Abstract
Artificially selected lines are widely used to investigate the genetic basis of quantitative traits and make inferences about evolutionary trajectories. Yet, the relevance of selected traits to field fitness is rarely tested. Here, we assess the relevance of thermal stress resistance artificially selected in the laboratory to one component of field fitness by investigating the likelihood of adult Drosophila melanogaster reaching food bait under different temperatures. Lines resistant to heat reached the bait more often than controls under hot and cold conditions, but less often at intermediate temperatures, suggesting a fitness cost of increased heat resistance but not at temperature extremes. Cold-resistant lines were more common at baits than controls under cold as well as hot field conditions, and there was no cost at intermediate temperatures. One of the replicate heat-resistant lines was caught less often than the others under hot conditions. Direct and correlated patterns of responses in laboratory tests did not fully predict the low performance of the heat selected lines at intermediate temperatures, nor the high performance of the cold selected lines under hot conditions. Therefore, lines selected artificially not only behaved partly as expected based on laboratory assays but also evolved patterns only evident in the field releases.
Keywords: field releases, genotype×environment interactions, resource location, thermal stress resistance, selection experiments
1. Introduction
In experimental physiology and evolutionary biology, artificial and natural laboratory selection experiments are often used to study evolutionary processes. They provide an opportunity to observe evolution as it occurs and can contribute to an understanding of physiological mechanisms and specific genes under selection (Gibbs 1999; Harshman & Hoffmann 2000; Brakefield 2003; Conner 2003). Furthermore, direct and correlated responses can be studied and yield information on the pleiotropic basis of evolutionary constraints resulting from tradeoffs between traits (Rose et al. 1992) and insights about the genetic variance and covariance structure within and between traits can be obtained (Brakefield 2003; Sgrò & Blows 2004).
Selection experiments on stress resistance traits have received much attention recently, partly because they can indicate processes that might be involved in adaptation to climate change (reviewed in Hoffmann et al. 2003). Drosophila has often been the organism of choice in such investigations because (i) it is possible to maintain replicate lines including unselected control lines and (ii) selective conditions can be easily defined in the laboratory. In addition, the genomic information available for several Drosophila species helps to link variation at different levels of biological organization and assists in detecting specific genes involved in phenotypic shifts.
Yet, despite the popularity of laboratory selection experiments, they have limitations for making physiological and evolutionary inferences (Gibbs 1999; Harshman & Hoffmann 2000). Similar selection regimes have often produced different patterns of direct and correlated responses, and results can be highly dependent on the genetic background and the environmental conditions under which selection is performed (Rose et al. 1992; Chippindale et al. 1994; Tower 1996; Harshman et al. 1999; Partridge et al. 1999; Bubliy & Loeschcke 2005). The importance of genotype×environment interactions means that results from one laboratory might not be extrapolated to another one or to field conditions (Harshman & Hoffmann 2000). Given that field conditions are more complex than laboratory environments, selection experiments might yield only limited insights into adaptive processes in the field (Santos et al. 2005).
One way of linking laboratory experiments to adaptive processes in the field is to examine the performance of lines generated by laboratory selection under field conditions. If selected phenotypes influence fitness in nature, there should be predictable fitness benefits and costs under field conditions. Location of field resources has previously been adopted to investigate physiological condition in Drosophila (Turelli & Hoffmann 1988) and the association between acclimation and field performance in parasitoids (West et al. 1996; Thomson et al. 2001). This approach has been used to test the beneficial acclimation hypothesis and the effects of crowding during development and adult size in Drosophila melanogaster in the field (Hoffmann & Loeschcke 2006; Loeschcke & Hoffmann in press). An advantage of this approach is that performance can be repeatedly measured with lines under different environmental conditions, although only one component of fitness is measured.
Here, we test if D. melanogaster lines artificially selected for cold and heat resistance in the laboratory differ in their ability to locate field resources under a range of temperature conditions. We released both sets of lines across field temperatures that encompass the low-temperature flight threshold in Drosophila as well as approaching the upper thermal limit for survival. We test the hypothesis that flies from the cold-resistant lines have higher frequencies of appearance at food resources in the field under cold conditions, and flies from heat-resistant lines have higher frequencies of appearance at food resources at the opposite extreme. We also address whether cold-resistant lines are (relatively) less likely to appear at baits than control lines under particularly hot conditions, and vice versa for the heat-resistant lines: in other words, is there a tradeoff for performance at the two temperature extremes?
2. Material and methods
(a) Experimental populations
The lines were derived from a genetically diverse massbred population of D. melanogaster established in September 2002 at the University of Aarhus, Denmark, by crossing several populations collected in Denmark, Australia and The Netherlands. Non-selected control lines (C) and lines selected for cold-shock resistance (CS) and heat-shock resistance (HS) were established by flies from the massbred population. The lines have previously been used in assaying direct and correlated responses to selection in the laboratory (Bubliy & Loeschcke 2005), and to compare gene expression patterns among control and selected lines (Nielsen et al. 2006). Details about the lines are provided in Bubliy & Loeschcke (2005). Briefly, the CS lines were selected for cold resistance every second generation following exposure to 11°C for 5 days to acclimate the flies. Acclimated individuals were chilled at 0.5°C for 27–50 h (depending on generation of selection) and surviving individuals selected. The HS lines were also selected every second generation following hardening for 30 min at 36°C, by exposure to 38°C for 1 h (this was increased to 38.5°C to maintain similar selection intensity across generations). The lines were selected for 34 generations with a census population size surviving selection above 500 individuals per generation in all lines. The intensity of selection in all selection lines was around 0.80. In generations with no selection, population sizes were always above 1000 individuals.
(b) Laboratory assessments
Prior to starting field releases, three C, CS and HS lines (ID's: C3, C4, C5, CS1, CS3, CS4, HS1, HS4, HS5) were assayed for heat and cold resistance on two occasions (September 2005 and January 2006) after moving the lines to the University of Melbourne, Australia. Prior to the two assessments, the lines had not been selected for, respectively, 6 and 14 generations. All lines were assessed for cold and heat resistance without prior acclimation using mortality assays. Cold resistance was assessed on 3-day-old flies exposed to 0.5°C in a water bath for 48 (September 2005) or 60 h (January 2006) in empty vials. The cold stressed flies were allowed to recover for 24 h at 25°C in vials with medium, and mortality was then scored. Heat resistance was assayed by exposing 3-day-old flies to 38.5°C for 1 h in a water bath. Heat stressed flies were left for 1 h in empty vials before mortality was scored. In both cold and heat assays, flies were scored as alive if they were able to move any part of their body. In all the assessments, five replicates each with 10 flies were assayed per sex and line.
(c) Releases
Lines were maintained in 600 ml bottles each containing 100 ml of sugar–agar–dead yeast medium with propionic acid and methyl p-hydroxybenzoate as preservatives (except during the periods of selection as described above). In two generations, prior to the release experiments, density was partly controlled in bottles by leaving 50–60 adults per bottle to lay eggs for a day. This ensured that flies developed at an intermediate density (300–500 offspring per bottle). After emergence, flies were transferred (without anaesthesia) to bottles with the medium and held at 25°C. On the day before release, 24–48 h old flies were transferred to vials each with 5 ml of medium (50 flies per vial) and kept at 25°C until just before release. Flies were not sexed. A subsample of flies to be released was tested and we found no deviation from a 1 : 1 sex ratio (results not shown).
We compared lines in six releases in cold conditions (cold 1–6), three at intermediate temperatures (intermediate 1–3) and four in hot conditions (hot 1–4). In each release, one CS, HS and C line were compared. Temperature conditions during each release and lines compared are given in table 1. In the cold releases, each of the three combinations of lines was tested twice. The fourth hot release represented a repeat of hot 3 because we noted that one of the heat lines (HS5) was caught less frequently than the other lines, and we wanted to test the repeatability of this pattern. In addition, we undertook three releases at intermediate temperatures with control flies (control 1–3). For the six releases undertaken at cold conditions (cold 1–6), capture points were 5 m apart starting 5 m from the release site and extending up to 30 m in opposite directions from the release point. For the other releases, capture points were 10 m apart extending up to 60 m in opposite directions from the release point. At each capture point, three buckets were placed 2–3 m apart running perpendicular to the release line. In total, 36 buckets were used per release (for further details see Loeschcke & Hoffmann in press). Temperatures were recorded with data loggers (Tinytalk II) placed in the shadow approximately 1 m above the ground close to the release site (table 1). Releases took place in woodland in Victoria, Australia, without soft fruit where Drosophila might feed and breed. The woodlands consisted of eucalyptus or pine trees with flowering shrubs.
Table 1.
Release number, lines, number of flies (N) released per line, average capture (%), time of release, time of last capture and temperature data presented for all releases. (Temperatures are given for the time of release, maximum and minimum during the capture period, and average temperatures in the time periods 14.00–17.00. For all hot and control releases, captures were performed only on the day of release and stopped between 18.30 and 19.00. For intermediate temperature and cold releases, flies were released at night and captures were performed on the next day only (intermediate temperature releases) or over 2 days (cold releases). In cells with two temperatures (cold releases), numbers above the ‘/’ are temperatures on the first day of capture and numbers below the ‘/’ are temperature on the second day of capture. ‘″’ indicates similar values as in the cell above.)
| temperatures (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| release number | lines released | N per line | average capture (%) | time released (day 0) | last capture | at release | maximum | minimum | 1400–1700 |
| cold | |||||||||
| 1 | C4, CS3, HS4 | 2200 | 12.1 | 2300 | 1400 (day 2) | 9.2 | 14.2/18.3 | 3.9/0.6 | 12.5/15.0 |
| 2 | C3, CS1, HS1 | 2200 | 9.6 | 2300 | 1400 (day 2) | ″ | ″ | ″ | ″ |
| 3 | C5, CS4, HS5 | 2200 | 15.3 | 2300 | 1400 (day 2) | ″ | ″ | ″ | ″ |
| 4 | C4, CS3, HS4 | 2200 | 10.8 | 2300 | 1400 (day 2) | 9.2 | 12.8/17.7 | 4.6/0.8 | 12.1/14.4 |
| 5 | C3, CS1, HS1 | 2200 | 16.6 | 2300 | 1400 (day 2) | ″ | ″ | ″ | ″ |
| 6 | C5, CS4, HS5 | 2200 | 11.7 | 2300 | 1400 (day 2) | ″ | ″ | ″ | ″ |
| intermediate | |||||||||
| 1 | C4, CS3, HS4 | 3000 | 27.7 | 2100 | 1330 (day 1) | 11.6 | 24.0 | 9.8 | 23.1 |
| 2 | C3, CS1, HS1 | 3000 | 42.9 | 2200 | 1300 (day 1) | 11.9 | 24.2 | 10.7 | 23.4 |
| 3 | C5, CS4, HS5 | 3000 | 36.6 | 0200 | 1300 (day 1) | 13.0 | 26.1 | 11.9 | 24.5 |
| hot | |||||||||
| 1 | C4, CS3, HS4 | 2500 | 18.7 | 1500 | 1900 (day 0) | 36.5 | 40.1 | 33.6 | 36.5 |
| 2 | C3, CS1, HS1 | 2500 | 1.0 | 1500 | 1900 (day 0) | ″ | ″ | ″ | ″ |
| 3 | C5, CS4, HS5 | 3000 | 3.2 | 1430 | 1730 (day 0) | 32.5 | 38.0 | 28.7 | 33.1 |
| 4 | C5, CS4, HS5 | 3000 | 17.3 | 1430 | 1730 (day 0) | ″ | ″ | ″ | ″ |
| control | |||||||||
| 1 | C3, C4, C5 | 2500 | 21.6 | 1315 | 1715 (day 0) | 20.1 | 21.1 | 15.7 | 20.0 |
| 2 | C3, C4, C5 | 2500 | 9.8 | 1345 | 1745 (day 0) | ″ | ″ | ″ | ″ |
| 3 | C3, C4, C5 | 2200 | 2.8 | 1300 | 1700 (day 0) | 18.3 | 19.9 | 13.1 | 16.3 |
Flies were transported by car to the release sites in insulated styrofoam boxes kept at 24–26°C. Just before the release, groups of 100 flies (two vials each with 50 flies) were transferred into vials with 0.0015 (±0.0005) g of fluorescent micronized dust (Radiant) and lightly shaken according to the recommendations in Moth & Barker (1975). Dust colours were randomly assigned to the different lines, and changed between releases. Between 2000 and 3000 flies were released per treatment group; 6000–9000 flies per release in total (table 1). To release flies, vials with flies from the different lines were randomly arranged in a container and foam stoppers were removed from the vials.
For hot and control releases, flies were released around the hottest time of the day (table 1). For the intermediate temperature and cold releases, flies were released at night (when temperatures were below 15°C). For hot and control releases, flies were captured from resources (buckets with mashed banana) by netting and/or aspirating, starting 1 h after release and every hour subsequently until at least four rounds of captures were performed. For the intermediate temperature releases, flies were captured from early morning until early afternoon on the day following the release. For the cold releases, flies were captured over 2 days. On the first day following the release, captures started at 14.00 (no flies were observed in the buckets before 14.00) and continued every subsequent hour until 17.00. On the second day, the first capture was at 11.00 and the last one was initiated at 14.00 (table 1).
Captured flies were held on ice in the field to incapacitate them and thereby minimize transfer of dust colour between the flies. They were then transported to the lab and frozen until colours were scored under ultraviolet light (Blak-Ray). Far less than 1% of captured flies were unmarked and were discarded.
(d) Statistical analysis
Analyses were carried out in SPSS for Windows v. 14. ANOVAs on resistance data were carried out on arcsine transformed proportions and involved a nested design with the terms sex, experiment, selection regime and line (random factor) nested within selection regime.
Capture data were treated as categorical and analysed separately for each release. We assessed the difference between lines in capture success using logit log-linear models where the effect of the line and sex were tested. The sample of flies that was not captured was estimated assuming a 1 : 1 sex ratio, a ratio that had been experimentally validated (data not presented). The logit analysis assumes that each fly represents an independent data point for a selected line and sex. The assumption of independence is likely to be justified because flies were pooled across multiple culture bottles (to minimize culture effects) and released in a randomized position at the field site (to minimize any point of release effects). Apart from using a logit analysis, we also undertook a simpler analysis where capture rates were compared directly between the lines in each release, and tested using the chi-square statistic against an expectation of equal numbers of individuals being captured from each line in a release.
We then considered only the capture data to assess capture success at various time points after release and distances from the release point using a hierarchical log-linear model design (Sokal & Rohlf 1981, p. 747ff). Models involved the variables sex, distance from release point, (time of) capture and selection line. Log-linear models were chosen based on both backward elimination and forward selection. When there were interactions between capture, distance and selection line treatments, we undertook contingency analysis to further investigate the association between these variables for each sex (this analysis also assumes that the data points are independent).
To indicate the relative capture success of the selected line treatments compared to the controls, we computed the ‘relative risk’ of each selection line being caught relative to the control lines, following the approach outlined in Loeschcke & Hoffmann (in press). For this measure of relative capture success, values greater than 1 imply that the likelihood of capture is higher for a selected line relative to a control line. We also computed relative capture success of males relative to females. For the selected line comparisons, data were combined across sexes unless there was a significant sex effect in the logit models.
Finally, we undertook Friedman tests to look for consistent patterns in the performance of the three types of lines across the releases. The relative number of flies captured was ranked for the three types of lines and then analysed.
3. Results
(a) Assessment of heat and cold resistance in the laboratory
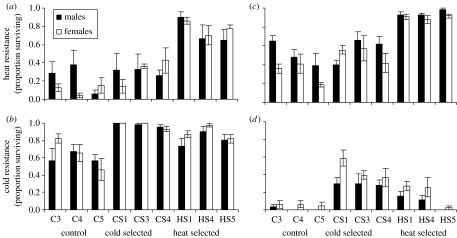
In the nested permutation ANOVA on heat resistance, there was a highly significant difference between the September 2005 and the January 2006 assessments (F1,156=42.79, p<0.001) as well as a highly significant effect of selection regime (F2,6=45.38, p<0.001) with the HS lines having a higher survival rate compared with the C lines in both assessments (figure 1). Sex influenced heat resistance (F1,6=10.02, p=0.02) and there were no significant interaction terms or differences among the replicate lines. There was also a tendency towards the CS lines having heat resistance levels higher than the control lines in both assessments (figure 1); this is non-significant in a nested ANOVA comparing only these lines (F1,4=3.79, p=0.12), although it is significant when increasing the number of degrees of freedom by testing over the combined error term that includes the non-significant replicate line and interaction terms (F1,116=8.88, p=0.004).
Figure 1.
Heat and cold resistance (proportion surviving±s.e.) in control, cold- and heat-selected lines for males and females. (a,b) Data from the September 2005 assessment. (c,d) Data from the January 2006 assessment.
For cold resistance, the nested ANOVA indicated highly significant effects of selection regime (F2,6=30.75, p<0.001) and a large difference between the experiments (F1,15=389.06, p<0.001). The CS lines were more resistant than the C lines in both assessments (figure 1). There was a tendency for HS lines to be more resistant than the C lines in both assessments (figure 1), and a nested ANOVA comparing CS and HS lines confirmed a significant difference between the selection treatments (F1,4=8.64, p=0.042). The effect of sex was significant (F1,6=12.83, p=0.011) showing that the proportion of females surviving cold stress was higher than males.
(b) Releases
The number of flies caught varied from 1 to 42.9% (table 1) with a tendency towards catching more flies in releases at intermediate temperatures. For all analyses, flies caught in the two directions were combined (e.g. flies caught 10 m from the release point in the two directions), as were flies caught more than 15 (cold releases) or 30 m (all other releases) from the release sites owing to the low numbers of flies retrieved further away than these points. Data for the releases were analysed and presented separately as different interactions were significant in the releases.
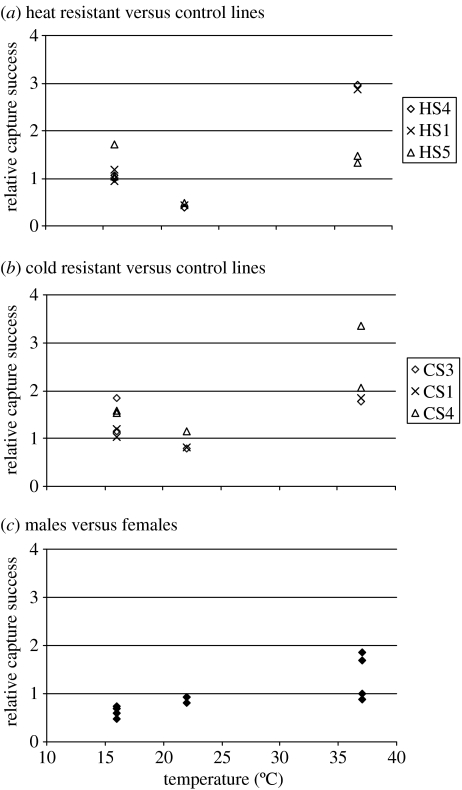
Logit analyses (table 2) showed that sex by line interactions were usually not significant, suggesting that the proportion of males and females caught in the different lines were similar. There were two exceptions and for these cases, relative capture success was estimated separately for the sexes. Sex effects were evident for all releases under cold conditions and for three of the other releases. Males were less frequently caught than females under cold conditions (relative risks below 1; figure 2). However, this pattern changed in two of the four releases under hot conditions.
Table 2.
Results of logit model analyses testing the effects of sex and line on the probability of capture under cold, intermediate and hot environmental temperatures, and for control lines. (Relative risks comparing the likelihood of capture of males versus females and selected lines versus control lines and significance values are also presented (*p<0.05; **p<0.01; ***p<0.001).)
| likelihood ratio due to | relative risks | |||||
|---|---|---|---|---|---|---|
| release | line (d.f.=2) | sex (d.f.=1) | line by sex (d.f.=2) | males | HS line | CS line |
| cold | ||||||
| 1 | 4.30 | 47.98*** | 3.66 | 0.59*** | 1.00 | 1.15 |
| 2 | 4.28 | 15.60*** | 2.79 | 0.48*** | 1.70*** | 1.54*** |
| 3 | 58.32*** | 27.73*** | 5.15 | 0.73*** | 1.18 | 1.034 |
| 4 | 66.31*** | 42.55*** | 0.43 | 0.59*** | 1.07 | 1.84*** |
| 5 | 15.27*** | 32.15*** | 1.41 | 0.69*** | 1.04 | 1.59*** |
| 6 | 41.58*** | 85.23*** | 3.66 | 0.68*** | 0.93 | 1.2** |
| intermediate | ||||||
| 1 | 281.61*** | 20.65*** | 0.89 | 0.80*** | 0.38*** | 0.79*** |
| 2 | 253.09*** | 2.29 | 5.94 | 0.94 | 0.44*** | 0.81*** |
| 3 | 307.79*** | 2.16 | 10.04** | 0.94 | 0.47*** | 1.16** |
| hot | ||||||
| 1 | 223.41*** | 82.37*** | 51.70*** | 1.70*** | 2.96*** | 1.78*** |
| 2 | 12.16** | 6.76** | 3.91 | 1.86*** | 2.87*** | 1.85 |
| 3 | 9.56** | 0.01 | 0.71 | 0.87 | 1.46* | 2.06*** |
| 4 | 23.53*** | 1.43 | 0.69 | 1.00 | 1.33 | 3.35** |
| control | ||||||
| 1 | 5.19 | 1.51 | 4.11 | 0.90 | 0.90 | |
| 2 | 2.06 | 0.74 | 1.46 | 0.94 | 1.06 | |
| 3 | 1.24 | 0.36 | 1.12 | 0.93 | 1.13 | |
Figure 2.
Capture success (relative risk) of male and female flies from the (a) heat-and (b) cold-selected lines relative to flies from the control lines and (c) for males expressed relative to females. Values greater than 1 indicate that the likelihood of capture is higher for flies from a selected line relative to flies from a control line or for males relative to females.
Relative capture success for the CS and the HS flies was plotted as a function of maximum temperature registered from the time of release to the last capture time (figure 2) and interpreted in terms of results from logit analyses (table 2). Flies from the HS lines were captured with similar or higher likelihood than the C lines when mean environmental temperatures were low as in the cold releases. One of the relative risks was significantly greater than 1. At intermediate temperatures, the proportion of HS flies caught was lower relative to C flies, a difference that was significant in all the three releases. Conversely, under hot conditions, HS flies were caught relatively more often than the C flies, and relative capture success was significantly larger than 1 in three of the four releases (table 2). One of the HS lines (HS5) had a lower relative risk than the others and, therefore, we repeated the release with this line (hot 4). In this repeat release, the relative capture success of HS5 was also low.
Flies from the CS lines were caught more often than flies from the C lines in the cold releases and this difference was significant in four of the six releases (table 2). There was no consistent difference between the CS and the C flies in the likelihood of capture under intermediate temperature conditions, while under hot environmental conditions CS flies were caught more frequently than the flies from the C lines (figure 2). This difference was significant in three of the four releases (table 2). Chi-square tests were undertaken to compare the number of flies in the capture sample from the three lines. Since the same number of flies were released per line, this number was also expected to be the same in the capture sample. However, the difference between the observed and the expected number was significant (p<0.01) in 11 of the 13 releases. The only exceptions were two cold releases (cold 1 and 3) when no line differences were detected in the logit analysis (table 2). Overall, Friedman tests indicated consistent differences in the capture rates of the three types of lines across releases undertaken in the hot and the cold conditions (p<0.05 in each case). For the intermediate temperature releases, when only three releases were completed, the Friedman test was marginally non-significant (p<0.10).
In releases with the control lines, no significant effects were detected based on the results from the logit analysis (table 2) or comparisons of capture rates (p>0.05 in all the three releases). Relative risks comparing the lines were all around 1 (table 2). Control lines were therefore caught at a similar rate in these three releases.
Terms included in the log-linear analysis testing for significance of sex, distance, line and capture time on numbers of flies caught are presented in table 1 of electronic supplementary material. For the cold releases, the interaction term distance×time was significant in all releases except cold 1 and for females cold 6 (see table 1 in electronic supplementary material). Over time, there was a tendency for flies to be caught further away from the release point. Furthermore, the line by time effect was significant in three releases (see table 1 in electronic supplementary material). In each case, the number of flies caught in the first capture was higher for the CS line relative to the other lines. For the intermediate temperature releases, the interaction term distance×time was significant in all releases and again reflected the tendency for flies to be caught further out later. The line by capture term was also significant in all releases and in each case, flies from the C lines tended to be caught earlier than those from the other lines (see table 1 in electronic supplementary material). For the hot releases, log-linear analyses were not undertaken for releases 2 and 3 due to low numbers captured. The interaction term distance×time was significant in hot 1 (see table 1 in electronic supplementary material) because flies were more widely dispersed at later times.
4. Discussion
The laboratory assessments of heat and cold resistance revealed results that are qualitatively similar to those obtained on the same lines by Bubliy & Loeschcke (2005), despite the fact that selection had been relaxed for several generations prior to the assessments performed here. Previously, only females had been assessed (Bubliy & Loeschcke 2005). We found that the cold and the heat resistance of both sexes had been increased by selection, and that there was a tendency towards the CS lines being more heat resistant than the C lines and the HS lines being more cold resistant than the C lines. In the literature, there is some disagreement about cross-resistance between cold and heat resistance (reviewed in Hoffmann et al. 2003). Correlations between thermal extremes appear to depend on the selection procedures and the type of assay used to assess lines (reviewed in Hoffmann et al. 2003).
A comparison between the laboratory and the field results suggests that there are three cases where differences in laboratory resistance predict field performance. Firstly, selection for increased heat resistance has enhanced the likelihood of locating resources under hot conditions in the field. Flies from the HS lines were caught in consistently higher numbers than those from the controls under hot conditions. This difference between the HS and the C lines may reflect the ability of flies to reach food (and perhaps high humidity provided by the food layer) under extreme hot conditions as well as their ability to survive these conditions. In previous releases, under hot conditions to test acclimation effects, we have shown that only flies hardened by exposure to a sub-lethal high temperature are caught in appreciable numbers (Loeschcke & Hoffmann in press). When baits are left out, no additional flies are caught at later times when temperatures become cooler again, suggesting that flies die when ambient temperatures are higher than 38°C unless the flies locate food and high humidity conditions provided at the baits. Secondly, the relatively higher capture rates of CS flies compared with C flies under cold conditions suggest that laboratory cold resistance predicts field performance under cold conditions. Thirdly, the similar levels of laboratory cold resistance of the HS and the C lines predict their similar capture success under cold conditions.
However, there were also three differences in the field performance of the lines that were not evident from the laboratory results. Firstly, we found that at intermediate temperatures, the HS lines were less likely to be caught than the C lines, suggesting a tradeoff between high performance under extreme and benign conditions. Resistance towards stresses has traditionally been assumed to infer a fitness cost (Bergelson & Purrington 1996; Taylor & Feyereisen 1996; but see Coustau et al. (2000)). For thermal stress, heat-shock proteins may be involved in observed tradeoffs. This group of proteins is known to be of adaptive importance under heat stress (Feder & Hofmann 1999; Sørensen et al. 2003). However, it is costly to possess extra copies of Hsp70 genes under benign conditions (Feder et al. 1996; Roberts & Feder 2000) and patterns of Hsp70 induction in field populations indicate that populations exposed to heat stress have low levels of Hsp70 (Sørensen et al. 2001). Secondly, the replicate HS lines did not behave consistently in the field, unlike in the laboratory. One of the replicate lines (HS5) had a lower relative capture success compared with other HS lines under hot conditions, whereas flies from this same line are caught with one of the highest likelihoods (relative to C lines) under cold environmental conditions (figure 2). Although we only tested one C line and one of each of the selected lines in each release, C lines behaved similarly when compared directly. This indicated that the results are due to an effect of selection on the likelihood of locating resources in nature, and not due to differences between the C lines. The ‘odd behaviour’ of HS5 emphasizes the importance of biological replication in this type of studies. Thirdly, the relatively high capture success of the CS lines under hot conditions was not predicted from the laboratory results. Although the CS lines were somewhat more resistant to heat than controls in our assays and those carried out previously (Bubliy & Loeschcke 2005), this difference was much smaller than the difference in laboratory resistance between the HS and the C lines. Yet, the capture success of the CS and the HS lines in the field relative to the controls was similar under hot conditions. Laboratory selection for cold resistance appears to be as effective as selection for heat resistance in increasing field performance under hot conditions.
In other field release experiments with similar designs to the ones used here, it has been shown that size and crowding during development (Hoffmann & Loeschcke 2006) and hardening (Loeschcke & Hoffmann in press) affect the likelihood of locating resources in the field. The results from the study reported here show that some results from laboratory selection experiments on climatic traits can be related to field performance, emphasizing the ecological relevance of laboratory selection studies. However, our study also reveals some surprising results not predicted from the laboratory assays. The differences emphasize the importance for further development of efficient methods testing questions of physiological and evolutionary interest in the field. The approaches used and conclusion drawn from release experiments are based on the assumption that capture success at baits in a habitat that is otherwise free of natural resources reflects differential survival probabilities under different temperatures and provides a field fitness correlate in Drosophila. However, we have not proven that is the case, and we are aware that this is just one component of fitness, although finding breeding resources may also be relevant to finding mating partners, as flies are often seen mating around resources (Partridge et al. 1987). Fitness components such as mating success, reproduction and survival are not directly investigated in field releases. Nevertheless, the assay represents a valuable addition to the laboratory assays that are normally used by Drosophila researchers to evaluate fitness. Another way of testing the ecological relevance of results obtained from selection experiments in the laboratory is to investigate the field distribution of alleles at candidate genes identified from selection experiments. Most knowledge on mechanisms involved in thermal resistance has so far been obtained from laboratory studies. The present study represents one attempt to move beyond the limitations of the laboratory environment.
Acknowledgments
We are grateful to Maddie Barton, Mark Blacket, Sarah DeGaris, Rebecca Hallas, Vanessa Kellermann, Maria S. Madsen, Elise Norberg, Rhonda Rawlinson, Jennifer Shirriffs, Anders Christian Sørensen, Belinda van Heerwaarden and Ben Wegener for their help with catching flies, to two anonymous reviewers for their critical comments on earlier versions of the manuscript, and to the Danish and Australian Research Councils for financial support by centre grants (V.L. and A.A.H.), a postdoc position to T.N.K. and a Federation Fellowship to A.A.H.
Supplementary Material
Terms included in log linear analysis testing for significance of sex, distance, line and recapture time on numbers of flies caught
References
- Bergelson J, Purrington C.B. Surveying patterns in the cost of resistance in plants. Am. Nat. 1996;148:536–558. doi:10.1086/285938 [Google Scholar]
- Brakefield P.M. Artificial selection and the development of ecologically relevant phenotypes. Ecology. 2003;84:1661–1671. [Google Scholar]
- Bubliy O.A, Loeschcke V. Correlated responses to selection for stress resistance and longevity in a laboratory population of Drosophila melanogaster. J. Evol. Biol. 2005;18:789–803. doi: 10.1111/j.1420-9101.2005.00928.x. doi:10.1111/j.1420-9101.2005.00928.x [DOI] [PubMed] [Google Scholar]
- Chippindale A.K, Hoang D.T, Service P.M, Rose M.R. The evolution of development in Drosophila melanogaster selected for postponed senescence. Evolution. 1994;48:1880–1899. doi: 10.1111/j.1558-5646.1994.tb02221.x. doi:10.2307/2410515 [DOI] [PubMed] [Google Scholar]
- Conner J.K. Artificial selection: a powerful tool for ecologists. Ecology. 2003;84:1650–1660. [Google Scholar]
- Coustau C, Chevillon C, ffrench-Constant R. Resistance to xenobiotics and parasites: can we count the cost? Trends Ecol. Evol. 2000;15:378–383. doi: 10.1016/s0169-5347(00)01929-7. doi:10.1016/S0169-5347(00)01929-7 [DOI] [PubMed] [Google Scholar]
- Feder M.E, Hofmann G.E. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. doi:10.1146/annurev.physiol.61.1.243 [DOI] [PubMed] [Google Scholar]
- Feder M.E, Cartano N.V, Milos L, Krebs R.A, Lindquist S.L. Effect of engineering Hsp70 copy number on Hsp70 expression and tolerance of ecologically relevant heat shock in larvae and pupae of Drosophila melanogaster. J. Exp. Biol. 1996;199:1837–1844. doi: 10.1242/jeb.199.8.1837. [DOI] [PubMed] [Google Scholar]
- Gibbs A.G. Laboratory selection for the comparative physiologist. J. Exp. Biol. 1999;202:2709–2718. doi: 10.1242/jeb.202.20.2709. [DOI] [PubMed] [Google Scholar]
- Harshman L.G, Hoffmann A.A. Laboratory selection experiments using Drosophila: what do they really tell us? Trends Ecol. Evol. 2000;15:32–36. doi: 10.1016/s0169-5347(99)01756-5. doi:10.1016/S0169-5347(99)01756-5 [DOI] [PubMed] [Google Scholar]
- Harshman L.G, Moore K.M, Sty M.A, Magwire M.M. Stress resistance and longevity in selected lines of Drosophila melanogaster. Neurobiol. Aging. 1999;20:521–529. doi: 10.1016/s0197-4580(99)00091-3. doi:10.1016/S0197-4580(99)00091-3 [DOI] [PubMed] [Google Scholar]
- Hoffmann A.A, Loeschcke V. Are fitness effects of density mediated by body size? Evidence from Drosophila field releases. Evol. Ecol. Res. 2006;8:813–828. [Google Scholar]
- Hoffmann A.A, Sorensen J.G, Loeschcke V. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 2003;28:175–216. doi:10.1016/S0306-4565(02)00057-8 [Google Scholar]
- Loeschcke, V. & Hoffmann, A. A. In press. Consequences of heat hardening on a field fitness component in Drosophila depend on environmental temperature. Am. Nat [DOI] [PubMed]
- Moth J.J, Barker J.S.F. Micronized fluorescent dusts for marking Drosophila adults. J. Nat. Hist. 1975;9:393–396. [Google Scholar]
- Nielsen M.M, Sørensen J.G, Kruhøffer M, Justesen J, Loeschcke V. Phototransduction genes are up-regulated in a global gene expression study of Drosophila melanogaster selected for heat resistance. Cell Stress Chaperones. 2006 doi: 10.1379/CSC-207.1. doi:10.1379/CSC-207.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Hoffmann A.A, Jones J.S. Male size and mating success in Drosophila melanogaster and D. pseudoobscura under field conditions. Anim. Behav. 1987;35:468–476. doi:10.1016/S0003-3472(87)80272-5 [Google Scholar]
- Partridge L, Prowse N, Pignatelli P. Another set of responses and correlated responses to selection on age at reproduction in Drosophila melanogaster. Proc. R. Soc. B. 1999;266:255–261. doi: 10.1098/rspb.1999.0630. doi:10.1098/rspb.1999.0630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S.P, Feder M.E. Changing fitness consequences of hsp70 copy number in transgenic Drosophila larvae undergoing natural thermal stress. Funct. Ecol. 2000;14:353–357. doi:10.1046/j.1365-2435.2000.00429.x [Google Scholar]
- Rose M.R, Vu L.N, Park S.U, Graves J.L. Selection on stress resistance increases longevity in Drosophila melanogaster. Exp. Gerontol. 1992;27:241–250. doi: 10.1016/0531-5565(92)90048-5. doi:10.1016/0531-5565(92)90048-5 [DOI] [PubMed] [Google Scholar]
- Santos M, Cespedes W, Balanya J, Trotta V, Calboli F.C.F, Fontdevila A, Serra L. Temperature-related genetic changes in laboratory populations of Drosophila subobscura: evidence against simple climatic-based explanations for latitudinal clines. Am. Nat. 2005;165:258–273. doi: 10.1086/427093. doi:10.1086/427093 [DOI] [PubMed] [Google Scholar]
- Sgró C.M, Blows M.W. The genetic covariance among clinal environments after adaptation to an environmental gradient in Drosophila serrata. Genetics. 2004;167:1281–1291. doi: 10.1534/genetics.103.026120. doi:10.1534/genetics.103.026120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. 2nd edn. W. H. Freeman; San Francisco, CA: 1981. Biometry. [Google Scholar]
- Sørensen J.G, Dahlgaard J, Loeschcke V. Genetic variation in thermal tolerance among natural populations of Drosophila buzzatii: down regulation of Hsp70 expression and variation in heat stress resistance traits. Funct. Ecol. 2001;15:289–296. doi:10.1046/j.1365-2435.2001.00525.x [Google Scholar]
- Sørensen J.G, Kristensen T.N, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 2003;6:1025–1037. doi:10.1046/j.1461-0248.2003.00528.x [Google Scholar]
- Taylor M, Feyereisen R. Molecular biology and evolution of resistance to toxicants. Mol. Biol. Evol. 1996;13:719–734. doi: 10.1093/oxfordjournals.molbev.a025633. [DOI] [PubMed] [Google Scholar]
- Thomson L.J, Robinson M, Hoffmann A.A. Field and laboratory evidence for acclimation without costs in an egg parasitoid. Funct. Ecol. 2001;15:217–221. doi:10.1046/j.1365-2435.2001.00516.x [Google Scholar]
- Tower J. Aging mechanisms in fruit flies. Bioassays. 1996;18:799–807. doi: 10.1002/bies.950181006. doi:10.1002/bies.950181006 [DOI] [PubMed] [Google Scholar]
- Turelli M, Hoffmann A.A. Effects of starvation and experience on the response of Drosophila to alternative resources. Oecologia. 1988;77:497–505. doi: 10.1007/BF00377265. doi:10.1007/BF00377265 [DOI] [PubMed] [Google Scholar]
- West S.A, Flanagan K.E, Godfray H.C.J. The relationship between parasitoid size and fitness in the field, a study of Achrysocharoides zwoelferi (Hymenoptera: Eulophidae) J. Anim. Ecol. 1996;65:631–639. doi:10.2307/5742 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Terms included in log linear analysis testing for significance of sex, distance, line and recapture time on numbers of flies caught