Abstract
Investigating the relative importance of multiple cues for mate choice within a species may highlight possible mechanisms that led to the diversification of closely related species in the past. Here, we investigate the importance of close-range pheromones produced by male Bicyclus anynana butterflies and determine the relative importance of these chemical cues versus visual cues in sexual selection by female choice. We first blocked putative androconial organs on the fore- and hindwings of males, while also manipulating the ability of females to perceive chemical signals via their antenna. We found that male chemical signals were emitted by both fore- and hindwing pairs and that they play an important role in female choice. We subsequently tested the relative importance of these chemical cues versus visual cues, previously identified for this species, and found that they play an equally important role in female choice in our laboratory setting. In addition, females will mate with males with only one signal present and blocking both androconial organs on males seems to interfere with male to male recognition. We discuss the possible functions of these signals and how this bimodal system may be used in intra- and interspecific mate evaluation.
Keywords: Bicyclus anynana, pheromones, female choice, sexual selection, chemical cues, visual cues
1. Introduction
Elucidating current mechanisms of sexual selection within a species may help us understand past selective forces driving the processes of assortative mating, reproductive isolation, divergence and speciation (Darwin 1981; West-Eberhard 1983; Coyne & Orr 2004). Much of the empirical work investigating sexual selection has focused on visual and acoustic signals such as plumage or calls in organisms such as birds, fishes and invertebrates (Wing 1946; Armstrong 1965; Gilliard 1969; Milinski & Bakker 1990; Snedden & Sakaluk 1992; Kotiaho 2000), whereas comparatively few studies have looked at the importance of chemical cues such as pheromones in mate choice (Pivnick et al. 1992; Jones & Hamilton 1998; Marco et al. 1998; Martin & Lopez 2000).
Pheromones are species-specific chemical compounds predominantly used for intraspecific communication, yet they may also communicate information among heterospecific individuals of closely related species (Phelan & Baker 1987; Löfstedt et al. 1991). Pheromones often function as mate attractors among conspecifics (Eisner & Meinwald 1995) and may convey information about the prospective mates, such as quality and quantity of nuptial gifts (Dussord et al. 1991), developmental stability (Thornhill 1992), dominance status (Moore et al. 1997), body size (Shine et al. 2003) and degree of relatedness (Smith 1983).
Studies investigating the role of sex pheromones in the Lepidoptera have mostly focused on moths, where a total of 1500 species have known sex attractants (Eisner 1980; Arn et al. 1986). Sex pheromones in lepidopterans are divided into long-range and close-range signals. The former are involved in the attraction of mates over long distances, whereas the latter play a role in courtship behaviour (Hartlieb & Anderson 1999). When visual cues are not the primary means of mate location, females may release the long-range sex pheromones from abdominal glands that attract males to fly towards the source from long distances (Hartlieb & Anderson 1999). Males, on the other hand, usually release the close-range sex pheromones from glands on the wings accompanied by visual and mechanosensory cues when courting females (Grant 1987). Aside from their role in courtship behaviour and as anti-aphrodisiacs (Eisner 1980; Pivnick et al. 1992; Schulz et al. 1993; Anderson et al. 2000, 2004), these close-range sex pheromones can also facilitate reproductive isolation between closely related species (Phelan & Baker 1987; Löfstedt et al. 1991). Although not directly examined, many studies have inferred the use of chemical cues as an additional signal to visual cues in mate recognition in lepidopterans (Van Dongen et al. 1998; Jiggins et al. 2001; Fordyce et al. 2002).
Male sex pheromones in lepidopterans are usually associated with morphological structures called androconia, many containing modified scales in the form of hair pencils and folds on the wings (Wuest 1998; Hall & Harvey 2002). The scents are emitted from pheromone glands in the androconia during wing fluttering in courtship (Grant & Brady 1975; Baker & Carde 1979; Rutowski 1980; Pivnick et al. 1992; Schulz et al. 1993).
Males from the 80-species-rich African butterfly genus, Bicyclus, are primarily differentiated on the basis of the location and number of the scent glands and modified hair-like scales that cover these glands (Condamin 1973; Larsen 2005), presumably to assist in spreading male scents. As such, it is plausible to hypothesize that diversification of male androconial organs and associated scents may have been important during the speciation and divergence within this genus. To date, however, sexual selection mechanisms operating within the genus, Bicyclus, have been investigated solely for visual cues in a single member, Bicyclus anynana. It was found that females exert stabilizing selection on the dorsal pattern of the forewings in males, preferring to mate with males with intermediate-sized UV-reflective pupils, present in the centre of the eyespots (Robertson & Monteiro 2005). The use of multiple signals in mating systems in Bicyclus and the importance of these visual cues relative to other potential cues such as chemical cues remains unknown.
Here, we investigate whether putative androconial organs present on both the wing pairs of male B. anynana butterflies play an important role in female mate choice. In addition, we determine the relative importance of visual versus olfactory cues in sexual selection in these butterflies. Specifically, we ask whether (i) androconial organs present on the hindwing and/or (ii) androconial organs present on the forewing of males affect sexual preference in the females, (iii) androconial organs from the hind- and forewings are equally important in B. anynana female choice, and (iv) olfactory cues are more or less important relative to visual cues in B. anynana female choice. We address each question in a series of laboratory experiments.
2. Material and methods
(a) General animal husbandry and experimental design
A B. anynana (Lepidoptera, Nymphalidae, Satyrinae) laboratory colony was established in Buffalo, New York in 2002 from hundreds of eggs collected from a laboratory colony in Leiden, The Netherlands, originally established from 80 gravid females collected in Malawi in 1988. Larvae were raised on young maize plants in a climate room at 27°C, 12L : 12D light : dark cycle and 80% relative humidity. All sexual selection experiments with the adults took place in a greenhouse with a mean temperature of 28°C. Male and female adult butterflies were separated on the day of eclosion from pupa (day 0). Females of this species often do not mate until at least 1 day following eclosion. Males and females used in each trial were 2 days old.
We tested female choice inside a greenhouse by placing single virgin females in cylindrical hanging net cages (30 cm diameter×40 cm height) with two virgin males. The two males within one experimental unit were randomly chosen with the exception of having similar wing sizes. The butterfly treatments were performed 24 h prior to observations, and a female was added to each cage immediately prior to mate-choice experiments.
The time to mating and the female mate choice were recorded for each trial. All three butterflies were then sacrificed and kept in a freezer for later analysis. Trials in which no mating occurred within 3 h were excluded from the analysis. This time limit was arbitrarily chosen in a previous study (Robertson & Monteiro 2005) and was kept the same in this study so that the results could be compared across studies. All observations were done in a greenhouse, only on sunny days to control for the effect of weather on butterfly behaviour (Scott 1973; McDonald & Nijhout 2000).
A sign rank test was used to analyse differences in female mate choice in all the four experiments. The number of trials the females chose each of the two male treatments was recorded, and the probability of the difference between these values being significantly different from zero was obtained. A two-sample t-test was used to analyse differences in the length of time it took females to mate in the first experiment.
(b) Experiment 1
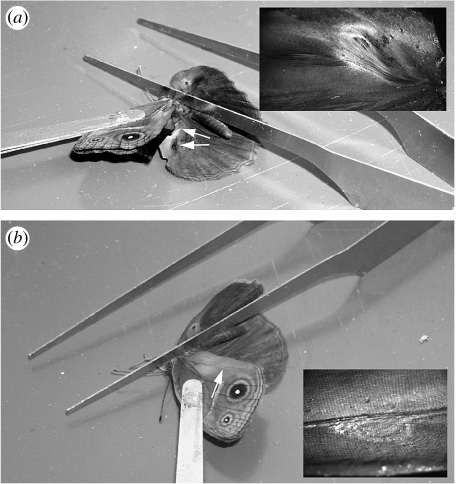
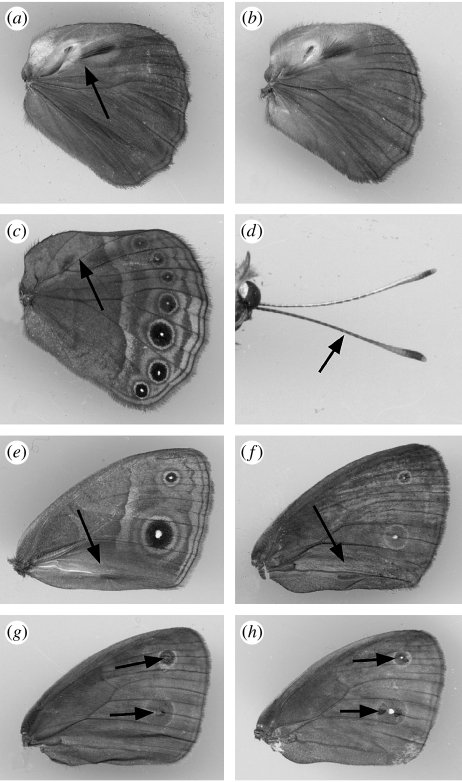
This experiment investigated the role of androconia present on the dorsal side of the hindwing in males. Each cage contained a ‘control’ male and a ‘blocked pheromone’ male. All the treatments were prepared by applying a transparent, non-viscous, nail solution (Revlon Liquid Quick Dry containing cyclomethicone, isopropyl alcohol, ethylhexyl palmitate, mineral oil and fragrance) to the wings of males. This solution makes filter paper impermeable to water, is not visible when dry, and was here tested for the first time as a ‘scent-blocking’ solution. The blocked males were prepared by applying the solution over the putative androconial organs (including hair pencils) on the dorsal side of the hindwings (figures 1a and 2a). In order to control for confounding effects such as odour of the solution, the control males (figure 2b) received a sham treatment of the same solution on the ventral side of the hindwings (figure 2c). These male treatments were crossed with two female treatments, a ‘control’ female and a ‘blocked antennae’ female. The blocked antennae females had their antennae painted over with the nail solution (figure 2d). This treatment was meant to block their most important sensory organs from detecting any chemical cues. The control females were prepared by applying nail solution to an elongated region on the anterior wing margin adjacent to where the antennae lie. There were 42 replicates of each blocked antennae and control female treatments.
Figure 1.
Methods used to prepare male treatments for (a) blocking androconia (including hair pencils) on the dorsal surface of the hindwing. Inset shows the proximal straw-coloured and the distal dark-coloured hair pencils covering gland-producing structures (not visible) and (b) blocking androconia on the ventral surface of the forewing. Inset shows modified scales marking the location of the androconia.
Figure 2.
(a) Clear nail solution treatment applied to the dorsal surface of the male hindwing. (b) Untouched dorsal surface of control male hindwing. (c) Ventral hindwing of control males with applied solution. (d) Nail solution applied to the single antenna marked with an arrow (both female antennae were treated in the experiments). (e) Nail solution applied to the androconia present on the ventral forewing. (f) Dorsal forewing of control males with applied solution. (g) Black paint applied to ‘scented’ males, blocking pupils on the dorsal surface of forewing. (h) Black paint applied to ‘visual’ males on scales surrounding pupil.
(c) Experiment 2
This experiment investigated the role of androconia present on the ventral side of the forewing of males not covered by any hair pencils. Fifty virgin females were individually placed in cages, each with two alternative virgin males, a control male and a blocked pheromone male. The blocked pheromone males had their putative androconial organs on the ventral side of the forewing painted over (figures 1b and 2e), whereas the control males received a sham treatment on the dorsal side of the forewings (figure 2f). In this experiment, we only used females without blocked antennae and did not record the time to mating. To determine the relative contribution of pheromones emitted from the hind- and forewing glands in female choice, we performed a Z-test on the proportion of females that mated with males without their pheromone glands blocked in both of our pheromone studies (experiments 1 and 2).
(d) Experiment 3
To determine the relative strength of female preference between visual and chemical cues, we performed a mate-choice experiment in which both male fore- and hindwing androconial organs were blocked and then compared these results to those of Robertson & Monteiro's (2005) mate-choice study where wing patterns were manipulated.
Forty-four virgin females were placed individually in a cage, each with two alternative virgin males. The ‘blocked’ males had both fore- and hindwing androconial organs blocked with identical methods used in experiments 1 and 2 (figure 2a,e). The control males received sham treatments on both fore- and hindwings with methods identical to experiments 1 and 2 (figure 2c,f). In addition to the sign test, a Z-test was performed on the proportion of females that chose the males without their androconia blocked (wild-type) and the proportion of females that chose the wild-type wing pattern from the female choice wing pattern manipulation conducted by Robertson & Monteiro (2005). Both of these experiments were conducted with identical methods other than the treatments administered (see Robertson & Monteiro 2005). Each experiment compared consisted of mate-choice trials with one virgin female and two alternative virgin males.
(e) Experiment 4
Additionally, to more directly investigate the relative importance of visual versus olfactory cues in a single choice experiment, 50 virgin females were individually placed in cages, each with two alternative virgin males, a control male and a blocked pheromone male. The control males had normal scent signals but had their visual signals blocked. The eyespot pupils on the dorsal side of the forewing were blocked with black Testers model enamel paint (flat black no. 1149; figure 2g) following Robertson & Monteiro (2005), whereas a sham treatment of nail solution was applied on both fore- and hindwings on the opposite side of the androconial organs. The blocked pheromone males had normal visual signals but had their pheromone signals blocked. Black paint was applied to the black scales surrounding the pupil (figure 2h), whereas all pheromone glands were blocked with the nail solution.
3. Results
In all four experiments, observations concluded that it was female choice that dictated which males mated with the respective females rather than male–male competition. In most trials, both males courted the females with behaviours such as wing fluttering and wing vibrations while slowly approaching the females. Females illustrated a preference behaviourally in that at times they would refuse a male's courtship attempt, while being receptive to the other male's courting. Once the female started copulating with a male, the other male would sometimes attempt to disrupt copulation, but was always unsuccessful. In experiment 3, we observed that control males would often attempt to court the other male in the cage that had all of its androconia blocked. It is possible that the males emitting no male scent were perceived by the other males as a female.
(a) The role of androconia on the dorsal hindwings
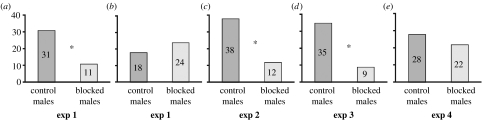
In experiment 1, an equal proportion of blocked or scented control males were chosen by females that had their antennae blocked (sign test, p>0.44; figure 3b). Females that did not have their antennae blocked, however, preferred to mate with the scented males (sign test, p=0.0029; figure 3a). The time to mating was not significantly different between the females that were blocked (62.17±8.62 min) or unblocked (52.85±7.34 min) (t82=0.973, p>0.05). These results indicate that pheromone cues from the hindwing are important in female choice, but if females are prevented from perceiving these cues they will still mate with a male in approximately the same amount of time as when able to perceive the olfactory cues.
Figure 3.
Number of females that mated with ‘blocked’ and ‘control’ males across all experiments. All blocked males had androconia blocked for each respective experiment, whereas the control males had respective sham treatments. Figures with an asterisk indicate a significant difference between treatments analysed through a sign test. (a, b) experiment 1 testing androconia on the hindwing, (c) experiment 2 testing androconia on the forewing, (d) experiment 3 testing androconia on the hind- and forewing and (e) experiment 4 testing visual versus olfactory cues.
(b) The role of androconia on the ventral forewings
In experiment 2, the majority of females chose the scented control males without their forewing androconia blocked (sign test, p=0.0003; figure 3c). These results indicate that androconia present on the forewing of males are also important in female choice.
(c) The combined role of androconia on the fore- and hindwings
In experiment 3, the majority of females mated with scented control males without their androconia blocked on both the fore- and hindwings (sign test, p=0.0002; figure 3d).
(d) The relative roles of androconia on the dorsal hindwings and ventral forewings
There was no difference between the proportion of females that chose forewing-scented males versus hindwing-scented males (controls; Z-test, p>0.05). Additionally, there was no significant difference in the proportion of females that mated with males with only one wing blocked versus both (Z-test, p>0.05).
(e) The relative roles of visual versus chemical cues in female mate choice
In experiment 4, an equal proportion of females chose scented males without UV-reflective pupils (controls) over the ‘visual’ unscented males (blocked pheromone; sign test, p=0.48; figure 3e). In addition, the proportion of females that chose males without pupils relative to control males (16 versus 34 from experiment G from Robertson & Monteiro 2005) was not significantly different from the proportion of females that chose males without pheromones in our experiment (9 versus 35; Z-test, p>0.05). These results suggest that there is no significant difference between the relative importance of visual versus olfactory cues in female mate choice in this species in our experimental setting.
4. Discussion
Behavioural observations throughout the trials suggest that it is female mate choice rather than male–male competition driving sexual selection in B. anynana in our laboratory setting. We found that androconia present in both the fore- and hindwings of male B. anynana butterflies play a role in female choice. These pheromones are perceived, at least for the most part, by the female antennae, because when these are blocked the females no longer discriminate between scented and unscented males. Surprisingly, females with blocked antennae take a similar amount of time to mate with soliciting mates as females with unblocked antennae. This result seems to indicate that when multiple stimuli are available to females to evaluate males, the presence of a correct stimulus in males (in this case the visual stimulus) is sufficient to lead females to copulation. When both stimuli are available, however, the males with the correct scent and visual stimuli are favoured to those only with the correct visual stimulus.
Pheromone glands and pheromone receptors can occur in a variety of locations in the Lepidoptera. In Colias butterflies, the glands are also found on the wing and the pheromones are believed to be secreted during the ‘wing clapping’ male courtship behaviour (Rutowski 1980). In moths, glands were found on the femur and tibia (Percy & Weatherston 1974), and the thorax and genitalia (Atkinson 1982). In addition, other Bicyclus species, but not B. anynana (Condamin 1973), have patches of velvety scales on the dorsal surface of the forewing. It is possible that these patches of scales represent androconia as well, but this has not been investigated for any of these species. Furthermore, female sensory organs are not limited to the antennae: in some lepidopteran groups, females are receptive to olfactory or chemical cues via their mouthparts as well (Bloomquist & Vogt 2003). It is possible that these additional sensory organs are also present in B. anynana, but our experiments (experiment 1) showed that just by blocking antennae, the strong preference towards ‘scented’ males was completely eliminated, suggesting the importance of antennae as primary sensory organs in the reception of male chemical signals in this species. The potential presence of additional pheromone glands in male B. anynana, not investigated in this study, could contribute, however, to us having underestimated the importance of olfactory cues in mate choice in B. anynana.
Assuming that we targeted the most important scent organs in the males, the results of our experiments combined with those of Robertson & Monteiro (2005) suggest that olfactory and visual cues play important roles in female mate choice in B. anynana. In addition, the results of our fourth experiment along with no detection of a difference in female preference between males with no olfactory cues (experiment 3) and males with no visual cues (experiments conducted by Robertson & Monteiro (2005)) suggest that these signals are relatively equal in their importance in our laboratory setting. While the relative importance of olfactory versus visual cues has been quantified in butterflies in other ecological contexts such as flower visitation and in other organisms in the context of mate choice (Kordric-Brown & Strecher 2001; Candolin 2003; Omura & Honda 2005), this is the first study to address the relative importance of multiple signals in sexual selection in butterflies. It is possible, however, that in this laboratory study, we have underestimated the importance of the visual cues relative to natural field conditions and, as a result, may also have underestimated their relative importance to olfactory cues relative to field conditions. We specifically targeted the UV-reflective pupils, present in the centre of the eyespots of the males as the visual cues because these were shown to be the only visual traits that influenced a female's mate choice (Robertson & Monteiro 2005). However, UV reflectance inside the mesh cages might have been reduced due to the reduction of UV light reaching the inside of these cages. In the greenhouse, the total UV content at 08.00 on September 18 was 1.48 mW cm−2, whereas outside the greenhouse it was more than double, 3.8 mW cm−2. Inside the mesh cages, total UV was further reduced to 0.29 mW cm−2. UVB content of light, however, was not so affected by the greenhouse setting (0.02 mW cm−2 both outside and inside the greenhouse and 0.01 mW cm−2 inside the cage). These measurements were similar at 13.00 only differing for UVB content outside the greenhouse which became 0.15 mW cm−2. Until we know more about the visual system of B. anynana, it may be difficult to extrapolate about the relative contribution of these important cues to the field situation.
It has been proposed that the magnitude of female preference may be positively related to the number of concurrent male sexual traits as in the three-spined stickleback fish (Künzler & Bakker 2001). Our study and that of Robertson & Monteiro (2005) also support the idea that females prefer to mate with males with both visual and olfactory cues present relative to males that carry only either a visual or a chemical signal. Despite the presence of two male signals, B. anynana females will mate with males producing only one of these signals, suggesting that the presence of both of these signals simultaneously is not vital for mate discrimination. Similarly, female Cycnia tenera moths will mate with courting males when chemical or acoustic signals were presented either alone or together (Conner 1987). Although in our study female Bicyclus butterflies will mate with males with only one signal present (experiment 4), they prefer to mate with males when they can perceive both signals (experiment 1).
Although we have identified the importance of chemical and visual cues in female mate choice in Bicyclus butterflies, the function of these cues has yet to be identified. Theoretical models presented by Van Doorn & Weissing (2004) suggest that female preference for multiple traits may evolve when the cost of choice is low and when different signals indicate different components of quality. It is possible that these two signals communicate different components of male quality, although a more thorough investigation of the function of these cues is needed. However, because females will mate with males with only one signal (experiment 4), the information conveyed by each signal, whether it may be redundant or not, is not necessary for a successful mating. Furthermore, it would be interesting to determine whether female Bicyclus would mate with males with both of the visual and olfactory cues blocked in further studies. This would not only confirm that Bicyclus can exhibit strong sexual selection in the experimental cages, but may also detect the existence of other cues and possibly a multimodal system.
The function of multiple signals may also differ on a spatial and temporal scale. Certain signals may also function over long distances or in close range (Candolin 2003; Lopez & Martín 2001), or function during different times in the courtship ritual (Shine & Mason 2001). In butterflies, it is suggested that visual cues function in mate location and that tactile and chemical cues follow during courtship (Siberglied 1984; Rutowski et al. 1991; Fordyce et al. 2002). Our study did not specifically look at the relative importance of these cues over long- versus close-range mate location and discrimination but does illustrate that they both are important in close-range interactions.
One last aspect that deserves consideration refers to the observation that in butterfly species in mimicry complexes (Schulz et al. 1993; Vane-Wright & Boppré 1993), there is often a shift from dependence on visual cues during intraspecific mate choice towards chemical cues such as pheromones. In the milkweed butterfly genus Amauris, for instance, there is a shift towards chemical communication among closely related Müllerian mimics. Although these chemical signals may not solely mediate species isolation, they can provide information on the identity of the male to the female and aid in species recognition in mimicry complexes where visual communication can be problematic (Schulz et al. 1993; Vane-Wright & Boppré 1993).
Members of the African endemic genus, Bicyclus, are not currently understood as belonging to mimicry complexes either with each other or with closely related genera, but perhaps they should. In support of this idea, we observe that all 80 Bicyclus species share fairly similar ventral wing patterns many occupy the same forests and some of the males in the genus are quickly identifiable in the field by their strong musky odour. For instance, it is possible to collect over 20 different species within a small patch (1 mile2) of rainforest in at least two different localities (Kibale Forest in Uganda, and the Dja Reserve in Cameroon; A. Monteiro 1997, 1998, personal observation). Bicyclus anynana, in particular, can be trapped with at least four other members of the genus in Malawi displaying similar wing patterns (Condamin 1973; Windig et al. 1994; Roskam & Brakefield 1999). In Lepidoptera, wing patterns have been suggested to aid in reinforcement of recently diverged species (Howard 1993; Fordyce et al. 2002; Lukhtanov et al. 2005; Mavárez et al. 2006). The ability of members of the genus Bicyclus not to rely exclusively on visual cues in sexual selection would decrease the chance of maladaptive matings in forests populated with heterospecifics. Therefore, the use of multiple signals in Bicyclus may benefit the female's intra- and interspecific mate evaluation.
In conclusion, this study found that in addition to visual cues such as wing patterns, chemical cues also play a role in intraspecific communication for mate choice in B. anynana. In addition, we found that both signals are equally important in the process of sexual selection by female choice in the laboratory and that females will mate with males exhibiting only one of these signals. The function of these cues and the adaptive significance of multiple signals in this mating system need to be further evaluated.
Acknowledgments
We thank Kendra Robertson for advice on the practical aspects of these experiments and William Piel, Diane Ramos, Daniel Poland, Derrick Dupois and three anonymous reviewers for comments on the manuscript. We also thank Amanda Diaz, Bin Chen, Gary Glaser, Kyle Golden, Andrew Goldman and Diane Ramos for the maintenance of laboratory butterflies used in this study, and Dennis Pietras for the plant husbandry. This study was funded by NSF award IOB-0316283 to A.M.
References
- Anderson J, Borg-Karlson A.-K, Wiklund C. Sexual cooperation and conflict in butterflies: a male-transferred anti-aphrodisiac reduced harassment of recently mated females. Proc. R. Soc. B. 2000;267:1271–1275. doi: 10.1098/rspb.2000.1138. doi:10.1098/rspb.2000.1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J, Borg-Karson A.K, Wiklund C. Sexual conflict and anti-aphrodisiac titre in a polyandrous butterfly: male ejaculate tailoring and absence of female control. Proc. R. Soc. B. 2004;271:1765–1770. doi: 10.1098/rspb.2003.2671. doi:10.1098/rspb.2003.2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong E.A. Dover Publications; New York, NY: 1965. The ethology of bird display and behavior. [Google Scholar]
- Arn H, Toth M, Preisner E. OILB-SROP; Wadenswill, Switzerland: 1986. List of sex pheromones of Lepidoptera and related attractants. [Google Scholar]
- Atkinson P.R. Structure of the putative pheromone glands of Eldana saccharina Walker (Lepidoptera: Pyralide) J. Entomol. Soc. Afr. 1982;45:93–104. [Google Scholar]
- Baker T.C, Carde R.T. Courtship behavior of the oriental fruit moth (Grapholitha molesta): experimental analysis and consideration of the role of sexual selection in the evolution of courtship pheromones in the Lepidoptera. Ann. Entomol. Soc. Am. 1979;72:173–188. [Google Scholar]
- Bloomquist G.J, Vogt R.G. Biosynthesis and detection of pheromones and plant volatiles—introduction and overview. In: Bloomquist G.J, Vogt R.G, editors. Insect pheromone biochemistry and molecular biology. Elsevier Academic Press; London, UK: 2003. pp. 3–18. [Google Scholar]
- Candolin U. The use of multiple cues in mate choice. Biol. Rev. 2003;78:575–595. doi: 10.1017/s1464793103006158. doi:10.1017/S1464793103006158 [DOI] [PubMed] [Google Scholar]
- Condamin M. Monographie du genre Bicyclus (Lepidptera: Satyridae) Memoires de L'Institut Fondamental d'Afrique Noire. 1973;88:1–824. [Google Scholar]
- Conner W.E. Ultrasound: its role in the courtship of the arctiid moth, Cycnia tenera. Experientia. 1987;43:1029–1031. doi:10.1007/BF01952230 [Google Scholar]
- Coyne J.A, Orr H.A. Sinauer; Sunderland, MA: 2004. Speciation. [Google Scholar]
- Darwin C. John Murray; London, UK: 1981. The descent of man, and selection in relation to sex. [Google Scholar]
- Dussord D.E, Harvis C.A, Meinwald J, Eisner T. Pheromonal advertisement of a nuptial gift by a male moth (Utetheisa ornatrix) Proc. Natl Acad. Sci. USA. 1991;88:9224–9227. doi: 10.1073/pnas.88.20.9224. doi:10.1073/pnas.88.20.9224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner T. Chemistry, defense, and survival: case studies and selected topics. In: Locke M, Smith D.S, editors. Insect biology in the future. Academic Press; New York, NY: 1980. pp. 847–878. [Google Scholar]
- Eisner T, Meinwald J. The chemistry of sexual selection. Proc. Natl Acad. Sci. USA. 1995;92:50–55. doi: 10.1073/pnas.92.1.50. doi:10.1073/pnas.92.1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordyce J.A, Nice C.C, Forister M.L, Shapiro A.M. The significance of wing pattern diversity in the Lycaenidae: mate discrimination by two recently diverged species. J. Evol. Biol. 2002;15:871–879. doi:10.1046/j.1420-9101.2002.00432.x [Google Scholar]
- Gilliard E.T. Natural History Press; Garden City, NY: 1969. Birds of paradise and bower birds. [Google Scholar]
- Grant G.G. Copulatory behaviour of spruce budworm, Choristoneura funiferana (Lepidoptera: Tortricidae): experimental analysis of the role of sex pheromone and associated stimuli. Ann. Entomol. Soc. Am. 1987;80:78–88. [Google Scholar]
- Grant G.G, Brady U.E. Courtship behavior of Phycitid moths. I. Comparison of Plodia interpunctella and Codra cautella and the role of male scent glands. Can. J. Zool. 1975;53:813–826. [Google Scholar]
- Hall J.P.W, Harvey D.J. A survey of the androconial organs in the Riodinidea (Lepidoptera) Zool. J. Linn. Soc. 2002;136:171–197. doi:10.1046/j.1096-3642.2002.00003.x [Google Scholar]
- Hartlieb E, Anderson P. Olfactory-released behaviours. In: Hanson B.S, editor. Insect olfaction. Springer; Berlin, Germany: 1999. pp. 315–349. [Google Scholar]
- Howard D.J. Reinforcement: origin, dynamics, and fate of an evolutionary hypothesis. In: Harrison R.G, editor. Hybrid zones and the evolutionary process. Oxford University Press; New York, NY: 1993. [Google Scholar]
- Jiggins C.D, Naisbit R.E, Coe R.L, Mallet J. Reproductive isolation caused by colour pattern mimicry. Nature. 2001;411:302–305. doi: 10.1038/35077075. doi:10.1038/35077075 [DOI] [PubMed] [Google Scholar]
- Jones T.M, Hamilton J.G.C. A role for pheromones in mate choice in a lekking sandfly. Anim. Behav. 1998;56:891–898. doi: 10.1006/anbe.1998.0857. doi:10.1006/anbe.1998.0857 [DOI] [PubMed] [Google Scholar]
- Kordric-Brown A, Strecher U. Responses of Cytrpinodon maya and C. labiousus females to visual and olfactory cues of conspecific and heterospecific males. Biol. J. Linn. Soc. 2001;74:541–548. doi:10.1006/bijl.2001.0598 [Google Scholar]
- Kotiaho J.S. Testing the assumption of conditional handicap theory: costs and condition-dependence of a sexually selected trait. Behav. Ecol. Sociobiol. 2000;43:188–194. doi:10.1007/s002650000221 [Google Scholar]
- Künzler R, Bakker T.C.M. Female preferences for single and combined traits in computer animated stickleback males. Behav. Ecol. 2001;10:681–685. doi:10.1093/beheco/12.6.681 [Google Scholar]
- Larsen T.B. Apollo Books; Stenstrup, Denmark: 2005. Butterflies of West Africa. [Google Scholar]
- Löfstedt C, Herrebout W.M, Menken S.B.J. Sex pheromones and their potential role in the evolution of reproductive isolation in small ermine moths (Yponomeutidae) Chemoecology. 1991;2:20–28. doi:10.1007/BF01240662 [Google Scholar]
- Lopez P, Martín J. Pheromonal recognition of females takes precedence over the chromatic cue in male Iberian wall lizards. Ethology. 2001;107:901–912. doi:10.1046/j.1439-0310.2001.00724.x [Google Scholar]
- Lukhtanov V.A, Kandul N.P, Plotkin J.B, Dantchenko A.V, Haig D, Pierce N.E. Reinforcement of pre-zygotic isolation and karyotype evolution in Agrodiaetus butterflies. Nature. 2005;436:385–389. doi: 10.1038/nature03704. doi:10.1038/nature03704 [DOI] [PubMed] [Google Scholar]
- Marco A, Chivers D.P, Kiesecher J.M, Blaustein A.R. Mate choice by chemical cues in western redback (Plethodon vehiculum) and Dunn's (P. dunni) salamanders. Ethology. 1998;104:781–788. [Google Scholar]
- Martin J, Lopez P. Chemoreception, symmetry, and mate choice in lizards. Proc. R. Soc. B. 2000;267:1265–1269. doi: 10.1098/rspb.2000.1137. doi:10.1098/rspb.2000.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavárez J, Salazar C.A, Bermingham E, Salcedo C, Jiggins C.S, Linares M. Speciation by hybridization in Heliconius butterflies. Nature. 2006;441:868–871. doi: 10.1038/nature04738. doi:10.1038/nature04738 [DOI] [PubMed] [Google Scholar]
- McDonald A.K, Nijhout H.F. The effect of environmental conditions on mating activity of the Buckeye butterfly, Precis coema. J. Res. Lepid. 2000;35:22–28. [Google Scholar]
- Milinski M, Bakker T.C.M. Female sticklebacks use male coloration and mate choice and hence avoid parasitized males. Nature. 1990;344:330–333. doi:10.1038/344330a0 [Google Scholar]
- Moore P.J, Reagan-Wallin N.L, Haynes K.F, Moore A.J. Odour conveys status in cockroaches. Nature. 1997;389:25. doi:10.1038/37888 [Google Scholar]
- Omura H, Honda K. Priority of color over scent during flower visitation by adult Vanessa indica butterflies. Oecologia. 2005;142:588–596. doi: 10.1007/s00442-004-1761-6. doi:10.1007/s00442-004-1761-6 [DOI] [PubMed] [Google Scholar]
- Percy J, Weatherston J. Gland structure and pheromone production in insects. In: Birch M, editor. Pheromones, frontier of biology. North Holland; Amsterdam, The Netherlands: 1974. [Google Scholar]
- Phelan L.P, Baker T.C. Evolution of male pheromones in moths: reproductive isolation through sexual selection. Science. 1987;235:205–207. doi: 10.1126/science.235.4785.205. doi:10.1126/science.235.4785.205 [DOI] [PubMed] [Google Scholar]
- Pivnick K.A, Lavoiedorinik J, McNeil J.N. The role of the androconia in the mating behavior of the European skipper, Thymelicus lineola, and evidence for a male sex-pheromone. Physiol. Etomol. 1992;17:260–268. [Google Scholar]
- Robertson K.A, Monteiro A. Female Bicyclus anynana butterflies choose males on the basis of their dorsal UV-reflective eyespot pupils. Proc. R. Soc. B. 2005;272:1541–1546. doi: 10.1098/rspb.2005.3142. doi:10.1098/rspb.2005.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskam J.C, Brakefield P.M. Seasonal polyphenism in Bicyclus (Lepidoptera: Satyridae) butterflies: different climates need different cues. Biol. J. Linn. Soc. 1999;66:345–356. doi:10.1006/bijl.1998.0268 [Google Scholar]
- Rutowski R.L. Male scent-producing structures in Colias butterflies. J. Chem. Ecol. 1980;6:15–27. doi:10.1007/BF00987523 [Google Scholar]
- Rutowski R.L, Dickinson J.L, Terkanian B. Behavior of male desert hackberry butterflies, Asterocampa leilia (Nymphalidae) at perching sites used in mate location. J. Res. Lep. 1991;30:129–139. [Google Scholar]
- Schulz S, Boppré M, Vane-Wright R.I. Specific mixtures from secretions of male sex scent-organs of African Milkweed butterflies (Danainae) Phil. Trans R. Soc. B. 1993;342:161–181. [Google Scholar]
- Scott J.A. Mating in butterflies. J. Res. Lepid. 1973;1:99–127. [Google Scholar]
- Shine R, Mason R.T. Courting male garter snakes (Thamnophilis sirtalis parietalis) use multiple cues to identify potential mates. Behav. Biol. Sociobiol. 2001;49:465–473. doi:10.1007/s002650100334 [Google Scholar]
- Shine R, Phillips B, Waye H, LeMaster M, Mason R.T. Chemosensory cues allow courting male garter snakes to assess body length and body condition of potential mates. Behav. Ecol. Sociobiol. 2003;54:162–166. [Google Scholar]
- Siberglied R.E. Visual communication and sexual selection among butteflies. In: Vane-Wright R.I, Ackery P.R, editors. The biology of butterflies. Academic; London, UK: 1984. pp. 207–233. [Google Scholar]
- Smith B.H. Recognition of female kin by male bees through olfactory signals. Proc. Natl Acad. Sci. USA. 1983;80:4551–4553. doi: 10.1073/pnas.80.14.4551. doi:10.1073/pnas.80.14.4551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedden W.A, Sakaluk S.K. Acoustical signaling and its relation to male mating success in sagebrush crickets. Anim. Behav. 1992;44:633–639. doi:10.1016/S0003-3472(05)80291-X [Google Scholar]
- Thornhill R. Female preference of the pheromone of males with low fluctuating asymmetry in the Japanese scorpionfly Panopra japonica mecoptera. Behav. Ecol. 1992;1:277–283. [Google Scholar]
- Van Dongen S, Matthysen E, Sprengers E, Dhondt A.A. Mate selection by male winter moths Operophtera brumata (Lepidoptera Geometridae): adaptive male choice or female control? Behaviour. 1998;135:29–42. [Google Scholar]
- Van Doorn G.S, Weissing F.J. The evolution of female preferences for multiple indicators of quality. Am. Nat. 2004;164:173–186. doi: 10.1086/422203. doi:10.1086/422203 [DOI] [PubMed] [Google Scholar]
- Vane-Wright R.I, Boppré M. Visual and chemical signaling in butterflies: functional and phylogenetic perspectives. Phil. Trans R. Soc. B. 1993;340:197–205. [Google Scholar]
- West-Eberhard M.J. Sexual selection, social competition, and speciation. Q. Rev. Biol. 1983;58:155–183. doi:10.1086/413215 [Google Scholar]
- Windig J.J, Brakefield P.M, Reitsma N, Wilson J.G.M. Seasonal polyphenism in the wild: survey of wing patterns in five species of Bicyclus butterflies in Malawi. Ecol. Entomol. 1994;19:285–298. [Google Scholar]
- Wing L. Drumming flight in the blue grouse and courtship characters of the Tetraonidae. Condor. 1946;48:154–157. [Google Scholar]
- Wuest J. The pheromone dispersing apparatus in some Hesperiinae (Lepidoptera: Hesperiidae) Swiss J. Zool. 1998;105:813–822. [Google Scholar]