Abstract
The North Sea has warmed in recent years and there is an ongoing debate into how this is affecting the distribution of fishes and other marine organisms. Of particular interest is the commercially important Atlantic cod (Gadus morhua L.), which has declined sharply in abundance in the North Sea over the past 20 years. Observations of the temperature experienced by 129 individual cod throughout the North Sea were made during a large-scale electronic tagging programme conducted between 1999 and 2005. We asked whether individual cod fully occupied the thermal habitat available to them. To this end, we compared the temperature experience of cod with independently measured contemporaneous sea-bottom temperature data. The majority of cod experienced a warmer fraction of the sea than was potentially available to them. By summer, most of the individuals in the south experienced temperatures considered superoptimal for growth. Cooler waters were within the reach of the cod and a small number of individuals migrated to areas that allowed them to experience lower temperatures, indicating that the cod had the capacity to find cooler water. Most did not, however, suggesting that the changing thermal regime of the North Sea is not yet causing adult cod to move to cooler waters.
Keywords: fishes, electronic tags, temperature, movement, distribution, population structure
1. Introduction
Climate change is predicted to have an increasing effect over the coming decades (Meehl et al. 2005). Forecasts of the effect of climate change on marine ecosystems vary (Hulme et al. 2002; ICES 2006), with predicted increases in average sea temperature much higher in some regions than others. The North Sea, being a relatively shallow shelf ecosystem within the northeast Atlantic, is perhaps particularly vulnerable to climate change. Since 1970, it has warmed by approximately 1.0°C (ICES 2006). Annual surface temperatures of the North Sea are forecast to warm by between 1 and 2.5°C during the next 50 years (Clark et al. 2003). Many ectothermic marine species, including crustaceans (Groeneveld & Branch 2002), cephalopods (Sims et al. 2001) and a number of commercially important fish species such as cod, plaice and mackerel (Metcalfe et al. 2002) are highly mobile and undertake extensive migrations. In response to warming, the geographical ranges of cold-adapted marine species are predicted to become compressed or move towards the poles (Parmesan & Yohe 2003). As one of the most heavily fished seas in the world (Jennings et al. 1999; Kaiser et al. 2002), the consequences of changes in the distribution of commercial fish species in the North Sea will be significant (Beare et al. 2004).
The Atlantic cod (Gadus morhua L.) is distributed along the continental shelves of the North Atlantic between 40 and 80° of latitude. The North Sea cod stock has declined markedly in abundance in recent decades (Daan et al. 1994; Hislop 1996) and is precariously close to collapse (Cook et al. 1997; Christensen et al. 2003). Over-fishing has been blamed for the decline (Becker & Pauly 1996; Cook et al. 1997). However, the North Sea has also warmed significantly in the past decades (O'Brien et al. 2000; Hulme et al. 2002; Hughes et al. 2003; ICES 2006) and this too has been implicated in the decline of the North Sea cod, be it on the basis of recruitment failure (O'Brien et al. 2000; Clark et al. 2003; Ottersen et al. 2006) or changes in the distribution of preferred habitat (Blanchard et al. 2005). Perry et al. (2005) reported a long-term northward shift of cod (and several other demersal fish species) in the North Sea that has coincided with its recent warming. Understanding the relative contribution of fishing pressure and climate change to the changes in stock distribution and abundance underpins effective fisheries management in the North Sea and elsewhere (Rose 2004; Horwood et al. 2006).
Throughout the geographical range of cod, the many stocks occupy mean annual seabed temperatures between 1 and 11°C, although the full thermal range extends from −1 to 19°C (Sundby 2000). Temperature influences vital processes such as growth rate (Pedersen & Jobling 1989; Brander 1995; Jobling 1988; Bjornsson et al. 2001; Björnsson & Steinarsson 2002), metabolic rate (Claireaux et al. 2000; Lannig et al. 2004) and maturation (Yoneda & Wright 2005). Most fishes, being ectothermic, conform to their thermal environment and they must move if they are to maximize their exposure to suitable temperatures and optimize vital processes such as growth. Defining thermal optima is, however, complicated; laboratory studies suggest that the optimal temperature for cod growth decreases with size and age, with estimates varying from 17°C for newly hatched juveniles to 7°C for adults (Pedersen & Jobling 1989; Jobling 1988; Boutilier 1998; Bjornsson et al. 2001; Björnsson & Steinarsson 2002). Furthermore, the specific effect of temperature will depend on a number of biotic factors that affect growth rate, such as food availability and haemoglobin genotype, both of which have been shown to underlie thermal preference in cod (Petersen & Steffensen 2003). Nevertheless, cod clearly has thermal distribution limits and, being a highly mobile species (Robichaud & Rose 2004), individuals would be expected to move either horizontally or vertically in order to remain at or close to a temperature optimal for growth.
In the face of global warming, cod populations are predicted to retreat from warmer areas and expand into cooler areas on yearly (Blanchard et al. 2005) or decadal time-scales (Drinkwater 2005) within certain constraints to movements (Heessen & Daan 1994). Cod are known to be capable of moving distances in excess of the dimensions of the North Sea (approx. 1000 km; Robichaud & Rose 2004). In theory, therefore, individual cod could occupy almost any location within the North Sea. Cod in the North Sea are probably not as vagile as this, however, and conventional mark and recapture experiments (Robichaud & Rose 2004; Wright et al. 2006a; Righton et al. 2007) and genetic evidence (Hutchinson et al. 2001) suggest that populations from the northern North Sea (further than 57° N) do not intermix significantly with those from the southern North Sea (below than 56° N). Within these areas, however, we expect cod to maximize their exposure to the most suitable thermal habitat. Previous studies addressing thermal influences on fish distribution (Heessen & Daan 1994; Blanchard et al. 2005; Perry et al. 2005) have been derived from surveys that cut across large (thousands of kilometres) geographical areas and long (decadal) temporal scales. Such studies are insensitive to short-term, individual and regional variations in distribution. This can be a problem when addressing distribution patterns of spatially structured stocks such as cod (Hutchinson et al. 2001; Metcalfe 2006; Wright et al. 2006b) or species like cod that show high individual variability in migratory behaviour (Righton et al. 2001; Palsson & Thorsteinsson 2003; Neat et al. 2006). Direct, individual-based evidence for thermal responses in fishes in the field is largely lacking and the consequences that individual behaviour could have for population-level range shifts have not been assessed.
The most direct means of evaluating whether or not cod actively moderate the range of temperatures they encounter is by comparing the temperatures experienced by individual cod with the range of temperatures available to them in their habitat. To this end, we attached electronic data storage tags (DSTs) to several hundred individual cod in the North Sea to record the temperatures they experienced while at liberty (Metcalfe & Arnold 1997). We compared the temperature experience of individual cod released at a number of locations throughout the North Sea between 1999 and 2005 with contemporaneous, independently collated hydrographic data for the region. Our study aimed to assess the extent to which cod occupy the thermal habitat potentially available to them. We predicted that if cod select thermal habitat to maximize growth rates, they should avoid superoptimal temperatures by seeking cooler waters and that this should be evidenced by the DST records.
2. Material and methods
(a) Data storage tags
Between 1999 and 2004, cod greater than 30 cm in length were captured in the North Sea from approximately 51° N in the south to 61° N in the north (table 1) and implanted with a DST. We used the ‘LTD_1200’ or ‘LTD_1400’ DST manufactured by Lotek Marine Technologies (Canada) or ‘Centi’ or ‘Milli’ DST manufactured by Star-Oddi Marine Device Manufacturing (Iceland). The DSTs recorded temperature to an accuracy of at least 0.1°C and a precision of at least 0.03°C. DSTs were programmed to record pressure and temperature every 10 or 20 min.
Table 1.
Summary statistics for number of tags deployed and DST temperature records successfully downloaded. (T, temperature (°C); s.d., standard deviation.)
| region | release site | number of tags deployed | number of cod recaptured | number of tags with data returned | number of days at liberty | longest record (days) | min T | max T | mean T | s.d. T |
|---|---|---|---|---|---|---|---|---|---|---|
| southern North Sea | eastern channel | 49 | 6 | 6 | 470 | 280 | 9.74 | 18.48 | 15.27 | 1.64 |
| Flamborough | 54 | 16 | 10 | 650 | 308 | 5.49 | 9.17 | 7.41 | 0.69 | |
| German Bight | 100 | 8 | 6 | 1895 | 540 | 2.03 | 17.2 | 8.37 | 2.85 | |
| southern Bight | 209 | 65 | 64 | 5131 | 308 | 5.07 | 19.45 | 11.42 | 3.71 | |
| sub total | 412 | 95 | 86 | 8146 | 540 | 2.03 | 19.45 | 10.61 | 3.79 | |
| northern North Sea | east Shetland | 30 | 7 | 6 | 2280 | 909 | 6.86 | 14.36 | 9.52 | 1.91 |
| west Shetland | 138 | 39 | 32 | 3674 | 337 | 6.27 | 13.29 | 9.08 | 1.84 | |
| Moray Firth | 94 | 5 | 3 | 233 | 131 | 6.85 | 9.61 | 7.79 | 0.85 | |
| Viking Bank | 7 | 3 | 2 | 278 | 152 | 5.64 | 8.51 | 7.63 | 1.86 | |
| sub total | 269 | 54 | 43 | 6465 | 909 | 5.64 | 14.36 | 8.51 | 1.43 | |
| grand total | 681 | 149 | 129 | 14 611 |
Since the southern and northern regions of the North Sea are physically and ecologically different and historical tagging experiments suggest a very limited mixing of cod between the two regions, we maintained this division in the design of our tagging experiment. Tagging was conducted during the autumn or spring and targeted locations were thought to contain discrete spawning groups based on past tagging studies and more recent genetic studies (table 1). Details of fish capture and tagging techniques can be found in Neat et al. (2006) and Righton et al. (2006). In brief, cod were captured by handline or with a modified trawl, anaesthetized and a DST implanted into the body cavity or attached externally to the dorsal musculature, and released. The long-term (more than 1 year) effect of tags on growth has been found to be negligible (Righton et al. 2006). All work was carried out under UK Home Office regulations (Animals (Scientific Procedures) Act 1986).
(b) Data sources and treatment
Temperature records from returned DSTs were downloaded and summarized to give daily averages per individual. These data were then used to derive monthly median values for each of the release sites and for each region as a whole. For each region, the range and interquartile range (25–75%) were calculated. For comparison with seabed temperature data, we used data from cod released in the Southern Bight (n=64) and west of Shetland (n=32), because these were most representative of each area in terms of numbers of cod at liberty and coverage throughout the year.
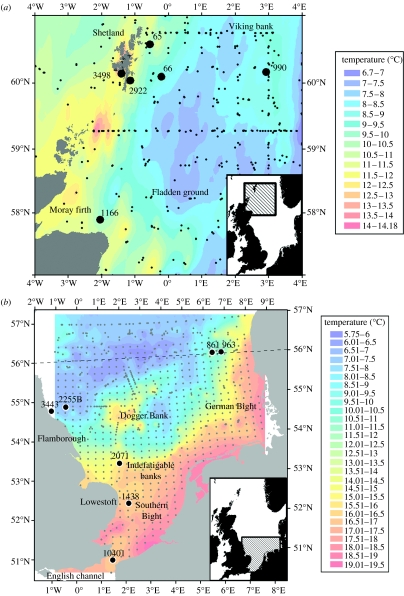
Data from the International Council for the Exploration of the Sea (ICES) conductivity and temperature at depth (CTD) database were used to estimate seabed temperatures in each region during the years that the cod were at liberty. The two regions were bounded by 4° W to 4° E and 57° N to 61° N in the north (figure 1a) and by 1° W to 8° E and 51° N to 56° N in the south (figure 1b). These boundaries were selected to encompass suitable depth habitat for cod and typical cod home ranges inferred from conventional tagging studies (Robichaud & Rose 2004; Wright et al. 2006a; Righton et al. 2007). The data were filtered by a depth limit of 300 m to coincide with the greatest depth experienced by a tagged cod (298 m). For each region, the CTD data were inspected for trends across the period of study (1999 through 2002 in the south and 2002 through 2004 in the north) using multifactorial ANOVA (quarter, year and their interaction). While significant differences (p<0.01) were detected between some quarters and years, there was no consistent trend in seabed temperature. The CTD data were thus pooled across years to achieve a better spatial coverage and split into quarters of the year. Temperature surfaces were created for each region and quarter of the year by interpolation with a long-range spherical (northern North Sea) or linear (southern North Sea) Kriging function. In the northern North Sea, a total of 1878 sample points between 2002 and 2005 gave good spatial (figure 1a) and temporal coverage for each quarter of the year with root mean square (RMS) less than 1°C in all the key areas. In the southern North Sea, despite 2256 sample points between 1999 and 2004, the CTD sampling stations in the waters between 50 and 53° N were widely spaced and relatively infrequently sampled (figure 1b), leading to high error estimates (RMS error >1°C) in poorly sampled areas. To counter this, the southern North Sea region was gridded into cells of 10′ north to south and 20′ east to west and daily seabed temperature values for 1999, 2001 to 2003 (data for 2000 were not available) from a variant of the Princeton Ocean Model (Young 2002; Young et al. 2003) were used to populate those grid cells for which there were no CTD casts in a given quarter. Average temperature values were then calculated for each grid cell for each quarter. RMS was less than 1°C at any point in the resulting surfaces.
Figure 1.
(a) Temperature surfaces for the seabed generated from CTD data for the northern North Sea in the third quarter of the year (July, August and September) averaged over 2002 to 2004. Black circles indicate recapture positions for cod profiled in figure 3a. Small grey circles show the location of CTD casts used to generate the temperature surface. Inset map shows the study area within the North Sea. (b) Equivalent plot for the southern North Sea for data averaged over 1999–2004. Black circles indicate summer location estimates for cod profiled in figure 3b. Small grey dots show the position of modelled data (regular grid) and CTD casts (outside the regular grid). Dashed grey line represents the northerly boundary of the region delimited as the southern North Sea.
The area of seabed at each 0.5°C increment was calculated from the quarterly temperature surfaces, and a frequency distribution generated. Based on the proportion of the seabed within each 0.5°C temperature interval, the number of days equivalent to that for which DST data were available was calculated. Then for each region, the frequency distribution of seabed temperature days was compared with the frequency distribution of DST temperature days. The Kolmogorov–Smirnov test for two samples was used to test for differences between the cumulative frequency distributions of seabed temperature and DST temperature.
3. Results
(a) DST recapture rates
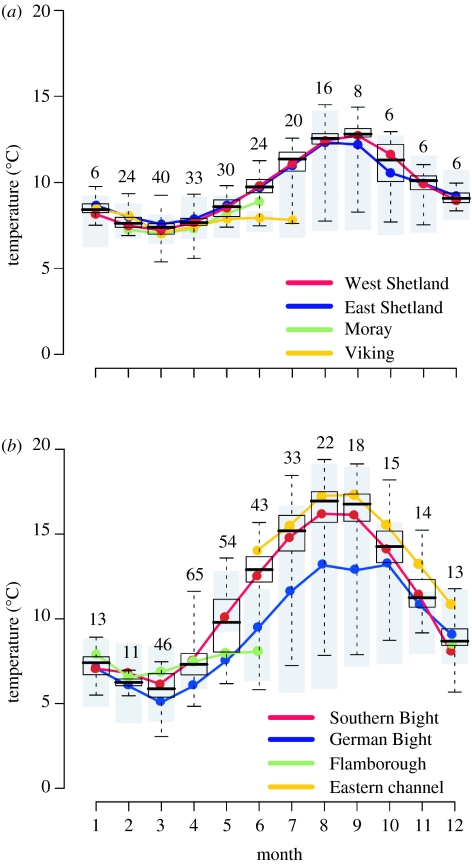
Out of 149 cod that were recaptured, 129 DSTs were returned and successfully downloaded (table 1). Forty-three of these tags were returned from the northern North Sea between 2002 and 2005 and yielded a total of 6465 days of temperature data. The longest record of temperature was 2.5 years (909 days) from a cod tagged east of Shetland. Eighty-six tags were returned from the southern North Sea from 1999 through 2004, and yielded 8146 days of temperature data. The longest record spanned 540 days from a cod tagged in the German Bight. During the 5 years of the study, data were available from at least six fishes at liberty in each region in each month of the year, peaking at 65 and 40 fishes in the southern North Sea and northern North Sea, respectively (figure 2a,b).
Figure 2.
(a) Sea bottom temperatures and the monthly thermal experience of cod in the northern North Sea. The limits of the boxes show 25 (upper) and 75% (lower) quartile temperature ranges, while the black line shows the median temperature. Error bars/whiskers show the full range of the temperature experience. Numbers above the error bars indicate the number of cod at liberty during each month. Average monthly temperature experiences of cod from each release site are shown by colours. The range of CTD data in each month is represented by filled grey bars. (b) Same plot for the southern North Sea.
(b) Annual thermal variation in the two regions
In the northern North Sea, temperatures were at their lowest around March and rose to a peak in September (figure 2a). This pattern varied across the region due to a number of thermally distinct areas; the areas to the northwest and northeast are heavily influenced by Atlantic inflow and were generally warmer, whereas the centrally located Fladden grounds were relatively colder, especially so in summer (figure 1a). In the southern North Sea, temperature dropped to a minimum in February before rising until September (figure 2b). From June onwards, the water column became stratified in the areas where water depth exceeded 50 m (generally north of 54° latitude; figure 1b). This resulted in the temperature range reaching a maximum, as deep areas remained cool and shallow areas warmed as high as 19°C (figures 1b and 2b).
(c) Temperature experience of cod
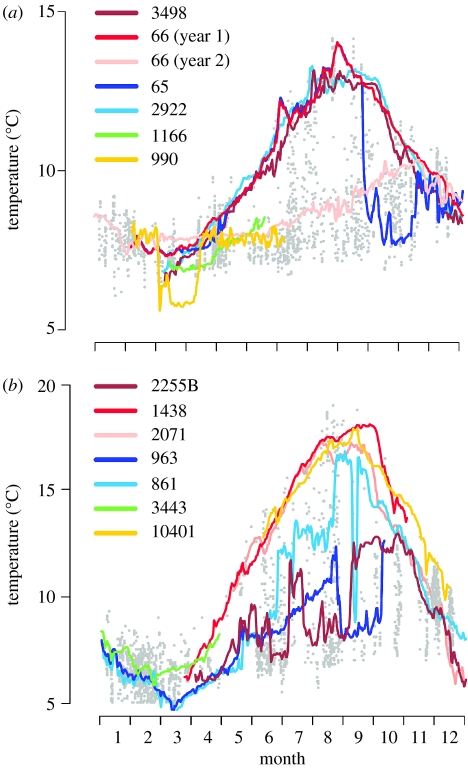
The annual range of temperatures was greater for cod in the southern North Sea than in northern North Sea (table 1). Overall, however, the majority of temperature experiences of cod in both of the regions closely followed the upper seasonal trend of sea bottom temperature (figure 2a,b). In the northern North Sea, experienced temperatures rarely exceeded 14°C and the cod released west of Shetland inhabited the warmest fraction of the area, particularly during the warmer months of the year (figure 2a). No cod from west Shetland moved to cooler waters and the variance between individuals was very low. In contrast, individuals released to the east of Shetland and on Viking Bank showed abrupt movements into cold fronts and prolonged occupancy of cooler areas (figure 3a). One cod (DST_66: figure 3a) was at liberty over 2 full years and experienced a very different thermal environment in each. In the first year, when the cod remained resident to its release site, it experienced the same seasonal rise and fall as its conspecifics. In the second year, the cod migrated and experienced much lower average temperatures with no obvious seasonal trend.
Figure 3.
(a) Individual cases of DST records illustrating variation in the mean daily thermal experience of cod in the northern North Sea. The legend refers to the tag ID number: 3498 (west Shetland), 66, 65 and 2922 (east Shetland), 1166 (Moray Firth) 990 (Viking bank). (b) DST records from the southern North Sea. Tag ID numbers: 2255B, 1438, 2071 (Southern Bight), 963, 861 (German Bight), 3443 (Flamborough), 10401 (Eastern Channel). CTD data (daily resolution) are represented by filled grey dots.
In the southern North Sea, all but two individuals released in the Southern Bight (figure 2b) migrated short distances and experienced temperatures well in excess of 14°C during late summer and autumn, with some experiencing temperatures up to 19°C (figure 3b). Individuals released in the English Channel also showed a similar pattern (figure 3b), but individuals released in the German Bight experienced relatively low average temperatures in what appeared to be a more variable environment in the far north and east of the region (figure 3b). Although none of the tagged cod released off Flamborough survived through to the end of the summer, they appeared to occupy lower than average temperatures (figure 3b). The lowest temperatures were experienced by the two individuals that were tagged off Lowestoft, but moved north into deeper water (more than 50 m) and by individuals tagged in the German Bight (figure 3b).
(d) Comparison of hydrographic data with DST data
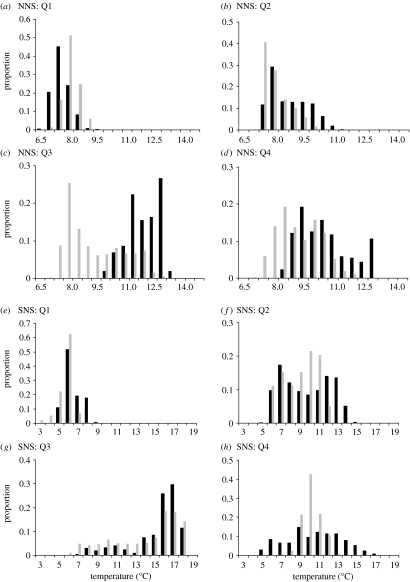
In both regions of the North Sea, cooler water was available for the cod to move into during all quarters of the year except the first, when temperatures were low and relatively uniform (figure 4). In the northern region during the third and fourth quarters of the year, a large fraction of the area, particularly around the Fladden ground (figure 1a), was cooler than the conditions that the majority of cod experienced. Cod released to the west of Shetland tended to be in the warmest fraction of water (figure 4), although this only approached statistical significance in the third quarter (table 2). In the southern North Sea, cooler areas were found in deep water north of 54° latitude (figure 1b). Most individuals, however, occupied the warm shallow waters in the south, and during the third and fourth quarters, they experienced significantly warmer temperatures than they would have done had they simply occupied a representative proportion of the thermal habitat available (figure 4, table 2).
Figure 4.
Frequency histograms representing proportion of DST data (black bars) and sea bottom data (grey bars) at 0.5°C intervals for each quarter (Q) of the year; (a)–(d) northern North Sea; (e)–(h) southern North Sea.
Table 2.
Results of Kolmogorov–Smirnov tests (KS) on equivalent frequency distributions for the seabed temperatures and DST records for each quarter of the year (Q) for cod from the Southern Bight and west Shetland.
| region | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|
| northern North Sea | KS =0.28, p=0.96 | KS =0.33, p=0.73 | KS=0.46, p=0.07 | KS=0.18, p=0.99 |
| southern North Sea | KS =0.31, p=0.58 | KS=0.33, p=0.19 | KS=0.48, p=0.003 | KS=0.56, p<0.001 |
4. Discussion
The North Sea has warmed in recent decades and there is evidence to suggest that the geographical distributions of several fish species, including cod, have shifted north towards cooler waters (Beare et al. 2004; Perry et al. 2005; Rindorf & Lewy 2006). Not all evidence is consistent with this scenario, however; Heessen & Daan (1994) observed that cod aged 3 and 4 years (equivalent to those in our study) tended to be found in higher numbers in the warmer fraction of the North Sea. The present study suggests that while cod were capable of finding cooler waters, the majority occupied the upper fraction of thermal habitat available. Although this does not contradict Perry et al.'s (2005) conclusion, we should consider why, if rising temperatures have pushed cod north, we did not observe any response to temperature at the individual level. Perry et al.'s (2005) and Rindorf & Lewy's (2006) data were drawn from across the entire North Sea. This can be a concern because there is mounting evidence that the North Sea cod stock shows a high level of population structure (Hutchinson et al. 2001; Metcalfe 2006; Wright et al. 2006b). What may seem like a distribution shift may, in fact, represent changes in the abundance and distribution of local populations as a consequence of the effects of population dynamics, fishing pressure and variation in migration (Hedger et al. 2004). The present study, however, being reliant on regionally explicit, individual-based data collected throughout the year, better captures the thermal habitat associated with the spatial dynamics of cod. If adult movement drives distribution shifts, then our data suggest that a shift has been unlikely and that the observation of Perry et al. (2005) is more likely to be explained by local depletion of cod in the south. If, on the other hand, cod has indeed shifted northward, our data suggest that the mechanism by which it has occurred is via recruitment or settlement rather than adult movement.
The northern and southern regions of the North Sea are hydrographically distinct. The northern region is strongly influenced by the Atlantic inflow (Turrell et al. 1992, 1996) and being deeper, has a lower and more stable thermal regime. Thermally distinct areas establish in the summer when the cooler water becomes isolated beneath a thermally stratified water column. In the south, where water depths rarely exceed 50 m, a high degree of mixing and a strong influence of continental air temperature lead to a highly variable thermal environment. The thermal variability across the two regions is clearly reflected in the DST records. At the extremes, a cod released in the Southern Bight experienced 14°C of annual temperature variation, whereas an individual released east of Shetland experienced less than a 3°C change over the year. In the shorter term, the greatest thermal variation (a 7°C decrease in temperature over 3 days, followed by a return to the previous temperature over 2 days) was experienced by cod in the German Bight during summer, where they occupied water at the interface of the stratified and mixed regions of the southern/central North Sea. Across the entire North Sea, however, it is the cod in the south that should be the most likely to exhibit behavioural thermoregulation and move north or into cool deep waters.
A key assumption in our argument is that the cod had the capacity to occupy any location within the delimited areas. In a week or two after release, the temperatures experienced will be locally biased to conditions within the vicinity of the release site. However, cod, like other large demersal fish species, e.g. plaice (Hunter et al. 2004) and sea bass (Pawson et al. 1987), is a highly mobile species capable of long migrations between feeding and spawning grounds (Robichaud & Rose 2004). Historical records of the tagged adult cod in the North Sea suggest average movement rates of 0.5 km per day and up to 11 km per day in the northern North Sea (Wright et al. 2006a), and movements rates of more than 1.5 km per day and up to 30 km per day in the southern North Sea (with a maximum distance between release and recapture of more than 650 km; Righton et al. 2007). In either region, cod needed to move only in the region of 50–200 km to find cooler water. Therefore, given the number of cod at liberty (129) and the number of days of observation in this study (nearly 15 000), we would expect that if the cod had cause to move to cooler water they had sufficient opportunity to do so. They did not, and so occupied the upper range of temperatures that were potentially available to them. This result is perhaps not surprising for cod from the northern North Sea where summer temperatures were rarely above 13°C, but many cod in the southern North Sea experienced periods of up to four months in temperatures (above 15°C) considered detrimental for adult growth (Jobling 1988). While a small number of individuals did move in such a way that caused them to experience a significantly lower range of temperatures, this really only serves to reinforce our assumption that the cooler waters were available for occupancy.
Our sampling of North Sea cod was inevitably biased by higher returns in some areas than in others for a variety of reasons including number of tags released and differences in fishing effort across the North Sea. As a consequence, the temperature experienced by the cod may have been biased by the propensity of the particular spawning group to migrate (Robichaud & Rose 2004). Cod in the southern North Sea show a range of migratory behaviours; off Flamborough, the German Bight and the Eastern Channel, they are largely resident, whereas Southern Bight cod can be highly migratory (Hutchinson et al. 2001; Robichaud & Rose 2004). Similarly, in the north, cod on the west of Shetland appear to be strongly site-attached throughout the year (Neat et al. 2006), whereas those on the east side are a mixture of residents and migrants (Wright et al. 2006b). However, this variability was equally represented in our study, and since cod tagged in the Southern Bight were especially well represented and they were the group predicted to migrate to avoid warm temperatures, this should not be a serious concern.
This lack of observed thermal response can be interpreted in a number of ways. First, that temperature is not the primary driver for cod habitat choice. Other biotic factors, such as prey availability, density-dependent effects (Swain 1999; Swain et al. 2003; Blanchard et al. 2005) and demographic change (Ottersen et al. 2006) may have important consequences for cod distribution and behaviour (Righton et al. 2001). Since the abundance of cod in the North Sea has remained below biologically safe limits since 1999, it is possible that reduced competition for habitat and food resources reduces movement rates and thereby influences habitat choice. Blanchard et al. (2005), however, suggest that cod are more likely to occupy the most suitable habitat when abundance is low. Addressing this issue seriously, however, would require reconstruction of the long-term feeding patterns of the individual fishes, which is not yet possible (Righton et al. 2001). Second, the costs of searching for and finding a narrow range of optimal temperatures in a highly variable environment like the North Sea may outweigh the benefits of finding them. Our knowledge of how fishes detect thermal gradients, or gradients in other aspects of their environment, is limited (Brown 2003; Sims et al. 2006). Our data show that fishes at different times of the year and in different areas of the North Sea can experience sometimes very similar and sometimes very different thermal conditions. This illustrates the temporal and spatial complexity of the thermal habitat and suggests that simple search strategies to find suitable temperatures would not necessarily succeed. Third, thermal tolerance and optima may have been inadequately described by laboratory experiments; natural or regional variance in thermal tolerance of cod may permit residence in apparently unsuitable areas. Studies of haemoglobin genotypes have shown that the Hb1-1 genotype is associated with warm water preference in juvenile cod (Petersen & Steffensen 2003) and the Hb1-1 allele is found at its highest frequency in cod from warmer areas, particularly the southern North Sea (Sick 1965; Husebø et al. 2004). Unfortunately, we were not able to determine Hb genotype of the tagged cod due to the difficulties of undertaking the analysis at sea and our concern that blood sampling may have compromised the survival of tagged fishes. Growth of cod of all Hb genotypes appears to be optimized at 14°C (Jordan et al. 2006). However, recent evidence suggests that the ability of cod to extract oxygen from seawater is maximized at around 12°C, regardless of Hb genotype (Colosimo et al. 2003). Combined, these studies suggest that cod in water significantly warmer than 14°C should benefit by moving to cooler water.
The North Sea is predicted to gradually warm for the next 50 years (Clark et al. 2003). If this will further exacerbate the decline of North Sea cod, this study concurs with conclusions of Heessen & Daan (1994), O'Brien et al. (2000), Beaugrande et al. (2003) and Ottersen et al. (2006) by suggesting that the mechanism by which it occurs must be at the level of recruitment or early settlement rather than adult behaviour. Our finding is also consistent with fishing mortality on cod in southern areas having been higher and as a consequence caused the distribution to contract in the south. There is a growing consensus that the North Sea cod stock is spatially structured (Hutchinson et al. 2001; Yoneda & Wright 2004; Wright et al. 2006a) and this study suggests that the cod populations it supports experience quite different thermal environments. We might therefore expect these populations to show different thermal tolerances and growth responses. Our data provide the first direct measurements of the temperature experienced by cod in the North Sea and as such provide an assessment of the occupancy of thermal habitat that accounts for spatial structuring and individual variation. They do not suggest any consistent pattern with respect to thermally selective behaviour or movement; therefore, they do not support the idea that adult cod are being forced north by rising sea temperatures.
Acknowledgments
We are indebted to many colleagues who contributed to this study, Julian Metcalfe, Peter Wright, Craig Mills, Iain Gibb, Kathryn Turner, Fiona Gibb, Paul Fernandes, Natasha Taylor, Bob Turner, Bill Turrell, the skipper and crew of FRV Clupea, FRV Scotia, the many skippers and the fishermen who have cooperated with us by helping us to tag cod and also by returning tags. We thank two anonymous referees for the constructive criticism of the study. The study was jointly funded by EU projects METACOD (Q5RS-2001-00953), CODYSSEY (QLRT-2001-00813), Defra (MF0154) and the Scottish Executive (MF 0756).
References
- Beare D, Burns F, Jones E, Peach K, Portilla E, Greig T, McKenzie E, Reid D. An increase in the abundance of anchovies and sardines in the north-western North Sea since 1995. Global Change Biol. 2004;10:1209–1213. doi:10.1111/j.1529-8817.2003.00790.x [Google Scholar]
- Becker G.A, Pauly M. Sea surface temperature changes in the North Sea and their consequences. ICES J. Mar. Sci. 1996;53:887–898. doi:10.1006/jmsc.1996.0111 [Google Scholar]
- Beaugrande G, Brander K.M, Lindley J.A, Souissi S, Reid P.C. Plankton effect on cod recruitment in the North Sea. Nature. 2003;426:661–664. doi: 10.1038/nature02164. doi:10.1038/nature02164 [DOI] [PubMed] [Google Scholar]
- Bjornsson B, Steinarsson A, Oddgeirsson M. Optimal temperature for growth and feed conversion of immature cod (Gadus morhua L.) ICES J. Mar. Sci. 2001;58:29–38. doi:10.1006/jmsc.2000.0986 [Google Scholar]
- Björnsson B, Steinarsson A. The food-unlimited growth rate of Atlantic cod (Gadus morhua) Can. J. Fish. Aquat. Sci. 2002;59:494–502. doi:10.1139/f02-028 [Google Scholar]
- Blanchard J.L, Mills C, Jennings S, Fox C.J, Rackham B.D, Eastwood P.D, O'Brien C.M. Distribution–abundance relationships for North Sea Atlantic cod (Gadus morhua): observation versus theory. Can. J. Fish. Aquat. Sci. 2005;62:2001–2009. doi:10.1139/f05-109 [Google Scholar]
- Boutilier R.G. Physiological ecology in cold ocean fisheries: a case study in Atlantic cod. In: Pörtner H.O, Playle R.C, editors. Cold ocean physiology. Cambridge University Press; Cambridge, UK: 1998. pp. 463–469. [Google Scholar]
- Brown B.R. Sensing temperature without ion channels. Nature. 2003;421:495. doi: 10.1038/421495a. doi:10.1038/421495a [DOI] [PubMed] [Google Scholar]
- Brander K.M. The effect of temperature on growth of Atlantic cod (Gadus morhua L.) ICES J. Mar. Sci. 1995;52:1–10. doi:10.1016/1054-3139(95)80010-7 [Google Scholar]
- Christensen V, Guénette S, Heymans J.J, Walters C.J, Watson R, Zeller D, Pauly D. Hundred year decline of North Atlantic predatory fishes. Fish Fish. 2003;4:1–24. [Google Scholar]
- Claireaux G, Webber D.M, Lagardère J.-P, Kerr S.R. Influence of water temperature and oxygenation on the aerobic metabolic scope of Atlantic cod (Gadus morhua) J. Sea Res. 2000;44:257–265. doi:10.1016/S1385-1101(00)00053-8 [Google Scholar]
- Clark R.A, Fox C.J, Viner D, Livermore M. North sea cod and climate change–modelling the effects of temperature on population dynamics. Global Change Biol. 2003;9:1669–1680. doi:10.1046/j.1365-2486.2003.00685.x [Google Scholar]
- Colosimo A, Giuliani A, Maranghi F, Brix O, Thorkildsen S, Fischer T, Knust R, Poertner H.O. Physiological and genetical adaptation to temperature in fish populations. Cont. Shelf Res. 2003;23:1919–1928. doi:10.1016/j.csr.2003.06.012 [Google Scholar]
- Cook R.M, Sinclair A, Stefànsson G. Potential collapse of North Sea cod stocks. Nature. 1997;385:521–522. doi:10.1038/385521a0 [Google Scholar]
- Daan N, Heesen H.J.L, Pope J. Changes in the North Sea cod stock during the twentieth century. ICES Mar. Sci. Symp. 1994;198:229–243. [Google Scholar]
- Drinkwater K. The response of Atlantic cod to future climate change. ICES J. Mar. Sci. 2005;62:1327–1337. doi:10.1016/j.icesjms.2005.05.015 [Google Scholar]
- Groeneveld J.C, Branch C.M. Long distance migration of South African deep water rock lobster Palinurus gilchristi. Mar. Ecol. Prog. Ser. 2002;232:225–238. [Google Scholar]
- Hedger R.E, McKenzie E, Heath M, Wright P.J, Scott B, Gallego A, Andrews J. Analysis of the spatial distributions of mature cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) abundance in the North Sea (1980–1999) using general additive modelling. Fish Res. 2004;70:17–25. doi:10.1016/j.fishres.2004.07.002 [Google Scholar]
- Heessen H, Daan N. Cod distribution and temperature in the North Sea. ICES Mar. Sci. Symp. 1994;198:244–253. [Google Scholar]
- Hislop J.R.G. Changes in the North Sea gadoid stocks. ICES J. Mar. Sci. 1996;53:1146–1156. doi:10.1006/jmsc.1996.0140 [Google Scholar]
- Horwood J, O' Brien O, Darby C. North Sea cod recovery? ICES J. Mar. Sci. 2006;63:961–968. doi:10.1016/j.icesjms.2006.05.001 [Google Scholar]
- Hughes S.L, Turrell W.R, Newton A. Hydrobiological variability on the northwest European continental shelf during the 1990s and its relation to changes in fish stocks. ICES Mar. Sci. Symp. 2003;219:421–425. [Google Scholar]
- Hulme, M. et al 2002. Climate change scenarios for the United Kingdom: the UKCIP02 scientific report. Tyndall Centre for Climate Change Research, Norwich.
- Hunter E, Metcalfe J.D, Holford B.H, Arnold G.P. Geolocation of free-ranging fish on the European continental shelf as determined from environmental variables II. Reconstruction of plaice ground tracks. Mar. Biol. 2004;144:787–798. doi:10.1007/s00227-003-1242-1 [Google Scholar]
- Husebø Å, Imsland A.K, Nævdal G. Haemoglobin variation in cod: a description of new variants and their geographical distribution. Sarsia. 2004;89:369–378. doi:10.1080/00364820410002631 [Google Scholar]
- Hutchinson W.F, Carvalho G.R, Rogers S.I. Marked genetic structuring in localised spawning populations of cod Gadus morhua in the North Sea and adjoining waters as revealed by microsatellites. Mar. Ecol. Prog. Ser. 2001;223:251–260. [Google Scholar]
- ICES. ICES report on ocean climate 2005. ICES Cooperative Res. Report. 2006;280:1–47. [Google Scholar]
- Jennings S, Alvsvågc J, Cottera A.J.R, Ehrich S, Greenstreet S.P.R, Jarre-Teichmann A, Mergardt N, Rijnsdorp A.D, Smedstad O. Fishing effects in northeast Atlantic shelf seas: patterns in fishing effort, diversity and community structure. III. International trawling effort in the North Sea: an analysis of spatial and temporal trends. Fish. Res. 1999;40:125–134. doi:10.1016/S0165-7836(98)00208-2 [Google Scholar]
- Jobling M. A review of the physiological and nutritional energetics of cod, Gadus morhua L., with particular reference to growth under farmed conditions. Aquaculture. 1988;70:1–19. [Google Scholar]
- Jordan A.D, Lampe J.F, Grisdale-Helland B, Helland S.J, Shearer K.D, Steffansen J.F. Growth of Atlantic cod (Gadus morhua L.) with different haemoglobin subtypes when kept near their temperature preferenda. Aquaculture. 2006;257:44–52. doi:10.1016/j.aquaculture.2006.03.015 [Google Scholar]
- Kaiser M.J, Collie J.S, Hall S.J, Jennings S, Poiner I.R. Modification of marine habitats by trawling activities: prognosis and solutions. Fish Fish. 2002;3:114–136. [Google Scholar]
- Lannig G, Bock C, Sartorius F.J, Portner H.O. Oxygen limitation of thermal tolerance in cod, Gadus morhua L., studied by magnetic resonance imaging and on-line venous oxygen monitoring. Am. J. Physiol. 2004;287:R902–R910. doi: 10.1152/ajpregu.00700.2003. [DOI] [PubMed] [Google Scholar]
- Meehl G.A, Washington W.M, Collins W.D, Arblaster J.M, Hu A, Buja L.E, Strand W.G, Teng H. How much more global warming and sea level rise? Science. 2005;307:1769–1772. doi: 10.1126/science.1106663. doi:10.1126/science.1106663 [DOI] [PubMed] [Google Scholar]
- Metcalfe J.D. Fish population structuring in the North Sea: evidence, processes and mechanisms. J. Fish. Biol. Suppl. C. 2006:48–65. [Google Scholar]
- Metcalfe J.D, Arnold G.P. Tracking fish with electronic tags. Nature. 1997;387:665–666. doi:10.1038/42622 [Google Scholar]
- Metcalfe J.D, Arnold G, McDowall R. Migration. In: Hart P.J.B, Reynolds J.D, editors. Handbook of fish biology & fisheries. vol. 1. Blackwell Scientific; Oxford, UK: 2002. pp. 175–199. [Google Scholar]
- Neat F.C, Wright P.J, Zuur A.F, Gibb I.M, Gibb F.M, Tulett D, Righton D.A, Turner R.J. Residency and depth movements of a coastal group of Atlantic cod (Gadus morhua L.) Mar. Biol. 2006;148:643–654. doi:10.1007/s00227-005-0110-6 [Google Scholar]
- O'Brien C.M.C, Fox J, Planque B, Casey J. Climate variability and North Sea cod. Nature. 2000;404:142. doi: 10.1038/35004654. doi:10.1038/35004654 [DOI] [PubMed] [Google Scholar]
- Ottersen G, Hjermann D.O, Stenseth N.Chr. Changes in the spawninf stock structure strengthen the link between climate and recruitment in a heavily fished cod (Gadus morhua) stock. Fish. Oceanogr. 2006;15:230–243. doi:10.1111/j.1365-2419.2006.00404.x [Google Scholar]
- Palsson O.K, Thorsteinsson V. Migration patterns, ambient temperature and growth of Icelandic cod (Gadus morhua): evidence from storage tag data. Can. J. Fish Aquat. Sci. 2003;60:1409–1423. doi:10.1139/f03-117 [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. doi:10.1038/nature01286 [DOI] [PubMed] [Google Scholar]
- Pawson M.G, Kelley D.F, Pickett G.D. The distribution and migrations of bass, Dicentrarchus labrax L., in waters around England and Wales as shown by tagging. J. Mar. Biol. Assoc. UK. 1987;67:183–217. [Google Scholar]
- Pedersen T, Jobling M. Growth rates of large, sexually mature cod Gadus morhua, in relation to condition and temperature during an annual cycle. Aquaculture. 1989;81:161–168. doi:10.1016/0044-8486(89)90242-1 [Google Scholar]
- Perry A.L, Lowe P.J, Ellis J.R, Reynolds J.D. Climate change and distribution shifts in marine fishes. Science. 2005;308:1912–1915. doi: 10.1126/science.1111322. doi:10.1126/science.1111322 [DOI] [PubMed] [Google Scholar]
- Petersen M.F, Steffensen J.F. Preferred temperature of juvenile Atlantic cod Gadus morhua with different haemoglobin genotypes under normoxia and moderate hypoxia. J. Exp. Biol. 2003;206:359–364. doi: 10.1242/jeb.00111. doi:10.1242/jeb.00111 [DOI] [PubMed] [Google Scholar]
- Righton D, Metcalfe J, Connolly P. Different behaviour of cod in the North and Irish Seas. Nature. 2001;411:156. doi: 10.1038/35075667. doi:10.1038/35075667 [DOI] [PubMed] [Google Scholar]
- Righton D, Kjesbu O.S, Metcalfe J. A field and experimental evaluation of the effects of data storage tags on the growth of cod. J. Fish Biol. 2006;68:385–400. doi:10.1111/j.0022-1112.2006.00899.x [Google Scholar]
- Righton, D., Quayle, V., Hetherington, S. & Burt, G. 2007 Movements and distribution of cod in the southern North sea and English Channel: results from conventional and electronic tagging experiments. J. Mar. Biol. Assoc
- Rindorf A, Lewy P. Warm, windy winters drive cod North and homing of spawners keeps them there. J. Appl. Ecol. 2006;43:445–453. [Google Scholar]
- Robichaud D, Rose G.A. Migratory behaviour and range in the Atlantic cod: inference from a century of tagging. Fish Fish. 2004;5:185–214. [Google Scholar]
- Rose G.A. Reconciling overfishing and climate change with stock dynamics of Atlantic cod (Gadus morhua) over 500 years. Can. J. Fish. Aquat. Sci. 2004;61:1553–1557. doi:10.1139/f04-173 [Google Scholar]
- Sick K. Haemoglobin polymorphism of cod in the North Sea and North Atlantic Ocean. Hereditas. 1965;54:49–69. doi: 10.1111/j.1601-5223.1965.tb02005.x. [DOI] [PubMed] [Google Scholar]
- Sims D.W, Genner M.J, Southward A.J, Hawkins S.J. Timing of squid migration reflects North Atlantic climate variability. Proc. R. Soc. B. 2001;268:2607–2611. doi: 10.1098/rspb.2001.1847. doi:10.1098/rspb.2001.1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims D.W, Witt M.J, Richardson A.J, Southall E.J, Metcalfe J.D. Encounter success of free-ranging marine predator movements across a dynamic prey landscape. Proc. R. Soc. B. 2006;273:1195–1201. doi: 10.1098/rspb.2005.3444. doi:10.1098/rspb.2005.3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundby S. Recruitment of Atlantic cod stocks in relation to temperature and advection of copepod populations. Sarsia. 2000;85:277–298. [Google Scholar]
- Swain D.P. Changes in the distribution of Atlantic cod (Gadus morhua) in the southern Gulf of St Lawrence–effects of environmental change or change in environmental preferences? Fish. Oceanogr. 1999;8:1–17. doi:10.1046/j.1365-2419.1999.00090.x [Google Scholar]
- Swain D.P, Sinclair A.F, Castonguay M, Chouinard G.A, Drinkwater K.F, Fanning L.P, Clark D.S. Density versus temperature-dependent growth of Atlantic cod (Gadus morhua) in the Gulf of St. Lawrence and on the Scotian Shelf. Fish. Res. 2003;59:327–341. doi:10.1016/S0165-7836(02)00027-9 [Google Scholar]
- Turrell W.R, Henderson E.W, Slesser G, Payne R, Adams R.D. Seasonal changes in the circulation of the northern North Sea. Cont. Shelf Res. 1992;12:257–286. doi:10.1016/0278-4343(92)90032-F [Google Scholar]
- Turrell W.R, Slesser G, Payne R, Adams R.D, Gillibrand P.A. Hydrography of the East Shetland Basin in relation to decadal North Sea variability. ICES J. Mar. Sci. 1996;53:899–916. doi:10.1006/jmsc.1996.0112 [Google Scholar]
- Wright P.J, Galley E, Gibb I.M, Neat F.C. Fidelity of adult cod to spawning grounds in Scottish waters. Fish. Res. 2006a;77:148–158. doi:10.1016/j.fishres.2005.10.008 [Google Scholar]
- Wright, P. J., Neat F. C., Gibb F. M., Gibb, I. M. & Thordarson, H. 2006b Evidence for metapopulation structuring in cod from the west of Scotland and North Sea. J. Fish Biol. Suppl. C, 181–199.
- Yoneda M, Wright P.J. Temporal and spatial variation in reproductive investment of Atlantic cod Gadus morhua in the northern North Sea and Scottish west coast. Mar. Ecol. Prog. Ser. 2004;276:237–248. [Google Scholar]
- Yoneda M, Wright P.J. Effects of varying temperature and food availability on growth and reproduction in first-time spawning female Atlantic cod. J. Fish Biol. 2005;67:1225–1241. doi:10.1111/j.1095-8649.2005.00819.x [Google Scholar]
- Young E. F. 2002 Tidal validation of a three-dimensional primitive equation model for the North Sea region. CEFAS internal report, Pakefield Rd, Lowestoft, UK.
- Young E.F, Brown J, Aldridge J.N, Horsburgh K.J, Fernand L. Development and application of a three-dimensional baroclinic model to the study of the seasonal circulation in the Celtic Sea. Cont. Shelf Res. 2003;24:13–36. doi:10.1016/j.csr.2003.09.003 [Google Scholar]