Abstract
The causes of variation in animal species richness at large spatial scales are intensively debated. Here, we examine whether the diversity of food plants, contemporary climate and energy, or habitat heterogeneity determine species richness patterns of avian frugivores across sub-Saharan Africa. Path models indicate that species richness of Ficus (their fruits being one of the major food resources for frugivores in the tropics) has the strongest direct effect on richness of avian frugivores, whereas the influences of variables related to water–energy and habitat heterogeneity are mainly indirect. The importance of Ficus richness for richness of avian frugivores diminishes with decreasing specialization of birds on fruit eating, but is retained when accounting for spatial autocorrelation. We suggest that a positive relationship between food plant and frugivore species richness could result from niche assembly mechanisms (e.g. coevolutionary adaptations to fruit size, fruit colour or vertical stratification of fruit presentation) or, alternatively, from stochastic speciation–extinction processes. In any case, the close relationship between species richness of Ficus and avian frugivores suggests that figs are keystone resources for animal consumers, even at continental scales.
Keywords: Africa, coevolution, community assembly, macroecology, plant–frugivore interactions, spatial autoregressive model
1. Introduction
A large number of hypotheses have been proposed to explain patterns of species richness at broad spatial scales (Willig et al. 2003). Based on high correlations with species richness, contemporary climate and energy variables (e.g. precipitation, temperature and/or evapotranspiration) are often thought to explain spatial variation in species richness better than any other non-climatic variable (Wright 1983; Hawkins et al. 2003a; Currie et al. 2004). However, a number of other factors also determine broad-scale patterns of species richness, including topography, habitat diversity, or regional and evolutionary history (e.g. Rahbek & Graves 2001; Jetz & Rahbek 2002; Willig et al. 2003). Despite a century of debate about the primary determinants of species richness, the underlying causal mechanisms behind the patterns still remain vague (Willig et al. 2003; Currie et al. 2004; Rahbek et al. 2007).
For vascular plants, it is widely argued that precipitation and ambient energy are the main drivers of species richness (Hawkins et al. 2003a; Field et al. 2005). Water availability, heat and light directly influence plant growth and productivity and are essential to plant physiological processes (Waide et al. 1999; Field et al. 2005). Higher productivity might result in more species because physiological tolerances of individual species vary for different climatic conditions (‘physiological tolerance hypothesis’; Currie et al. 2004), or, alternatively, because more productive areas are warmer and evolutionary rates might be faster at higher ambient temperatures (‘speciation rate hypothesis’; Allen et al. 2006). For animals, especially for endotherms, the relationships between species richness and water, energy and climate are less pronounced than for plants (Rahbek & Graves 2001; Jetz & Rahbek 2002; Hawkins et al. 2003a,b). One likely explanation is that energy might not directly influence animal species richness via its effect on animals' physiological requirements or evolutionary rates, but rather indirectly via trophic relationships (Wright 1983; Hawkins et al. 2003a,b; Currie et al. 2004). This hypothesis assumes that richness of animals is determined by the abundance, distribution and diversity of food resources (e.g. plant biomass for herbivores, fruits for frugivores).
At small spatial scales, animal species richness can be associated with the abundance, diversity or partitioning of food resources (e.g. Herrera 1985; Siemann et al. 1998; Novotny et al. 2006). This relationship is however difficult to test at large spatial extents because it is difficult to map food resources for animal groups at continental scales (e.g. insects for insectivorous birds). One possibility to test for a link between animal species richness and resources is to relate the species richness of animals to that of their food items (e.g. food plants; Hawkins & Porter 2003; Márquez et al. 2004; Novotny et al. 2006). However, correlations between animal and plant species richness can also result from both groups responding similarly to the same environmental variables. After accounting for these environmental variables, a convincing dependency of animal on plant species richness has not been demonstrated so far at broad spatial scales (Hawkins & Porter 2003; Hawkins & Pausas 2004; Márquez et al. 2004).
Plant–frugivore interactions might be an ideal model system for continental analyses of animal and plant species richness. Most frugivorous animals heavily rely on fruits, particularly in the tropics (Fleming et al. 1987). In a number of fine-scale field studies, it has been shown that the richness of frugivorous animals is largely dependent on fruit availability (e.g. Herrera 1985; Fleming et al. 1987; Bleher et al. 2003). Among the fruiting plants, the fig genus (Ficus) has been considered to be a keystone plant resource for many frugivores owing to large crop sizes and asynchronous fruiting patterns throughout the year (Terborgh 1986; Lambert & Marshall 1991; Shanahan et al. 2001a; Bleher et al. 2003; Harrison 2005; but see Gautier-Hion & Michaloud 1989). Thus, the diversity and abundance of figs might set the carrying capacity for frugivorous animals in the tropics. Correspondingly, Goodman & Ganzhorn (1997) proposed that avian frugivore richness might depend directly on species richness of Ficus trees. However, no rigorous test of this ‘fig–frugivore-richness hypothesis’ has been conducted at a large regional scale such as a continent.
In this study, we examine whether the richness of Ficus species at a continental scale (i.e. sub-Saharan Africa) influences avian consumer richness by examining a comprehensive database with a resolution of 1° latitude and longitude, summarizing the distribution of all breeding birds (n=1771), all Ficus species (n=86) and five climatic and environmental variables (precipitation, temperature, productivity, topography and ecosystem diversity). We classify frugivorous birds into three classes (obligate, partial and opportunistic fruits eaters) and predict the association between frugivore and Ficus richness to be stronger for those frugivores that are more specialized on fruit eating. We apply path analysis to disentangle inter-correlations between variables and compare the results of this non-spatial method with those of spatial regression models that account for the spatial autocorrelation structure within our dataset.
2. Material and methods
(a) Bird data
We used an updated version (29 September 2005) of the comprehensive distribution database of African breeding birds compiled by the Zoological Museum, University of Copenhagen (see Burgess et al. (1998) and Brooks et al. (2001) for methodology; Jetz & Rahbek (2002) for sources used for mapping). Maps for each species represent a conservative extent-of-occurrence extrapolation of the breeding range at a resolution of 1°×1° cells (latitude–longitude). Data were compiled from the standard reference works and dozens of other published references (including recent atlases and unpublished research) and, for difficult regions and taxa, experts' opinions were sought (the full list of sources is available at http://www.zmuc.dk/commonweb/research/biodata.htm). Most of the northern part of continental Africa, the Sahara, is marked by extreme species scarcity (Jetz & Rahbek 2002) and almost all species in it and North of it belong to the Eurasian biome. We thus focused our analyses on all 1771 breeding bird species south of the Saharan desert ecoregion (figure 1e) with ecoregion boundaries for the South Sahara as northern boundary (Olson et al. 2001). Our sub-Saharan database contains 434 789 records on 1737 cells. The extent of the grid was chosen to be similar to the one used by Jetz & Rahbek (2002) to make the results comparable. We therefore excluded cells containing less than 50% dry land. Cell size varies only slightly with latitude, ranging from 10 188 to 12 308 km2. The Worldmap computer program, v. 4.20.24 (1999, P. H. Williams, Natural History Museum, London) was used to overlay the distributional data.
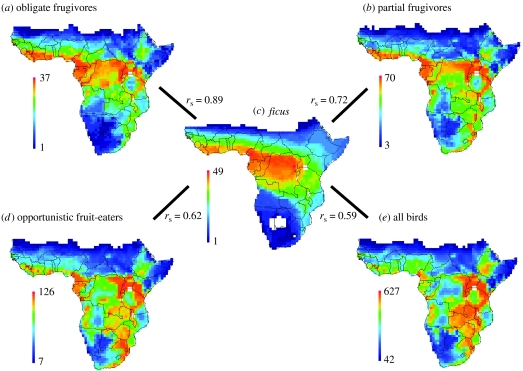
Figure 1.
Geographical patterns of species richness in sub-Saharan Africa. (a) Obligate frugivores (92 species), (b) partial frugivores (200 species), (c) all Ficus trees (86 species), (d) opportunistic fruit eaters (290 species) and (e) all breeding birds (1771 species). Equal frequency classification is shown, with colour ramps indicating minimum (dark blue, bottom of legend) and maximum (dark red, top of legend) species richness. Note that the scale of richness differs among figures.
(b) Frugivore classification
The diets of all bird species in our sub-Saharan database were determined from a comprehensive literature survey (see electronic supplementary material S1 for references and classification procedure). We classified all species into three frugivore guilds depending on diet preference for fruits: (i) obligate frugivores (species that primarily feed on fruits, i.e. the only major food items are fruits), (ii) partial frugivores (species that have, beside fruits, other major food items, e.g. terrestrial invertebrates), and (iii) opportunistic fruit eaters (species that only occasionally eat fruits as supplementary food). The three frugivore guilds were characterized by the degree of avian specialization on fruits, with obligate frugivores being most dependent and opportunistic fruit eaters being least dependent on the availability of fruits. Our classification of frugivorous bird species integrates the best knowledge currently available on the feeding behaviour of African birds (see electronic supplementary material S1). For the interested reader, we also provide lists of species of all African frugivores (see electronic supplementary material S2).
(c) Ficus data
Individual distribution maps for all Ficus species were provided by the Iziko Museums of Cape Town (2005, S. van Noort and J.-Y. Rasplus, available at http://www.figweb.org/Ficus/Species_index/afrotropical_species.htm). The maps are based on country records and the extent of species occurrence is approximated based on habitat affiliations of each species (S. van Noort 2005, Iziko Museums of Cape Town, personal communication). To create a Ficus richness map for sub-Saharan Africa, we first georeferenced the maps of each species and digitized the geographical ranges. The ranges of all individual Ficus species were then overlaid on a 1°×1° grid cell map. For each species, we assigned the value 1 indicating species presence for each 1° grid cell when the cell contained more than 10% distribution cover. Ficus richness values were then calculated for each cell by adding all presence values. We tested the sensitivity of the 10% distribution cover threshold by calculating Ficus richness values from Ficus presence maps based on thresholds of 0, 5, 15 and 20% distribution cover. All of the resulting Ficus richness patterns were highly correlated with each other (Spearman rank correlations, rs>0.98) indicating that the arbitrarily chosen threshold of 10% did not distort the overall Ficus richness pattern. Geoprocessing was done with the software ArcView v. 3.2 and ArcGIS v. 9. Taxonomy of Ficus follows Berg & Wiebes (1992) and Shanahan et al. (2001a; see their Appendix 1). The geographical distributions of different subspecies were pooled as one species. Ficus thonningii was used as a synonym for Ficus petersii and Ficus burkei. A total of 86 Ficus species were thus finally distinguished in our study (see electronic supplementary material S3).
(d) Environmental variables
Besides species richness of Ficus, we included five environmental variables as potential determinants of the richness pattern of avian frugivores. The environmental variables included two climatic variables related to water input (precipitation) and ambient energy (temperature), a measure of productivity, a measure of topographic heterogeneity and habitat diversity (see table 1 for details). These variables have previously been shown to be strongly correlated with species richness of birds and woody plants at continental scales (Waide et al. 1999; Rahbek & Graves 2001; Jetz & Rahbek 2002; Hawkins et al. 2003a,b; Field et al. 2005). Data for precipitation and temperature were extracted from the mean monthly climatic database for the period 1961–1990 provided by the Intergovernmental Panel on Climate Change (IPCC), available online at http://ipcc-ddc.cru.uea.ac.uk/obs/get_30yr_means.html (see New et al. (1999) for methodology). We used mean annual precipitation (mm yr−1) and mean daily maximum temperature (°C) (following Jetz & Rahbek 2002), degraded from 0.5 to 1° resolution. For productivity, we chose net primary productivity (NPP) predictions from the DOLY global model (Woodward et al. 1995). Topographic heterogeneity was quantified as altitudinal range (difference between maximum and minimum elevation) of the 1 min digital elevation model presented by Hutchinson et al. (1996). Ecosystem diversity was estimated by counting the number of distinct ecosystems in each cell from a recently published map of global ecosystems (Olson 1994; available at http://edcsns17.cr.usgs.gov/glcc/). While both ecosystem diversity and topographic relief are potentially important predictors in their own right (Rahbek & Graves 2001), they are also rough surrogate variables for habitat heterogeneity.
Table 1.
Predictor variables used to explain spatial variation in richness of avian frugivore species across sub-Saharan Africa.
| mnemonic | predictor variables (units) | hypothesis (reference) |
|---|---|---|
| FigRich | number of Ficus species per 1° cell (count) | food plant diversity (Goodman & Ganzhorn 1997; Bleher et al. 2003) |
| Prec | mean annual precipitation (mm yr−1) | water availability (Rahbek & Graves 2001; Jetz & Rahbek 2002; Field et al. 2005; Hawkins et al. 2003a) |
| MaxTemp | mean daily maximum temperature (°C) | ambient energy (Jetz & Rahbek 2002; Hawkins et al. 2003a) |
| NPP | net primary productivity (t C ha−1 yr−1) | productivity (Waide et al. 1999; Jetz & Rahbek 2002; Hawkins et al. 2003a) |
| AltRange | topographic relief (altitudinal range in m) | topographic heterogeneity (Rahbek & Graves 2001; Jetz & Rahbek 2002) |
| EcoDiv | number of ecosystems in cell (count) | ecosystem diversity (Rahbek & Graves 2001) |
(e) Statistical analysis
To disentangle the relative roles of predictor variables, many of which covaried (see electronic supplementary material, table S5), and to assess the potential influence of spatial autocorrelation on the robustness of our results, the analysis comprised a three-step process.
In the first step, we calculated Spearman rank correlations (rs) between all variables in our dataset to examine the strength of the relationships between predictor variables, and between predictor and response variables. In the second step, we applied path analysis (Mitchell 1992; Quinn & Keough 2002), which allows the consideration of hypothesized causal relationships in datasets with more than one dependent variable and effects of dependent variables on one another. Whereas path analysis cannot replace experimental manipulations for detecting causal links between variables, it is one of the few methods to test ecological and evolutionary hypotheses at broad spatial scales (Hawkins & Porter 2003; Márquez et al. 2004). Path models are usually presented in path diagrams, where the effect of one variable on another is measured by standardized partial regression coefficients (Mitchell 1992; Quinn & Keough 2002). Path analysis further allows the partitioning of correlation between predictor and response variables (so called ‘total effects’) into direct and indirect effects. Direct effects are measured by the standardized partial regression coefficients between a predictor variable and a response variable (i.e. the direct link), whereas indirect effects are calculated by adding the products of all standardized partial regression coefficients over all paths between a predictor and a response variable (i.e. including indirect links via other correlated predictor variables; see Mitchell 1992; Quinn & Keough 2002).
Our path models were designed to represent hypotheses of how predictor variables might interact with each other to influence avian frugivore richness, and the links were thus based on a priori knowledge or logical relationships among our predictor variables (see references in table 1). Since our main focus was on the potential influence of Ficus richness on frugivore richness, we first generated a path model that excluded Ficus richness followed by a model to which Ficus richness was added. Comparison of the first model with the second model allowed us to evaluate whether Ficus richness had a significant effect on frugivore richness itself, or whether it only acted upon frugivore richness through causal relationships with other environmental variables. We assessed the path models using structural equation modelling (SEM), which is an extension of path analysis (see Mitchell (1992) for an introduction). Model evaluation was done by comparing the fitted path models to a baseline model, where observed variables were assumed to be uncorrelated with each other (Arbuckle (2003), see his Appendix C). We used the normed fit index (NFI) as a fit measure (Bentler & Bonett 1980), which ranges between 0 and 1, with values close to 1 indicating a good fit (Arbuckle 2003). The χ2 goodness of fit test (which is often used to assess the null hypothesis that a path model fits to the data) is invalid in our case, because the large sample size (n=1737) would have almost certainly resulted in significant departures from the null hypothesis (Arbuckle 2003, see his Appendix C). We additionally tested whether multiple regression models with all explanatory variables (table 1) explained frugivore species richness better than multiple regression models, where Ficus richness was excluded as an explanatory variable. These model comparisons were done with the Akaike Information Criterion (AIC), a model selection criterion which accounts for both model fit and model complexity (Burnham & Anderson 2002).
In our third analysis, we tested for the presence of spatial autocorrelation because our data violate the assumption of independently distributed errors in regression models (Legendre & Legendre 1998), and, as a consequence, the effects of explanatory variables might thus be exaggerated (Lichstein et al. 2002). To quantify the pattern of autocorrelation in our dataset, we calculated Moran's I values (i.e. a measure of autocorrelation) across 20 distance classes (one distance class corresponds to 112 km) and plotted them in so-called correlograms (Legendre & Legendre 1998). We first calculated Moran's I for all raw bird richness data (i.e. obligate frugivores, partial frugivores, opportunistic fruit eaters and all birds), and then fitted multiple regression models with all predictor variables (i.e. models which are equivalent to all direct effects on avian richness in our path models) and recalculated Moran's I on the residuals. Since fitting the multiple regression models with all predictor variables did not remove all of the spatial autocorrelation in our richness variables, we fitted spatial autoregressive models (Cliff & Ord 1981; Cressie 1993) which augment the multiple regression models with an additional term that accounts for patterns in the response variable that are not predicted by explanatory variables, but are instead related to values in neighbouring locations. We then compared the standardized partial regression coefficients (Quinn & Keough 2002) from the spatial autoregressive models to those of our path models (i.e. direct effects on avian richness) to assess whether the relative importance of parameter estimates changes when the spatial autocorrelation structure in our response variables is removed.
All statistical analyses were done with the free software R (R Development Core Team 2005) except for the path models which were calculated with the AMOS software (Arbuckle 2003). The spatial models were calculated as ‘spatial simultaneous autoregressive error models’ using the R library ‘spdep’, v. 0.3–25 (2006, R. Bivand, available at http://cran.r-project.org/src/contrib/Descriptions/spdep.html). These models are a special type of simultaneous autoregressive models and assume that the response at each location (i) is a function not only of the explanatory variable at i, but also of the values of the response at neighbouring locations (j) as well (Cliff & Ord 1981; Anselin 1988; Cressie 1993). We defined the spatial neighbourhood with a distance of 112 km including the four neighbouring cells that directly join each focal cell (the rook's case). The spatial weights matrix was calculated with a row standardized coding scheme that scales the covariances based on the number of neighbours of each region (see R library ‘spdep’ for details, reference above). Moran's I values and correlograms were calculated with the R library ‘ncf’, v. 1.0–9 (2006, O. N. Bjørnstad, available at http://asi23.ent.psu.edu/onb1/). To improve the normality of distributions, we transformed all endogenous variables (i.e. those with incoming arrows in the path models) and used transformed values in all regression analyses. Precipitation, maximum temperature and NPP+1 were log transformed, whereas all richness measures (i.e. species richness of Ficus, obligate frugivores, partial frugivores, opportunistic fruit eaters, all birds and ecosystem diversity) were square-root transformed. These transformations yielded the best approximations of normal distributions and were performed to meet the normality of errors assumption (Mitchell 1992; Quinn & Keough 2002). Analyses with untransformed values gave qualitatively similar results.
3. Results
(a) Geographical patterns of species richness
Species richness of obligate avian frugivores (n=92) across sub-Saharan Africa is highest in tropical rainforest regions at equatorial latitudes (figure 1a), particularly, in the coastal areas of West Africa and in the Congo Basin, but also within the East African mountains. Hotspots of obligate frugivore richness are thus not congruent with hotspots of overall bird species richness, which are mainly found in the eastern parts of sub-Saharan Africa (figure 1e). Geographical patterns of species richness of partial frugivores (n=200; figure 1b) are more similar to obligate frugivore richness (figure 1a) than to overall bird species richness (n=1771; figure 1e), whereas opportunistic fruit eaters (n=290; figure 1d) closely resemble overall bird species richness (figure 1e) rather than obligate frugivore richness (figure 1a). The species richness of Ficus trees (n=86) is highest in the Congo Basin and relatively low in South Africa and along the eastern parts of Africa (figure 1c), and thus largely congruent with obligate frugivore richness patterns (figure 1a).
(b) Determinants of frugivore richness
Simple correlations between Ficus and bird species richness indicated that they positively covary across sub-Saharan Africa (figure 1 and see electronic supplementary material, figure S4). As expected, the relationship was strongest for obligate frugivores (rs=0.89), intermediate for partial frugivores (rs=0.72) and lowest for opportunistic fruit eaters (rs=0.62) and overall bird species richness (rs=0.59). Precipitation, NPP, ecosystem diversity and maximum temperature were also strongly correlated (rs>0.60) with avian species richness in almost all cases, and precipitation and NPP highly covaried with each other and with species richness of Ficus trees (rs>0.84). Maximum temperature, altitudinal range and ecosystem diversity generally showed weaker correlations (rs<0.50) with other predictor variables (see electronic supplementary material, table S5).
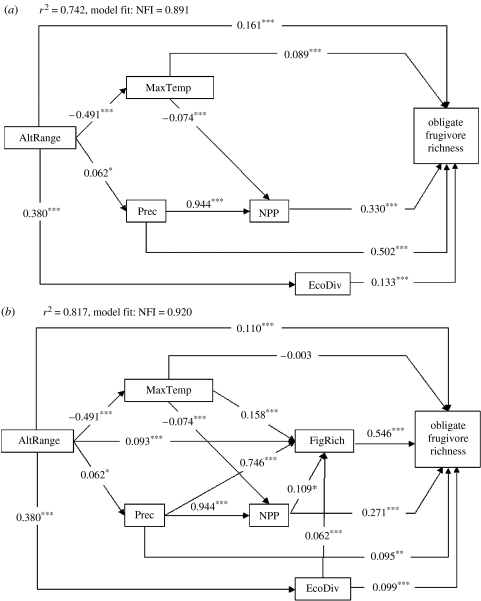
The path model without Ficus richness (figure 2a) explained 74.2% of the variance in richness of obligate frugivorous birds, and the measure of fit (NFI=0.891) indicated that the model adequately described the data structure. Precipitation had the strongest direct effect on richness of obligate frugivorous birds followed by NPP, altitudinal range, ecosystem diversity and maximum temperature (figure 2a). Including richness of Ficus trees in the path model improved the explanatory power (81.7%) and the overall fit of the model (NFI=0.920), and we thus consider this path model (figure 2b) a better description of obligate frugivore richness patterns. Model selection based on AIC values also indicated that a multiple regression model with Ficus species richness (AIC=2245) supported the obligate frugivore richness data better (i.e. had a lower AIC value) than a multiple regression where Ficus richness had been excluded (AIC=2838, ΔAIC=593). In the path model with Ficus richness (figure 2b), the direct effect of precipitation on richness of obligate frugivorous birds was very low (0.095) and richness of Ficus trees instead became the most important variable with the strongest direct effect (0.546) on richness of obligate frugivorous birds (table 2; figure 2b). When including indirect effects, the relative importance of precipitation increased (table 2), because it was very strongly correlated with NPP and Ficus richness (figure 2b). The total effects of other predictor variables were also higher than their direct effects (table 2), indicating that they indirectly affected frugivore richness via other variables.
Figure 2.
Path models for richness of obligate frugivorous bird species. (a) Ficus richness excluded and (b) Ficus richness included. Illustrated are direct effects (i.e. standardized partial regression coefficients) and their significance levels (*p<0.05; **p<0.01; ***p<0.001). r2 and normed fit index (NFI) are given for each model (see §2 for details).
Table 2.
Standardized direct and total effects of predictor variables on species richness of obligate frugivores (OBL), partial frugivores (PAR), opportunistic fruit eaters (OPP) and all birds (ALL). (Values are derived from path models (figure 2b), which include species richness of Ficus trees as predictor variable. Indirect effects are total effects minus direct effects and equal zero if total effects and direct effects have the same values. Mnemonics of predictor variables are explained in table 1.)
| direct effects | total effects | |||||||
|---|---|---|---|---|---|---|---|---|
| predictor variable | OBL | PAR | OPP | ALL | OBL | PAR | OPP | ALL |
| FigRich | 0.546 | 0.454 | 0.252 | 0.172 | 0.546 | 0.454 | 0.252 | 0.172 |
| Prec | 0.095 | 0.018 | 0.193 | 0.410 | 0.814 | 0.626 | 0.604 | 0.611 |
| MaxTemp | 0.003 | −0.362 | −0.421 | −0.264 | 0.064 | −0.311 | −0.398 | −0.243 |
| NPP | 0.271 | 0.236 | 0.209 | 0.058 | 0.330 | 0.285 | 0.236 | 0.077 |
| AltRange | 0.110 | 0.044 | 0.043 | 0.137 | 0.230 | 0.383 | 0.388 | 0.416 |
| EcoDiv | 0.099 | 0.250 | 0.220 | 0.267 | 0.133 | 0.278 | 0.235 | 0.278 |
Replacing obligate frugivores in our path model (figure 2b) with partial frugivores, opportunistic fruit eaters and all birds resulted in less-explained variance in avian species richness (partial frugivores, 73.7%; opportunistic fruit eaters, 70.3%; all birds, 66.4%) than the original path model (obligate frugivore richness, 81.7%). This trend is consistent with our expectation that the hypothesized causal relationships in the path models should be stronger for birds that are more specialized on fruit eating. Furthermore, these path models showed that direct effects of Ficus richness became weaker with decreasing specialization of birds on fruit eating (table 2) which also confirms our expectations. Correspondingly, the AIC values of multiple regression models with all explanatory variables increased with decreasing specialization on fruit eating (AICobligatefrugivores=2245; AICpartialfrugivores=3452; AICopportunisticfruiteaters=4275; AICallbirds=7214).
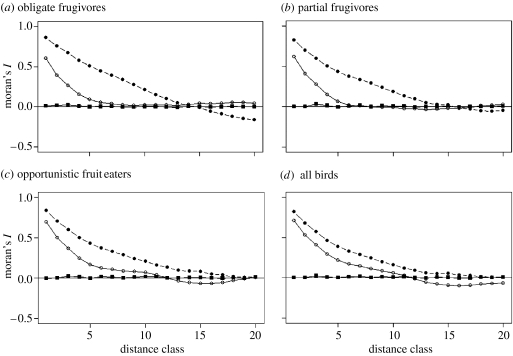
(c) Effect of spatial autocorrelation
All avian species richness data were spatially autocorrelated over more than 1000 km, although the extent (i.e. distance) differed slightly between frugivore guilds (figure 3). Fitting multiple regression models with all predictor variables (i.e. models with those variables that show direct effects on avian species richness in our path models) reduced spatial autocorrelation in all richness data, indicating that the spatial structure of explanatory variables accounted for some of the spatial autocorrelation structure in the avian richness data. However, our set of explanatory variables could not account for all of the observed spatial structures in our response variables (figure 3). We therefore fitted spatial autoregressive models, which removed almost all of the spatial autocorrelation in richness data across all distance classes (figure 3), indicating that the spatial structure can be explained by including information on the covariance structure from the four neighbouring cells directly joining each focal cell.
Figure 3.
Correlograms for raw data on species richness (solid circles), residuals of multiple regression models (open circles) and residuals of spatial autoregressive error models (solid squares). Both models included all predictor variables (table 1) and species richness of (a) obligate frugivores, (b) partial frugivores, (c) opportunistic fruit eaters and (d) all birds, respectively, as response variables. Multiple regression models thus include all direct effects of predictor variables on avian species richness from our path models. One unit distance class corresponds to 112 km.
The standardized partial regression coefficients of the spatial autoregressive models (table 3) differed from those of the path models (direct effects in table 2), demonstrating that the effects of predictor variables might be exaggerated when using traditional multiple regression or path models. However, despite the changes in parameter estimates, richness of Ficus trees still remained the strongest predictor variable to explain the richness pattern of obligate and partial frugivorous birds, respectively. Moreover, its effect still decreased with decreasing specialization of birds on fruit eating (table 3). Besides, the relative importance of other predictor variables to explain obligate frugivore richness did not change when using spatial autoregressive models except precipitation, which became more important in spatial analyses (compare direct effects in tables 2 and 3).
Table 3.
Standardized partial regression coefficients from spatial autoregressive error models (see §2 for details). All models were calculated as multiple regression models with avian species richness (OBL, obligate frugivores; PAR, partial frugivores; OPP, opportunistic fruit eaters; ALL, all birds) as response variable and all other variables as predictor variables (see table 1 for explanation of mnemonics).
| predictor variable | OBL | PAR | OPP | ALL |
|---|---|---|---|---|
| FigRich | 0.382 | 0.266 | 0.222 | 0.231 |
| Prec | 0.259 | 0.253 | 0.322 | 0.248 |
| MaxTemp | −0.023 | −0.082 | −0.032 | −0.037 |
| NPP | 0.165 | 0.162 | 0.160 | 0.163 |
| AltRange | 0.086 | 0.097 | 0.070 | 0.065 |
| EcoDiv | 0.077 | 0.083 | 0.081 | 0.123 |
4. Discussion
Our analyses indicate that a positive relationship between species richness patterns of figs (Ficus spp.) and avian frugivores exists across sub-Saharan Africa, which suggests that both are linked via resource–consumer interactions rather than being caused by similar responses to environmental variables. We thus provide evidence that food plant diversity is an important determinant of avian frugivore richness in tropical regions, even after controlling for confounding environmental variables and spatial autocorrelation. The results also underline the potential role of Ficus as a keystone plant resource for avian frugivores in the tropics (Shanahan et al. 2001a; Bleher et al. 2003; Harrison 2005).
There are a number of mechanisms that could potentially explain a positive relationship between food plant and animal consumer species richness. Some can be based on deterministic processes and niche assembly theory (Graves & Rahbek 2005), whereas others are based on stochastic processes and ecological drift (i.e. neutral theory; Hubbell 2001; see also Colwell et al. 2004). One possible explanation for a positive relationship between food plant and frugivore species richness is that a greater number of plant species could potentially provide more niches for the coexistence of animal species (‘niche assembly hypothesis’; Hutchinson 1959). This explanation assumes that animal species specialize on certain food plants or on specific types of resources provided by the plants (Price 2002). For instance, the latitudinal gradient in species richness of herbivorous insects from temperate to tropical regions has been suggested to be a direct function of an increase in plant species richness (Novotny et al. 2006). However, this ‘reciprocal specialization hypothesis’ is unlikely to be relevant for plant–frugivore interactions (Herrera 2002). Most fruit-eating bird species do not specialize on the fruits of a particular plant species. Instead, frugivorous bird species often treat fleshy fruited plant species as interchangeable (Zamora 2000; Herrera 2002). For our study system, we know of only one frugivorous bird species (Bruce's Green-pigeon, Treron waalia) that feeds particularly on one single fig species (Ficus platyphylla) with the ranges of the two species largely overlapping. Other examples might exist, but evidence for strong reciprocal specialization between frugivore species and fig or fleshy fruited plant species is generally scarce (Herrera 2002).
Alternatively, a greater number of food plant species could potentially provide more niches for animal consumer species by providing a larger range of resources types. For instance, fruit size is an important attribute of fruits and varies greatly between species (e.g. fruit sizes of Ficus species range from 0.5 to 10 cm in diameter; Berg & Wiebes 1992). If frugivores show some specialization on differently sized fruits, then frugivore species richness is likely to increase with a greater range of fruit sizes. There is some evidence for this ‘size-related coupling hypothesis’ (Herrera 2002; Githiru et al. 2002; Lord 2004), because fruit size sets limits to fruit ingestion, at least to relatively small-sized birds that swallow whole fruits. It is thus likely that a greater number of Ficus species is accompanied with a larger diversity of fruit sizes (Berg & Wiebes 1992), which may attract a greater size range of fruit-eating birds increasing frugivore species richness (Shanahan et al. 2001a). If size-related coupling of fruits and frugivores is the underlying mechanism in our study system, then the correlations between fig and frugivore species richness could result as a by-product of this relationship.
Similar to fruit size, other fruit traits could potentially influence food choice and partitioning of the available fruit spectrum among consumer species (Gautier-Hion et al. 1985; Herrera 2002). For instance, frugivorous birds can discriminate among fruits on the basis of colour and might exhibit distinct colour preferences (Herrera 2002). A larger number of Ficus species is likely to increase the range of fruit colours (fig colours vary greatly from red, yellow, orange, green, brown to black fruits; Berg & Wiebes 1992), and this might attract a wider range of frugivorous species (‘fruit colour-richness hypothesis’). There is some evidence that differences in fruit colour can explain differences in frugivore assemblage structure, at least when considering consumer species across taxa (e.g. when comparing primates and birds; Voigt et al. 2004). However, to our knowledge, no study has shown convincingly that certain frugivorous bird species specialize on specific fruit colours. Other fruit traits such as fruit pulp quality (i.e. nutrient composition) could also be critical in food selection of frugivorous animals, but there is generally little evidence that they play an important role in shaping mutual adaptations between fleshy fruited plants and frugivores (Herrera 2002).
Another potential mechanism underlying a positive relationship between fleshy fruited plant and frugivore species richness is that a larger number of food plants are likely to increase the diversity of fruit presentation. For instance, depending on the Ficus species, figs are presented at different heights above ground level and at different locations (e.g. at ground-level runners, on stems or trunks, or in leaf axis; Berg & Wiebes 1992). The architecture of fruit display is likely to determine fruit suitability for particular frugivores, especially if frugivores exhibit different feeding behaviours. The variability of fruit presentation within Ficus thus allows discrete guilds of Ficus species to attract different subsets of the total frugivore community (Shanahan & Compton 2001). This might result in a distinct vertical stratification of fig–frugivore communities (the ‘vertical stratification hypothesis’; Shanahan & Compton 2001) and could, at least partly, explain the positive relationship between Ficus and frugivore species richness.
All mechanisms outlined so far explain the positive relationship between food plant and animal consumer species richness with an increased availability of niches provided by a larger number of plant species (niche assembly hypothesis). In contrast, the species richness of trophically similar species (e.g. frugivores) competing for similar resources (e.g. fruits) could also result from stochastic ecological and evolutionary processes (Hubbell 2001). For instance, areas with high species richness of fleshy fruited plants could potentially produce more fruit biomass due to either more food plant individuals or higher total fruit production (e.g. Ortiz-Pulido & Rico-Gray 2000). If the total abundance of fruit resources increases with food plant species richness, then more individuals of frugivores could be sustained in areas with high food plant diversity. The high species richness of frugivorous birds could then be governed by neutral speciation and extinction processes, where differences in traits of food plant and animal consumer species might be irrelevant for structuring plant–frugivore assemblages (Hubbell 2001; see also Burns 2006). In our case, Ficus species richness would then be positively associated with species richness of frugivorous birds, because it also correlates with the overall abundance and availability of fruit resources (‘resource-abundance hypothesis’).
Finally, the spatial congruence in patterns of fig and frugivore richness could not only be driven by figs as resources for frugivores, but also vice versa if frugivores constrain the spatial distribution and species richness of figs at continental scales. We tested this idea by interchanging Ficus richness and obligate frugivore richness in our path model (figure 2b) and found an influence of similar magnitude between frugivores and figs (direct effect, 0.532). This pattern could result if the large-scale distribution of fig species and their colonization of new sites are constrained by seed dispersal of frugivorous birds (e.g. Shanahan et al. 2001b for fig colonization of new volcanic islands). With a greater species richness of frugivorous dispersers, the seeds are more likely to arrive in a greater variety of sites and at different distances, as different species of birds have different foraging behaviours, perching locations and movement patterns. Furthermore, a higher species richness of frugivores might lead to better seed dispersal, more long-distance dispersal events and the foundation of new Ficus populations, potentially resulting in higher speciation rates of Ficus species.
In contrast to our study, many thoroughly conducted studies on plant–frugivore interactions have failed to document strong adaptive relationships (e.g. through demographic sorting or coevolutionary processes) between fruits and frugivores (e.g. Herrera 1998), suggesting that non-adaptive processes such as climate, historical or phylogenetic effects constrain the development of mutual adaptations (Herrera 2002). For instance, similar to our study, Márquez et al. (2004) analysed plant–frugivore richness at the scale of major river basins across Europe and found that avian frugivore richness was more dependent on environmental factors than on fleshy fruited plant species richness. Fleming (2005) examined the relationship between species richness of fruit-eating birds and their food plants in New and Old World communities and found hemispheric differences in plant–frugivore mutualisms. Recent plant–frugivore research suggests that these kinds of differences are often generated by analyses at different spatio-temporal scales (Burns 2004; García & Ortiz-Pulido 2004).
To understand the causal mechanisms of animal species richness patterns at continental and global scales, predictions from competing mechanistic hypotheses should be tested (Willig et al. 2003; Currie et al. 2004; Rahbek et al. 2007), ideally across multiple spatial and temporal scales (Böhning-Gaese 1997; Burns 2004; Rahbek 2005). Our results demonstrate a close relationship between the species richness of Ficus and avian frugivores in sub-Saharan Africa, suggesting that figs are keystone resources for animal consumers at continental scales. This relationship might be driven by niche assembly mechanisms, e.g. coevolutionary adaptations to fruit size, fruit colour or vertical stratification of fruit presentation, or, alternatively, by a neutral speciation–extinction process. In both cases, however, the present study suggests that climatic variables influence frugivore species richness only indirectly via food webs rather than having a direct effect on the physiological tolerances of the organisms.
Acknowledgments
We are grateful to K. C. Burns and one anonymous referee for stimulating and constructive comments on a draft manuscript. E. Baker, N. Baker, F. Dowsett-Lemaire, R. Dowsett, J. Fjeldså, M. E. Gartshore, H. M. de Klerk, M. Languy, R. B. Payn, COC/Birdlife Cameroon and Birdlife International provided data for the ZMUC database, and S. van Noort gave information on Ficus distribution maps. P. H. Williams kindly provided the Worldmap software used to overlay the species distribution data. The study was financially supported by the Helmholtz Association through funding of a Virtual Institute. C.R. acknowledges Danish National Science Foundation grant J. no. 21-03-0221 for support of macroecological research. This is the first Ph.D. thesis paper of W.D.K. at the Institute of Zoology, University of Mainz, Germany.
Supplementary Material
S1, Classification of frugivores; S2, Frugivore species list; S3, Ficus species list; S4, Fig-frugivore richness correlations; S5, Correlation matrix
References
- Allen A.P, Gillooly J.F, Savage V.M, Brown J.H. Kinetic effects of temperature on rates of genetic divergence and speciation. Proc. Natl Acad. Sci. USA. 2006;103:9130–9135. doi: 10.1073/pnas.0603587103. doi:10.1073/pnas.0603587103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselin L. Kluwer; Dordrecht, The Netherlands: 1988. Spatial econometrics: methods and models. [Google Scholar]
- Arbuckle, J. L. 2003 Amos 5.0.1 URL http://amosdevelopment.com
- Bentler P.M, Bonett D.G. Significance tests and goodness of fit in the analysis of covariance structures. Psychol. Bull. 1980;88:588–606. doi:10.1037/0033-2909.88.3.588 [Google Scholar]
- Berg C.C, Wiebes J.T. Koninklijke Nederlandse Akademie van Wetenschappen; Amsterdam, The Netherlands: 1992. African fig trees and fig wasps. [Google Scholar]
- Bleher B, Potgieter C.J, Johnson D.N, Böhning-Gaese K. The importance of figs for frugivores in a South African coastal forest. J. Trop. Ecol. 2003;19:375–386. doi:10.1017/S0266467403003420 [Google Scholar]
- Böhning-Gaese K. Determinants of avian species richness at different spatial scales. J. Biogeogr. 1997;24:49–60. [Google Scholar]
- Brooks T, Balmford A, Burgess N, Fjeldså J, Hansen L.A, Moore J, Rahbek C, Williams P. Toward a blueprint for conservation in Africa. BioScience. 2001;51:613–624. doi:10.1641/0006-3568(2001)051[0613:TABFCI]2.0.CO;2 [Google Scholar]
- Burgess N, Fjeldså J, Rahbek C. Mapping the distributions of Afrotropical vertebrate groups. Species. 1998;30:16–17. [Google Scholar]
- Burnham K.P, Anderson D.R. Springer; New York, NY: 2002. Model selection and multimodel inference: a practical information-theoretic approach. [Google Scholar]
- Burns K.C. Scale and macroecological patterns in seed disperser mutualisms. Global Ecol. Biogeogr. 2004;13:289–293. doi:10.1111/j.1466-822X.2004.00108.x [Google Scholar]
- Burns K.C. A simple null model predicts fruit–frugivore interactions in a temperate rainforest. Oikos. 2006;115:427–432. doi:10.1111/j.2006.0030-1299.15068.x [Google Scholar]
- Cliff A.D, Ord J.K. Pion Limited; London, UK: 1981. Spatial processes: models and applications. [Google Scholar]
- Colwell R.K, Rahbek C, Gotelli N.J. The mid-domain effect and species richness: what have we learned so far? Am. Nat. 2004;163:E1–23. doi: 10.1086/382056. doi:10.1086/382056 [DOI] [PubMed] [Google Scholar]
- Cressie N.A.C. Wiley Series in Probability and Mathematical Statistics; New York, NY: 1993. Statistics for spatial data. [Google Scholar]
- Currie D.J, et al. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 2004;7:1121–1134. doi:10.1111/j.1461-0248.2004.00671.x [Google Scholar]
- Field R, O'Brien E.M, Whittaker R.J. Global models for predicting woody plant species richness from climate: development and evaluation. Ecology. 2005;86:2263–2277. [Google Scholar]
- Fleming T.H. The relationship between species richness of vertebrate mutualists and their food plants in tropical and subtropical communities differs among hemispheres. Oikos. 2005;111:556–562. doi:10.1111/j.1600-0706.2005.14272.x [Google Scholar]
- Fleming T.H, Breitwisch R, Whitesides G.H. Patterns of tropical vertebrate frugivore diversity. Annu. Rev. Ecol. Syst. 1987;18:91–109. doi:10.1146/annurev.es.18.110187.000515 [Google Scholar]
- García D, Ortiz-Pulido R. Patterns of resource tracking by avian frugivores at multiple spatial scales: two case studies on discordance among scales. Ecography. 2004;27:187–196. doi:10.1111/j.0906-7590.2004.03751.x [Google Scholar]
- Gautier-Hion A, Michaloud G. Are figs always keystone resources for tropical frugivorous vertebrates? A test in Gabon. Ecology. 1989;70:1826–1833. doi:10.2307/1938115 [Google Scholar]
- Gautier-Hion A, et al. Fruit characters as a basis of fruit choice and seed dispersal in a tropical forest vertebrate community. Oecologia. 1985;65:324–337. doi: 10.1007/BF00378906. doi:10.1007/BF00378906 [DOI] [PubMed] [Google Scholar]
- Githiru M, Lens L, Bennur L.A, Ogol C.P.K.O. Effects of site and fruit size on the composition of avian frugivore assemblages in a fragmented Afrotropical forest. Oikos. 2002;96:320–330. doi:10.1034/j.1600-0706.2002.960214.x [Google Scholar]
- Goodman S.M, Ganzhorn J.U. Rarity of figs (Ficus) on Madagascar and its relationship to a depauperate frugivore community. Rev. Ecol. Terre. Vie. 1997;52:321–329. [Google Scholar]
- Graves G.R, Rahbek C. Source pool geometry and the assembly of continental avifaunas. Proc. Natl Acad. Sci. USA. 2005;102:7871–7876. doi: 10.1073/pnas.0500424102. doi:10.1073/pnas.0500424102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R.D. Figs and the diversity of tropical rainforests. BioScience. 2005;55:1053–1064. doi:10.1641/0006-3568(2005)055[1053:FATDOT]2.0.CO;2 [Google Scholar]
- Hawkins B.A, Pausas J.G. Does plant richness influence animal richness? the mammals of Catalonia (NE Spain) Divers. Distrib. 2004;10:247–252. doi:10.1111/j.1366-9516.2004.00085.x [Google Scholar]
- Hawkins B.A, Porter E.E. Does herbivore diversity depend on plant diversity? The case of California butterflies. Am. Nat. 2003;161:40–49. doi: 10.1086/345479. doi:10.1086/345479 [DOI] [PubMed] [Google Scholar]
- Hawkins B.A, et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology. 2003a;84:3105–3117. [Google Scholar]
- Hawkins B.A, Porter E.E, Diniz-Filho J.A.F. Productivity and history as predictors of the latitudinal diversity gradient of terrestrial birds. Ecology. 2003b;84:1608–1623. [Google Scholar]
- Herrera C.M. Habitat–consumer interactions in frugivorous birds. In: Cody M.L, editor. Habitat selection in birds. Academic Press; Orlando, FL: 1985. pp. 341–365. [Google Scholar]
- Herrera C.M. Long-term dynamics of Mediterranean frugivorous birds and fleshy-fruits: a 12-year study. Ecol. Monogr. 1998;68:511–538. doi:10.2307/2657152 [Google Scholar]
- Herrera C.M. Seed dispersal by vertebrates. In: Herrera C.M, Pellmyr O, editors. Plant–animal interactions—an evolutionary approach. Blackwell; Oxford, UK: 2002. pp. 185–208. [Google Scholar]
- Hubbell S.P. University of Princeton Press; Princeton, NJ: 2001. The unified neutral theory of biodiversity and biogeography. [Google Scholar]
- Hutchinson G.E. Homage to Santa Rosalia, or why are there so many kinds of animals? Am. Nat. 1959;93:145–159. doi:10.1086/282070 [Google Scholar]
- Hutchinson M.F, Nix H.A, McMahon J.P, Ord K.D. Centre for Resource and Environmental Studies; Canberra, Australia: 1996. The development of a topographic and climate database for Africa. [Google Scholar]
- Jetz W, Rahbek C. Geographic range size and determinants of avian species richness. Science. 2002;297:1548–1551. doi: 10.1126/science.1072779. doi:10.1126/science.1072779 [DOI] [PubMed] [Google Scholar]
- Lambert F, Marshall A.G. Keystone characteristics of bird-dispersed Ficus in a Malaysian lowland rain forest. J. Ecol. 1991;79:793–809. doi:10.2307/2260668 [Google Scholar]
- Legendre P, Legendre L. Elsevier; Amsterdam, The Netherlands: 1998. Numerical ecology. [Google Scholar]
- Lichstein J.W, Simons T.R, Shriner S.A, Franzreb K.E. Spatial autocorrelation and autoregressive models in ecology. Ecol. Monogr. 2002;72:445–463. [Google Scholar]
- Lord J.M. Frugivore gape size and the evolution of fruit size and shape in southern hemisphere floras. Austral Ecol. 2004;29:430–436. doi:10.1111/j.1442-9993.2004.01382.x [Google Scholar]
- Márquez A.L, Real R, Vargas J.M. Dependence of broad-scale geographical variation in fleshy-fruited plant species richness on disperser bird species richness. Global Ecol. Biogeogr. 2004;13:295–304. doi:10.1111/j.1466-822X.2004.00100.x [Google Scholar]
- Mitchell R.J. Testing evolutionary and ecological hypotheses using path analysis and structural equation modelling. Funct. Ecol. 1992;6:123–129. doi:10.2307/2389745 [Google Scholar]
- New M, Hulme M, Jones P. Representing twentieth-century space-time climate variability. Part I: development of a 1961–90 mean monthly terrestrial climatology. J. Clim. 1999;12:829–856. doi:10.1175/1520-0442(1999)012<0829:RTCSTC>2.0.CO;2 [Google Scholar]
- Novotny V, Drozd P, Miller S.E, Kulfan M, Janda M, Basset Y, Weiblen G.D. Why are there so many species of herbivorous insects in tropical rainforests? Science. 2006;313:1115–1118. doi: 10.1126/science.1129237. doi:10.1126/science.1129237 [DOI] [PubMed] [Google Scholar]
- Olson, J. S. 1994 Global ecosystem framework-definitions U.S. Geological Society Earth Resources Observation Satellite Data Center Internal Report. Sioux Falls, IA: U.S. Geological Society Earth Resources Observation Satellite Data Center.
- Olson D.M, et al. Terrestrial ecoregions of the world: a new map of life on earth. BioScience. 2001;51:933–938. doi:10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2 [Google Scholar]
- Ortiz-Pulido R, Rico-Gray V. The effect of spatio-temporal variation in understanding the fruit crop size hypothesis. Oikos. 2000;91:523–527. doi:10.1034/j.1600-0706.2000.910314.x [Google Scholar]
- Price P.W. Species interactions and the evolution of biodiversity. In: Herrera C.M, Pellmyr O, editors. Plant–animal interactions—an evolutionary approach. Blackwell; Oxford, UK: 2002. pp. 3–25. [Google Scholar]
- Quinn G.P, Keough M.J. Cambridge University Press; Cambridge, UK: 2002. Experimental design and data analysis for biologists. [Google Scholar]
- R Development Core Team 2005 R: a language and environment for statistical computing Vienna, Austria: R foundation for Statistical Computing. URL http://www.R-project.org
- Rahbek C. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 2005;8:224–239. doi:10.1111/j.1461-0248.2004.00701.x [Google Scholar]
- Rahbek C, Graves G.R. Multiscale assessment of patterns of avian species richness. Proc. Natl Acad. Sci. USA. 2001;98:4534–4539. doi: 10.1073/pnas.071034898. doi:10.1073/pnas.071034898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbek C, Gotelli N.J, Colwell R.K, Entsminger G.L, Rangel T.F.L.V.B, Graves G.R. Predicting continental-scale patterns of bird species richness with spatially explicit models. Proc. R. Soc. B. 2007;274:165–174. doi: 10.1098/rspb.2006.3700. doi:10.1098/rspb.2006.3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan M, Compton S.G. Vertical stratification of figs and fig-eaters in a Bornean lowland rain forest: how is the canopy different? Plant Ecol. 2001;153:121–132. doi:10.1023/A:1017537707010 [Google Scholar]
- Shanahan M, So S, Compton S.G, Corlett R.T. Fig-eating by vertebrate frugivores: a global review. Biol. Rev. 2001a;76:529–572. doi: 10.1017/s1464793101005760. [DOI] [PubMed] [Google Scholar]
- Shanahan M, Harrison R.D, Yamuna R, Boen W, Thornton I.W.B. Colonization of an island volcano, Long Island, Papua New Guinea, and an emergent island, Motmot, in its caldera lake. V. Colonization by figs (Ficus spp.), their dispersers and pollinators. J. Biogeogr. 2001b;28:1365–1377. doi:10.1046/j.1365-2699.2001.00638.x [Google Scholar]
- Siemann E, Tilman D, Haarstad J, Ritchie M. Experimental tests of the dependence of arthropod diversity on plant diversity. Am. Nat. 1998;152:738–750. doi: 10.1086/286204. doi:10.1086/286204 [DOI] [PubMed] [Google Scholar]
- Terborgh J. Keystone plant resources in the tropical forest. In: Soule M.E, editor. Conservation biology: the science of scarcity and diversity. Sinauer; Sunderland, MA: 1986. pp. 330–344. [Google Scholar]
- Voigt F.A, Bleher B, Fietz J, Ganzhorn J.U, Schwab D, Böhning-Gaese K. A comparison of morphological and chemical fruit traits between two sites with different frugivore assemblages. Oecologia. 2004;141:94–104. doi: 10.1007/s00442-004-1654-8. doi:10.1007/s00442-004-1654-8 [DOI] [PubMed] [Google Scholar]
- Waide R.B, Willig M.R, Steiner C.F, Mittelbach G, Gough L, Dodson S.I, Juday G.P, Parmenter R. The relationship between productivity and species richness. Annu. Rev. Ecol. Syst. 1999;30:257–300. doi:10.1146/annurev.ecolsys.30.1.257 [Google Scholar]
- Willig M.R, Kaufman D.M, Stevens R.D. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu. Rev. Ecol. Syst. 2003;34:273–309. doi:10.1146/annurev.ecolsys.34.012103.144032 [Google Scholar]
- Woodward F.I, Smith T.M, Emanuel W.R. A global land primary productivity and phytogeography model. Global Biogeochem. Cycles. 1995;9:471–490. doi:10.1029/95GB02432 [Google Scholar]
- Wright D.H. Species-energy theory: an extension of species-area theory. Oikos. 1983;41:496–506. doi:10.2307/3544109 [Google Scholar]
- Zamora R. Functional equivalence in plant–animal interactions: ecological and evolutionary consequences. Oikos. 2000;88:442–447. doi:10.1034/j.1600-0706.2000.880222.x [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1, Classification of frugivores; S2, Frugivore species list; S3, Ficus species list; S4, Fig-frugivore richness correlations; S5, Correlation matrix