enclosures. Competition also increased the diet–morphology correlations, so that the frequency-dependent interactions were stronger in high competition. These results not only confirm that resource competition promotes niche variation within populations, but also show that this increased diversity can arise via behavioural plasticity alone, without the evolutionary changes commonly assumed by theory.
Keywords: diversification, Gasterosteus aculeatus, individual specialization, intraspecific competition, optimal foraging theory, negative frequency dependence
1. Introduction
Natural populations are typically variable for a wide variety of morphological, behavioural and physiological traits. Phenotypic variation represents a puzzle for evolutionary biologists, since natural selection is often thought to favour one optimal phenotype and eliminate all others in a population. Consequently, a major goal of evolutionary biology has been to identify processes that maintain phenotypic and genetic variation in natural populations. One possibility is that diversity is maintained by disruptive selection, arising from negative frequency-dependent processes that favour rare phenotypes (Wilson & Turelli 1986; Rueffler et al. 2006). In particular, many models propose that resource competition can promote ecological and phenotypic variation (e.g. Levene 1953; Rosenzweig 1978; Slatkin 1980; Taper & Case 1985; Bürger & Gimelfarb 2004; Dieckmann et al. 2004). This is because rare phenotypes may have access to alternative resources, thereby escaping competition with more common phenotypes (Pfennig 1992; Maret & Collins 1997; Swanson et al. 2003). Perhaps the most familiar consequence of competitive diversification is interspecific character displacement, in which coexisting species diverge in resource use to mitigate the effects of competition (Grant 1972; Dayan & Simberloff 2005). However, intraspecific competition is also thought to maintain intraspecific variation (Roughgarden 1972; Bolnick 2004; Bürger & Gimelfarb 2004), trophic polymorphism (Smith & Skulason 1996) or even drive speciation (Rosenzweig 1978; Dieckmann et al. 2004).
Despite its prominent role in recent theory (Dieckmann et al. 2004), the diversifying effect of intraspecific competition has received few experimental tests (Maret & Collins 1997; Bolnick 2001, 2004; MacLean 2005). Here, we present experimental evidence that competition can increase resource use diversity within a natural population. Resource use diversity, also known as ‘individual specialization’, occurs when a population is composed of ecologically heterogeneous individuals, each of which uses only a subset of the population's overall resource base (Bolnick et al. 2003). Although ecologists have traditionally assumed that conspecific individuals are ecologically equivalent, a large number of studies have shown that apparently generalized species are composed of relatively specialized individuals (Bolnick et al. 2003).
The proximate and ultimate causes of this among-individual variation remain poorly understood: why would conspecific individuals, inhabiting a common environment, choose to use different subsets of the available resources? To answer this question, we first need to examine why an individual might use only a subset of the resources available to it. Such diet selectivity is generally explained by optimal foraging theory (OFT), which assumes that individuals act to maximize their rate of energy intake (Stephens & Krebs 1986). OFT suggests that individuals may ignore certain types of prey when the time required to consume them could be more profitably spent searching for more valuable prey. This theory can then explain individual specialization if phenotypic variation causes individuals to differ in search or handling efficiencies for alternate prey (Bolnick et al. 2003; Svanbäck & Bolnick 2005).
One of the key predictions of OFT is that an individual predator should add new prey types to its diet as preferred prey become scarce (Stephens & Krebs 1986). By extension, these diet changes mean that resource competition may modify the degree of diet overlap among individuals (Svanbäck & Persson 2004; Svanbäck & Bolnick 2005). While the exact result depends on the strength of functional trade-offs, a number of scenarios predict that competition should lead to greater among-individual variation. This occurs when phenotypically different individuals have similar top-ranked prey, but resort to different back-up prey as their preferred ones become scarce (Robinson & Wilson 1998) or if phenotypic variation causes different individuals to add alternate prey more readily than others. As a result, models suggest that resource competition may generally lead to increased diet variation. Consistent with this expectation, the degree of individual specialization is positively related to population density in Eurasian Perch (Perca fluviatilis) over a 9-year natural fluctuation in density (Svanbäck & Persson 2004). A related prediction is that phenotypic variation will be more tightly correlated with diet variation at high competition, when morphological differences lead to greater niche variation among individuals.
The predicted positive relationship between competition and diet variation is a close analogue of the evolutionary theories of competitive diversification (Dieckmann et al. 2004). The key difference is that in the OFT model, competition can drive diversity via changes in foraging behaviour (plasticity) rather than changes in genetic variation (evolution; Svanbäck & Bolnick 2005). Note that plastic and evolutionary are not mutually exclusive, but will tend to operate on different time-scales. In this study, we tested whether the level of competition leads to behavioural diversification in foraging decisions, by experimentally manipulating a natural population of three-spine sticklebacks (Gasterosteus aculeatus). As predicted, the degree of diet variation and the strength of diet–morphology correlations increased with elevated competition.
2. Material and methods
In June 2005, we built ten 9 m2 enclosures made of 1/16 inch. seine net, set in approximately 2 m deep water in Blackwater Lake on northern Vancouver Island, British Columbia (125°35′28″ W, 50°10′19″ N). The surface area of Blackwater lake is 37.5 ha, the perimeter is 6000 m and maximum depth is 28 m. Enclosures were placed in pairs along 0.5 km of shoreline and stocked with wild-caught sticklebacks to generate paired low- and high-density treatments (either 30 or 90 fish per enclosure; LD or HD hereafter). These densities fall within natural densities of stickleback populations (Wooton et al. 2005). After 13 days, we quantitatively sampled prey (benthic invertebrates and zooplankton) in each enclosure by straining 33 l of the water column with a zooplankton net (100 μm mesh) and sifting through 800 cm2 of benthic sediment, 10 cm deep collected with a core sampler. Sticklebacks were trapped the following day, anesthetized and preserved in formalin. One milligram of muscle tissue was stored in RNAlater and used to measure RNA/DNA ratios as an indicator of growth rate (Ali & Wootton 2003). Tissue samples were thawed, rinsed in RNAlater and RNA/DNA ratios measured according to standard ethidium bromide fluorescence procedures (Caldarone et al. 2001). Sticklebacks and prey were also sampled from outside each enclosure pair to serve as a natural baseline (control). This research adhered to the Association for the Study of Animal Behaviour/Animal Behaviour Society Guidelines for the Use of Animals in Research, the legal requirements of the country in which the work was carried out and all institutional guidelines.
Stomach contents were identified to the lowest feasible taxonomic level (family or genus) and measured to estimate dry weight. For each enclosure, we used the proportion of dry weight of each prey type in an individual's stomach to estimate its diet breadth using Levins' D (Levins 1968). All stomach contents within an enclosure were then summed to measure the population's diet breadth, also using Levins' D. Finally, we quantified diet variation by calculating the mean overlap between each individual's diet and the population diet (IS; Bolnick et al. 2002). IS ranges from 1 (perfect overlap, no variation) down towards 0 (less overlap, more variation). Stomach contents provide a cross-sectional measure of an individual's diet, which may be biased if the forager is sampling from patchy prey or if the stomach can hold only a few diet items at a time. However, stickleback guts usually contain many items, and the small scale of our enclosures ensured that all individuals were capable of sampling all available prey in much less time than it takes to digest them (more than 6 h; R. Svanbäck 2005, unpublished work). Consequently, the spatial scale makes it unlikely that the observed diet variation is a result of patchy resources or stochastic variation. In addition, significant correlations between morphology and diet suggest that diet variation is not due to stochastic sampling effects. This has been confirmed by studies of stable isotope variation, demonstrating that cross-sectional gut content variation can be a good guide to long-term differences in resource use (Araujo et al. in press; Bolnick et al. submitted).
To check whether density manipulations affected resource competition, we compared benthic and pelagic prey density and diversity, gut fullness and RNA/DNA ratios in HD versus LD using paired t-tests. Gut fullness was measured by calculating the residuals of stomach content mass regressed against body mass. To test our central hypothesis, we used paired t-tests to evaluate whether mean diet overlap (IS) differed between HD and LD. We also compared the mean individual diet breadth and population diet breadth between paired enclosures. All of these tests were repeated to contrast HD or LD against ‘control’ samples taken immediately adjacent to each enclosure pair.
To measure whether competition increased the strength of the correlation between diet and morphology, we photographed each fish for landmark-based geometric morphometrics. We digitized 23 homologous landmarks on the left side of each fish and used TpsRelw (Rohlf 2005) to convert the landmarks to partial warps and uniform scores. Furthermore, we used the same set of landmark coordinates collected from benthic and limnetic sticklebacks from Paxton Lake (R. Svanbäck, unpublished work) and converted them into partial warps and uniform scores together with the experimental fish from Blackwater Lake. We then performed a discriminant function analysis on the basis of separation (classification) of the benthic and limnetic sticklebacks from Paxton Lake. To identify more benthic- and limnetic-like phenotypes from our experiment, we projected the partial warps and uniform scores from the experimental fish on to the benthic–limnetic axis from Paxton Lake sticklebacks (see Eklöv & Svanbäck 2006 for further details on these morphometric methods). We first used all experimental individuals to evaluate whether there was a correlation between morphology and diet. We then used regression to test whether morphologically divergent individuals (large distance from the population centroid) are also ecologically divergent (low diet overlap with the population; PSi; Bolnick et al. 2002) within each enclosure. The absolute values of these regression slopes represent the strength of morphology–diet associations and were subjected to paired t-tests contrasting HD and LD treatments.
3. Results
There was no difference in survival between LD and HD (t4=−0.769, p=0.49). Furthermore, at the end of the experiment, we found no difference in average morphology between LD and HD (t4=0.12, p=0.91) or any difference in morphological variance between LD and HD (t4=1.44, p=0.22). Morphological means and variances also did not differ between either experimental treatment (HD or LD) and wild-caught control fish at the end of the experiment (t4=0.017–1.69, p=0.17–0.99). Thus, the following results of our study are due only to behavioural plasticity and not to any morphological change caused by either differential mortality or morphological plasticity.
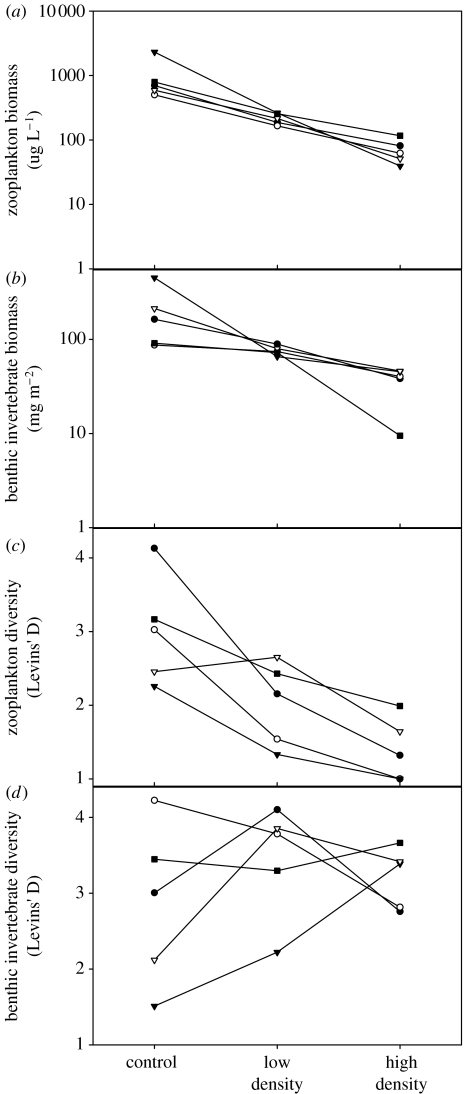
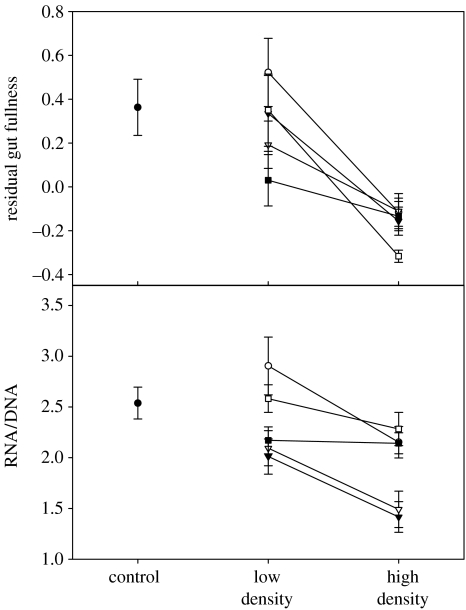
Several lines of evidence confirm that manipulating stickleback density modified the strength of resource competition. The HD treatment had significantly lower densities of benthic invertebrates (t4=2.96, p=0.042) and zooplankton (t4=5.64, p=0.005; figure 1). Benthic invertebrate diversity was unaffected by the density treatment (t4=0.53, p=0.62), whereas zooplankton diversity declined in HD treatment (t4=4.99, p=0.008). This reduced prey availability in HD led to reduced residual stomach content mass (t4=4.68, p=0.009) and reduced RNA/DNA ratios (t4=3.52, p=0.025; figure 2), suggesting that fish grew less in HD treatment. LD enclosures were not statistically different from adjoining control samples for all measures of competition, except for zooplankton density which was lower in enclosures (see electronic supplementary material for details). In contrast, HD enclosures showed significantly lower prey availability, stomach contents and growth rates than control samples.
Figure 1.
Prey responses to stickleback density manipulations. (a) Zooplankton and (b) benthic invertebrate biomass. (c) Zooplankton and (d) benthic invertebrate diversity, measured with Levins' D. Samples immediately outside each pair of enclosures are presented to indicate the natural base-line state. The different lines represent different pairs of enclosures and their controls.
Figure 2.
Tests of whether density manipulation resulted in resource competition among sticklebacks. (a) Relative stomach content mass as a measure of foraging rate. (b) RNA/DNA ratio as a measure of current growth rates in sticklebacks (Ali & Wootton 2003; Dahlhoff 2004). Results for wild-caught fish are presented to indicate the natural base-line state. The different lines represent different pairs of enclosures.
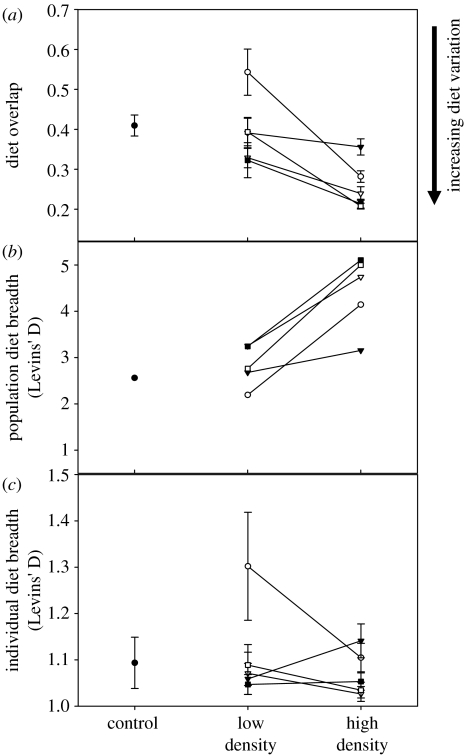
The increased competition in HD enclosures coincided with increased among-individual diet variation (t4=3.44, p=0.026; figure 3). This increased diet variation arose because different individuals shifted to use different previously under-used prey, so that individual diet breadth remained constant (t4=0.90, p=0.42) while the population's total niche breadth increased (t4=−5.23, p=0.006; figure 3). Diet variation and niche breadth in wild-caught fish were similar to that in LD fish and significantly different from HD fish (figure 3; also see supplementary material).
Figure 3.
Effect of population density on diet variation among sticklebacks. (a) Mean diet overlap (IS) between individuals and their population's total diet distribution (Bolnick et al. 2002), comparing paired low- and high-density populations in enclosures. Note that high diet overlap corresponds to low diet variation and vice versa. The mean diet overlap for wild-caught (control) fish in the same lake is presented for comparison. (b) Population diet breadth measured with Levins' D and (c) mean individual diet breadth. Note that the y-axis scale in (c) is smaller than in (b). The different lines represent different pairs of enclosures.
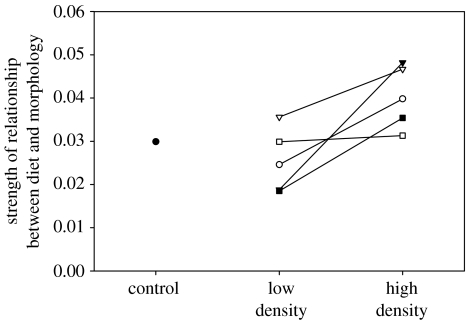
Diet variation among individuals was associated with morphological variation: individuals with deeper bodies and shorter gill rakers had a higher proportion of littoral macroinvertebrates in their diet (p<0.001) and a lower proportion of pelagic cladocerans in their diet (p<0.001). As a result, morphologically different individuals tend to be ecologically divergent as well, an association that was consistently stronger in HD than LD enclosures (t4=3.27, p=0.031; figure 4).
Figure 4.
Effect of competition on the linkage between phenotype variation and diet variation. The diet/morphology relationship was consistently stronger in high-density enclosures. The value for wild-caught control fish is provided for comparison. The different lines represent different pairs of enclosures.
4. Discussion
Our results confirm that resource competition can lead to increased diet variation among members of a single population (figure 3a). Since intraspecific competition is generally believed to be strong and widespread (Gurevitch et al. 1992), our findings suggest that the dynamics documented here may play a major role in maintaining ecological variation within populations. Such ecological variation is a prerequisite for the frequency-dependent interactions thought to underlie disruptive selection, which in turn maintains genetic diversity (Bürger & Gimelfarb 2004) and might drive speciation (Dieckmann & Doebeli 1999). However, models of competitive speciation tend to assume that the strength of frequency dependence is constant and that competition drives evolutionary divergence (Bürger & Gimelfarb 2004; Dieckmann et al. 2004; but see Taper & Case 1985; Ackermann & Doebeli 2004). In contrast, our results demonstrate that the level of diet variation can change depending on ecological conditions and can arise via behavioural rather than evolutionary divergence. Diet variation increased in under two weeks, commensurate with the time it takes a forager to detect changes in prey availability (Werner et al. 1981), but not with changes in genetic variance over generations. In LD treatments, phenotypically different individuals fed on similar prey types, whereas in HD the same phenotypic differences led to divergent diets. We suggest that this reflects shared resource preferences for prey that become depleted as stickleback density rises, causing phenotypically different individuals to shift onto different alternative prey (Svanbäck & Bolnick 2005). Thus, behavioural decisions made by individual sticklebacks account for the increased correlation between diet and morphology with increased competition.
These behavioural changes are consistent with several predictions of OFT. Although OFT is generally used to explain an individual's resource use, it may be extended to explain diet variation by assuming that search or handling times vary among individuals, perhaps as a function of morphological traits or experience. Foraging theory suggests that resource competition, by reducing the frequency of preferred prey, should lead to increased population diet breadth (Schoener 1971; Stephens & Krebs 1986). A more recent model suggested that this population-level niche expansion might arise via ecological divergence among individuals (Svanbäck & Bolnick 2005), similar to a classical verbal model known as the ‘niche variation hypothesis’ (Van Valen 1965). This should occur when different phenotypes resort to different alternate prey. Consider a simple model where two phenotypes share a single preferred resource, but have different alternate prey (Robinson & Wilson 1998). When the preferred prey are abundant, there is no diet–morphology association. As prey density declines, the two phenotypes increasingly rely on different resources. Thus, competition is expected to increase both diet variations, because increases in population niche breadth should outpace increases in individual diet breadth (Svanbäck & Bolnick 2005). As a result, the correlation between diet and morphology should increase with competition. All three of these predictions were confirmed in our study (figures 3a–c and 4). The only discrepancy with foraging theory was our observation that individual niche breadth remained constant rather than increasing as foragers add new prey types to their diet. This contradiction can be understood by realizing that foraging theory predicts an increase in the number of prey taxa that an individual would accept, if encountered. In contrast, our niche breadth measure reflects the diversity of prey actually eaten, which will also depend on prey availability.
In conclusion, our results confirm that resource competition is a diversifying force that can lead to increased niche variation among members of a single population. However, unlike the evolutionary diversification generally thought to result from competitive diversification (Ludwig 1950; Levene 1953; Rosenzweig 1978; Seger 1985; Christiansen 1988; Dieckmann & Doebeli 1999; Bürger & Gimelfarb 2004; Dieckmann et al. 2004), our results arose from changes in foraging behaviour, as the functional importance of morphological variation was exaggerated by declining prey densities. Such behavioural diversification is likely to arise much faster than evolutionary diversification and is easily reversible. It is not yet known how this rapid behavioural diversification might affect subsequent evolutionary dynamics, since existing models of diversification due to competition treat ecological parameters like niche width and frequency dependence as fixed constants (Dieckmann et al. 2004) or allow them to evolve (Taper & Case 1985; Ackermann & Doebeli 2004). On the contrary, our results show that both niche width and frequency dependence can change via behavioural plasticity.
It is possible that behavioural diversification may actually facilitate evolutionary diversification (West-Eberhard 2003). Greater diet variation increases the degree to which competition is frequency dependent, and it is this frequency dependence that drives disruptive selection (Dieckmann & Doebeli 1999; Bürger & Gimelfarb 2004). In addition, the increased correlation between morphology and diet in high competition means that any selection acting on resource use will be more efficiently translated into morphological evolution. In low-competition environments, phenotypic variation may be hidden from the effects of selection on resource use, since all individuals use similar resources. It should be noted that the selection itself may be weaker at low competition (Bolnick 2004). This may allow populations to accrue genetic variation for morphological traits in low-competition environments, because ecological (and fitness) effects only emerge when intraspecific competition intensifies. Our results therefore suggest that theories of competitive diversification and speciation would benefit from a more careful consideration of how changes in the level of niche variation and frequency dependence may affect evolutionary dynamics.
Acknowledgments
We thank B. Robinson, D. Schluter and two anonymous reviewers for their comments and D. Agashe, E. Caldarone, E. Caldera, M. Hartzler and O. Lau for their help in the laboratory and field. R.S. was funded by the Swedish Research Council, and D.I.B. was funded by the University of Texas at Austin and the National Science Foundation.
Supplementary Material
Tables with supporting statistics and data
References
- Ackermann M, Doebeli M. Evolution of niche width and adaptive diversification. Evolution. 2004;58:2599–2612. doi: 10.1111/j.0014-3820.2004.tb01614.x. doi:10.1554/04-244 [DOI] [PubMed] [Google Scholar]
- Ali M, Wootton R.J. Correlates of growth in juvenile three-spined sticklebacks: potential predictors of growth rates in natural populations. Ecol. Freshwat. Fish. 2003;12:87–92. doi:10.1034/j.1600-0633.2003.00003.x [Google Scholar]
- Arau´jo, M. S., Bolnick, D. I., Machado, G., Giaretta, A. A. & Reis, S.F. In press. Using d13C stable isotopes to quantify individual-level diet variation. Oecologia [DOI] [PubMed]
- Bolnick D.I. Intraspecific competition favours niche width expansion in Drosophila melanogaster. Nature. 2001;410:463–466. doi: 10.1038/35068555. doi:10.1038/35068555 [DOI] [PubMed] [Google Scholar]
- Bolnick D.I. Can intraspecific competition drive disruptive selection? An experimental test in natural populations of sticklebacks. Evolution. 2004;87:608–618. doi:10.1554/03-326 [PubMed] [Google Scholar]
- Bolnick, D. I., Caldera, E. J. & Matthews, B. Submitted. Migration load in a pair of ecologically divergent lacustrine stickleback populations.
- Bolnick D.I, Yang L.H, Fordyce J.A, Davis J.A, Svanbäck R. Measuring individual-level trophic specialization. Ecology. 2002;83:2936–2941. [Google Scholar]
- Bolnick D.I, Svanbäck R, Fordyce J.A, Yang L.H, Davis J.M, Hulsey C.D, Forrister M.L. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 2003;161:1–28. doi: 10.1086/343878. doi:10.1086/343878 [DOI] [PubMed] [Google Scholar]
- Bürger R, Gimelfarb A. The effects of intraspecific competition and stabilizing selection on a polygenic trait. Genetics. 2004;167:1425–1443. doi: 10.1534/genetics.103.018986. doi:10.1534/genetics.103.018986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarone, E. M., Wanger, M., Ogner-Burns, J. S. & Buckley, L. J. 2001 Protocol and guide for estimating nucleic acids in larval fish using a fluorescence microplate reader. Reference Document 01–11, Northeast Fisheries Science Center.
- Christiansen F.B. Frequency dependence and competition. Phil. Trans. R. Soc. B. 1988;319:587–600. doi: 10.1098/rstb.1988.0067. [DOI] [PubMed] [Google Scholar]
- Dahlhoff E.P. Biochemical indicators of stress and metabolism: applications for marine ecological studies. Annu. Rev. Physiol. 2004;66:183–207. doi: 10.1146/annurev.physiol.66.032102.114509. doi:10.1146/annurev.physiol.66.032102.114509 [DOI] [PubMed] [Google Scholar]
- Dayan T, Simberloff D. Ecological and community-wide character displacement: the next generation. Ecol. Lett. 2005;8:875–894. doi:10.1111/j.1461-0248.2005.00791.x [Google Scholar]
- Dieckmann U, Doebeli M. On the origin of species by sympatric speciation. Nature. 1999;400:354–357. doi: 10.1038/22521. doi:10.1038/22521 [DOI] [PubMed] [Google Scholar]
- Dieckmann U, Doebeli M, Metz J.A.J, Tautz D, editors. Adaptive speciation. Cambridge University Press; New York, NY: 2004. [Google Scholar]
- Eklöv P, Svanbäck R. Predation favors phenotypic divergence in sympatric perch populations. Am. Nat. 2006;167:440–452. doi: 10.1086/499544. [DOI] [PubMed] [Google Scholar]
- Grant P.R. Convergent and divergent character displacement. Biol. J. Linn. Soc. 1972;4:39–69. [Google Scholar]
- Gurevitch J, Morrow L.L, Wallace A, Walsh J.S. A meta-analysis of competition in field experiments. Am. Nat. 1992;140:539–572. doi:10.1086/285428 [Google Scholar]
- Levene H. Genetic equilibrium when more than one ecological niche is available. Am. Nat. 1953;87:331–333. doi:10.1086/281792 [Google Scholar]
- Levins R. Princeton University Press; Princeton, NJ: 1968. Evolution in changing environments: some theoretical explorations. [Google Scholar]
- Ludwig Zur theorie der konkurrenz. Neue Ergeb. Prob. Zool., Klatt-Festschrift. 1950:516–537. [Google Scholar]
- MacLean R.C. Adaptive radiation in microbial microcosms. J. Evol. Biol. 2005;18:1376–1386. doi: 10.1111/j.1420-9101.2005.00931.x. doi:10.1111/j.1420-9101.2005.00931.x [DOI] [PubMed] [Google Scholar]
- Maret T.J, Collins J.P. Ecological origin of morphological diversity: a study of alternative trophic phenotypes in larval salamanders. Evolution. 1997;51:898–905. doi: 10.1111/j.1558-5646.1997.tb03671.x. doi:10.2307/2411164 [DOI] [PubMed] [Google Scholar]
- Pfennig D.W. Polyphenism in spadefoot toad tadpoles as a locally adjusted evolutionary stable strategy. Evolution. 1992;46:1408–1420. doi: 10.1111/j.1558-5646.1992.tb01133.x. doi:10.2307/2409946 [DOI] [PubMed] [Google Scholar]
- Robinson B.W, Wilson D.S. Optimal foraging, specialization, and a solution to Liem's paradox. Am. Nat. 1998;151:223–235. doi: 10.1086/286113. doi:10.1086/286113 [DOI] [PubMed] [Google Scholar]
- Rohlf, F. J. 2005 TpwRelw. See http://life.bio.sunysb.edu/morph/
- Rosenzweig M.L. Competitive speciation. Biol. J. Linn. Soc. 1978;10:275–289. [Google Scholar]
- Roughgarden J. Evolution of niche width. Am. Nat. 1972;106:683–718. doi:10.1086/282807 [Google Scholar]
- Rueffler C, Van Dooren T.J.M, Leimar O, Abrams P.A. Disruptive selection and then what? Trends Ecol. Evol. 2006;21:238–245. doi: 10.1016/j.tree.2006.03.003. doi:10.1016/j.tree.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Schoener T.W. Theory of feeding strategies. Annu. Rev. Ecol. Syst. 1971;2:369–404. doi:10.1146/annurev.es.02.110171.002101 [Google Scholar]
- Seger J. Intraspecific resource competition as a cause of sympatric speciation. In: Greenwood P.J, Harvey P.H, Slatkin M, editors. Evolution: essays in honor of John Maynard Smith. Cambridge University Press; Cambridge, UK: 1985. pp. 43–53. [Google Scholar]
- Slatkin M. Ecological character displacement. Ecology. 1980;61:163–177. doi:10.2307/1937166 [Google Scholar]
- Smith T.B, Skulason S. Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annu. Rev. Ecol. Syst. 1996;27:111–133. doi:10.1146/annurev.ecolsys.27.1.111 [Google Scholar]
- Stephens D.W, Krebs J.R. Princeton University Press; Princeton, NJ: 1986. Foraging theory. [Google Scholar]
- Svanbäck R, Bolnick D.I. Intraspecific competition affects the strength of individual specialization: an optimal diet theory model. Evol. Ecol. Res. 2005;7:993–1012. [Google Scholar]
- Svanbäck R, Persson L. Individual specialization, niche width and population dynamics: implications for trophic polymorphisms. J. Anim. Ecol. 2004;73:973–982. doi:10.1111/j.0021-8790.2004.00868.x [Google Scholar]
- Swanson B.O, Gibb A.C, Marks J.C, Hendrickson D.A. Trophic polymorphism and behavioral differences decrease intraspecific competition in a cichlid, Herichthys minckleyi. Ecology. 2003;84:1441–1446. [Google Scholar]
- Taper M.L, Case T.J. Quantitative genetic models for the coevolution of character displacement. Ecology. 1985;66:355–371. doi:10.2307/1940385 [Google Scholar]
- Van Valen L. Morphological variation and width of ecological niche. Am. Nat. 1965;99:377–389. doi:10.1086/282379 [Google Scholar]
- Werner E.E, Mittelbach G.G, Hall D.J. The role of foraging profitability and experience in habitat use by the bluegill sunfish. Ecology. 1981;62:116–125. doi:10.2307/1936675 [Google Scholar]
- West-Eberhard M.J. Oxford University Press; New York, NY: 2003. Developmental plasticity and evolution. [Google Scholar]
- Wilson D.S, Turelli M. Stable underdominance and the evolutionary invasion of empty niches. Am. Nat. 1986;127:835–850. doi:10.1086/284528 [Google Scholar]
- Wooton R.J, Adams C.E, Attrill M.J. Empirical modelling of the population dynamics of a small population of the threespine stickleback, Gasterosteus aculeatus. Environ. Biol. Fishes. 2005;74:151–161. doi:10.1007/s10641-005-7690-3 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables with supporting statistics and data