Abstract
Big brains are hypothesized to enhance survival of animals by facilitating flexible cognitive responses that buffer individuals against environmental stresses. Although this theory receives partial support from the finding that brain size limits the capacity of animals to behaviourally respond to environmental challenges, the hypothesis that large brains are associated with reduced mortality has never been empirically tested. Using extensive information on avian adult mortality from natural populations, we show here that species with larger brains, relative to their body size, experience lower mortality than species with smaller brains, supporting the general importance of the cognitive buffer hypothesis in the evolution of large brains.
Keywords: brain evolution, behavioural flexibility, life-history theory, mortality rate
1. Introduction
The evolution of large brains, such as those of humans, presents evolutionary biologists with an unresolved problem: if growing an enlarged brain has a high cost of development and maintenance (Allman 2000; Iwaniuk & Nelson 2003), why do some animals have large brains relative to their body size? A classic answer to this question is that the costs are compensated for later in life by the benefits that a large brain provides to survive environmental challenges through flexible behaviours (Allman et al. 1993; Allman 2000; Deaner et al. 2002), a theory known as the ‘cognitive buffer’ hypothesis. Supporting evidence for the theory comes from the finding that big-brained animals have a higher propensity to innovate and learn (Lefebvre et al. 1997; Reader & Laland 2002; Byrne & Corp 2004) and such behavioural flexibility helps them face challenges presented by new or altered environments (Shultz et al. 2005; Sol et al. 2005a). Nonetheless, the hypothesis that large brains are associated with reduced adult mortality has not been tested in any group of animals.
We have investigated the relationship between brain size and mortality rate with a comparative analysis in birds, using information on avian adult mortality from more than 300 natural populations of 220 species from polar, temperate and tropical regions (Liker & Székely 2005). Birds are ideally suited for such a test as they represent one of the handful taxa for which the relationship between large brains and enhanced behavioural response to ecological challenges is best understood (Lefebvre et al. 2004; Sol et al. 2005a). Moreover, the unparalleled amount of data available on mortality from wild populations makes it possible to conduct a general test of the cognitive buffer hypothesis.
2. Material and methods
We gathered information on adult annual mortality rates (sexes averaged) for 319 populations of 236 species from published studies by searching extensively in reference books, species monographs and electronic databases that included biological abstracts (BIOSIS, 1975–2002; Liker & Székely 2005). Mortality rate is considered context dependent and subject to measurement error, and may thus vary between populations from the same species. However, we found a high repeatability in our mortality measures, with 80.5% of variation found among rather than within species (see Lessells & Boag 1987 for the method).
Information on brain and body masses (in grams) from published sources (see Sol et al. (2005a) for details) was available for 184 of these species; for 40 additional species, we estimated their brain size by using the average brain mass of species from the same genus (Sol et al. 2005a), a taxonomic level that predicts 91% of the species level variance. This yields a total of 303 populations and 224 species from polar, temperate and tropical regions (Liker & Székely 2005). Conclusions are qualitatively similar whether or not we include the 40 species for which brain size is inferred; for this reason, we only report the results with the larger dataset.
Previous work has shown that it is not brain size per se, but the extent to which the brain is either larger or smaller than that expected for a given body size which indicates adaptation for enhanced neural processing (Jerison 1973). Three general methods have been proposed to remove the allometric effect of body size on brain size (Deaner et al. 2000, 2002): (i) estimate the residuals of a log–log least-square linear regression of brain mass against body mass, (ii) calculate the fraction of the body mass that corresponds to brain mass, and (iii) include absolute brain size and body mass (both log-transformed) as covariates in a multivariate model. Since there is still no consensus on which is the most appropriate method (Reader & Laland 2002), we validated the cognitive buffer hypothesis using the three approaches. The three methods yielded qualitatively similar results, although the second method did not appropriately remove the effect of body size and the third created problems of colinearity due to the high correlation between brain and body masses (r=0.99). Thus, we report in the text the results obtained using the method of residuals.
We tested the cognitive buffer hypothesis using a hierarchical approach (Bennett & Owens 2002) in which mortality rate was modelled at the levels in which substantial variation in mortality rates exist: populations and families. The population-level analysis was used to test the relationship between brain size and mortality rate while controlling for the effect of the environment and clade-traits, whereas the family-level analysis served to validate the hypothesis at the taxonomic level where the most diversification in brain size has occurred.
A difficulty in population level analyses is the need to deal with the autocorrelation that may exist in mortality measures belonging to the same species, higher taxonomic levels or regions. Since it is not possible to deal with several sources of autocorrelation using classical phylogenetic-based techniques (e.g. independent contrasts), and a phylogenetic hypothesis for the studied populations is not available, we used generalized linear mixed models (GLMMs) to model mortality rate as a function of relative brain size while controlling for differences in mortality among species, higher taxonomic levels and regions (Blackburn & Duncan 2001; Sol et al. 2005a). We modelled the likely non-independence of mortality rates due to taxonomic or regional affiliations using a variance components model, assuming a common positive correlation between mortality rates of populations from the same taxon (species, family and parvorder) or region (Arctic, North temperate, tropical, South temperate and Antarctic), but a zero correlation between mortality rates involving different taxa or regions (Blackburn & Duncan 2001). This approach ensured that the significance tests for the fixed-effect predictors (i.e. residual brain size and other species-traits, see below) were not biased by non-independence of mortality rates belonging to the same species, higher taxonomic level or region. Given that after arcsine (square root) transformation mortality fit reasonably well to a normal distribution, we implemented models with the error structure defined as normal.
We identified four confounding effects that could potentially affect the relationship between brain size and mortality rate: migratory behaviour (Sol 2003; Winkler et al. 2004; Sol et al. 2005b), prolonged parental care (Gittleman 1994; Liker & Székely 2005), intense competition for mates (Madden 2001; Garamszegi et al. 2005; Liker & Székely 2005), and type of offspring development (Bennett & Harvey 1985; Iwaniuk & Nelson 2003). To measure migratory strategy, we classified each species as resident (no migration), short- and long-distance migratory (separated breeding and wintering areas; Sol et al. 2005b). The intensity of parental care was assessed by dividing it into five major activities and scoring each one separately for males and females as explained in Liker & Székely (2005). We used two surrogate measures of mating competition, testis sizes (in grams, corrected for body weight in the analyses using the residuals approach; see above) and the percentage of socially polygamous males (Liker & Székely 2005). Finally, to measure type of offspring development, we classified each species as precocial, semi-precocial, semi-altricial or altricial. Information on all these variables was collected from reference books, species monographs and published articles (see Liker & Székely 2005).
We further confirmed the population-level analyses in three ways. First, we used the GLMM approach to examine whether brain size was able to explain some of the variation that remains in mortality rate once the effect of body size has been removed (Allman et al. 1993; Deaner et al. 2000), using as response variable the residuals of a log–log regression of mortality rate against body size. Since errors in body size would create a bias in the same direction in both response and predictor (Deaner et al. 2002), we used different sets of body masses to remove body size effects on mortality rate and brain size. Second, we used the method of phylogenetic-independent contrasts to further validate the existence of a relationship between brain size and adult mortality at the species level. When several mortality rates were available for a species, we used the ones that were based on capture–recapture analyses, larger samples and/or longer study periods. Our phylogenetic hypothesis was that described in Liker & Székely (2005), with branch length set to unity. Equal branch lengths are commonly used in comparative studies such as ours when true lengths are unknown (Harvey & Pagel 1991). Independent contrasts were calculated with the software Caic v. 2.0 (Purvis & Rambaut 1995), tested for proper standardization by plotting absolute values against standard deviations and analysed with ordinary regressions forced through the origin (for statistical justifications see Harvey & Pagel (1991) and Garland et al. (1992)). In all cases, the contrasts were correctly standardized.
Finally, because body size is sensitive to nutritional and reproductive condition and varies considerably within individuals and among populations (Garamszegi et al. 2002; Iwaniuk & Nelson 2002), which could bias the results (Pagel & Harvey 1988; Dunbar 1992; Deaner et al. 2002), we repeated all analyses with an independent set of body masses. The results of all these confirmatory analyses were qualitatively similar to the main ones (see appendix in electronic supplementary material).
Since body mass was the only confounding variable significantly associated with mortality rate, we used path analysis (Li 1975) to further confirm that brain size affected mortality independently of body size at the species level. To evaluate the relative importance of each link in the path diagram, we calculated the path coefficients (standardized partial regression coefficients) using both raw data and independent contrasts (see above). Path coefficients estimate the strength of the hypothesized causal link that affect a given dependent variable, and hence allows one to separate direct effects from indirect or spurious effects.
For the family-level analysis, brain size was estimated as the residual from a log–log regression of brain size on body mass, using information on 1974 species. Then, mortality rate and residual brain size were calculated as the average value for all the species from the family. We controlled for similarities among taxa due to common ancestry with the method of phylogenetic-independent contrasts (Felsenstein 1985). To calculate the independent contrasts, we adopted the phylogeny proposed by Sibley & Ahlquist (1990) with branch lengths estimated as genetic distances and followed the same procedure already described.
We finally explored the role of evolutionary history in explaining variation in mortality rates and residual brains with two phylogenetic-based methods. First, we used nested ANOVAs to identify the taxonomic level or levels (genus, family and order) where major diversification in the traits has taken place during the evolutionary history of birds. Second, we estimated the phylogenetic correlation in residual brain and mortality rate using the spatial autocorrelation statistic Moran's I (Gittleman et al. 1996). Positive values would indicate that the variable at a particular taxonomic level is more similar than random, whereas negative values indicate that they are more different. We estimated Moran's I based on the phylogenetic classification proposed by Sibley & Monroe (1990), using the software APE developed by Emmanuel Paradis and Julien Dutheil.
3. Results
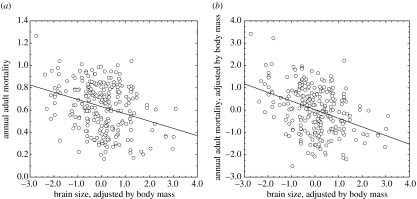
At the population level, there was a significant relationship between residual brain size and adult mortality rate; birds with larger brains relative to their body size had lower annual mortality rates than birds with smaller brains (GLMM: F1,81=13.82, p=0.0004; figure 1), when both taxonomic and regional autocorrelations were accounted for. The relationship did not change after the removal of the most widely represented order in our sample, the Passeriformes (estimate±s.e.=−0.459±0.130, F1,49=12.43, p<0.0001; the estimate is a linear parameter estimate from a GLMM).
Figure 1.
Relationship between residual brain size and mortality rate at the population level (a) without and (b) with control of allometric effects of body size on mortality rates. Brain size adjusted by body mass is estimated as the residuals of a log–log least-square linear regression of brain mass against body mass. Body size effects were removed from mortality rates estimates using the same approach.
To further confirm that the relationship between relative residual brain size and mortality rate was not confounded by environmental factors, we studied how adult mortality varied across major habitats (marine–coastal habitats, fresh-water habitats, open habitats, woodlands and forests and human-made habitats) within the regions we defined (see §2). Mortality was higher for species inhabiting agricultural and urban habitats than for those not using these habitats (GLMM, estimate±s.e.=−0.359±0.114, F1,81=9.91, p=0.002), perhaps because birds are not fully adapted to these new environments (Sax & Brown 2000) or they suffer there from greater human disturbances. However, residual brain size remained negatively associated with mortality rate when the effect of the habitat was included as a covariate in the model (GLMM, estimate±s.e.=−0.303±0.096, F1,81=9.94, p=0.002).
There was still the possibility that the link between residual brain size and mortality was inflated by species-traits effects we have not yet considered. Four attributes could potentially affect such a relationship. First, migratory behaviour is associated with small brains relative to body size (Sol et al. 2005b), and it may also increase mortality. Second, prolonged parental care is costly and may increase mortality of parents (Liker & Székely 2005). Third, intense competition for mates is a factor that has consistently been found to increase mortality (Owens & Bennett 1994; Liker & Székely 2005) and is also suggested to explain some variation in residual brain size (Madden 2001). Finally, the type of chick development is known to be associated with brain size in birds (Bennett & Harvey 1985; Iwaniuk & Nelson 2003) and could also be related to adult mortality. When all these variables were accommodated in the same model, the relationship between residual brain size and annual mortality rate remained statistically significant (table 1). Besides brain residual, body size was the only variable in the minimum adequate model retaining significant variables (GLMM: F1,81=46.68, p<0.0001).
Table 1.
Generalized linear mixed model (GLMM) accounting for variation in adult mortality rates between populations. (The significance of each fixed effect on annual adult mortality rate is tested while controlling for the other fixed effects and random effects. Migratory strategy and offspring development are categorical variables, so we provide the ranges for the parameter estimates.)
| fixed effects | parameter estimate | s.e. | type III, F | p |
|---|---|---|---|---|
| residual brain size | −0.062 | 0.019 | 10.69 | <0.001 |
| body mass | −0.126 | 0.031 | 17.01 | <0.0001 |
| migratory strategy | −0.003 to 0.014 | 0.027–0.048 | 0.46 | 0.631 |
| social polygamy | 0.009 | 0.009 | 0.90 | 0.346 |
| male parental care | −0.011 | 0.010 | 1.14 | 0.291 |
| female parental care | −0.001 | 0.006 | 0.01 | 0.909 |
| residual testis mass | 0.002 | 0.002 | 0.80 | 0.374 |
| offspring development | −0.009 to 0.056 | 0.072–0.089 | 0.10 | 0.961 |
| random effects | variance component | s.e. |
|---|---|---|
| among orders | 0.006 | 0.008 |
| among families within order | 0.007 | 0.004 |
| among species within family | 0.006 | 0.001 |
| among regions | 0.001 | 0.001 |
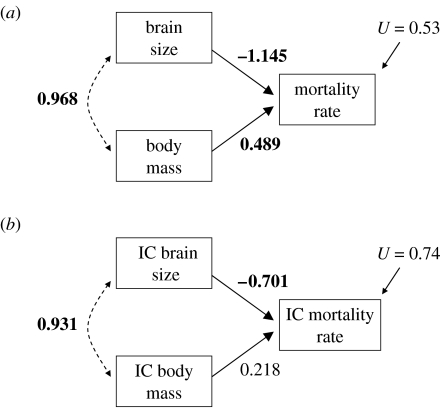
We further investigated the relationship between brain size and body size using path analysis, a technique that allows the deconstruction of causal relationships between traits (Li 1975). A path analysis revealed that most of the effect of brain mass (in absolute terms) on mortality rate is direct rather than being indirectly caused by its correlation with body mass (figure 2). The possibility that such a causal pattern arose as a statistical artefact of the higher measurement error in body mass compared with brain size (Pagel & Harvey 1988) was unlikely in our case, given the low error associated with our body mass measures (98% repeatability in our body mass measures).
Figure 2.
Path diagram of expected causal relationship between brain mass, body mass and mortality rate at the species level (a) without and (b) with phylogenetic corrections using the independent contrast method. Dashed lines with the double-ended arrow indicate correlations and solid lines with a single arrow indicate path coefficients. Bold numbers refer to significant values at 0.05 level.
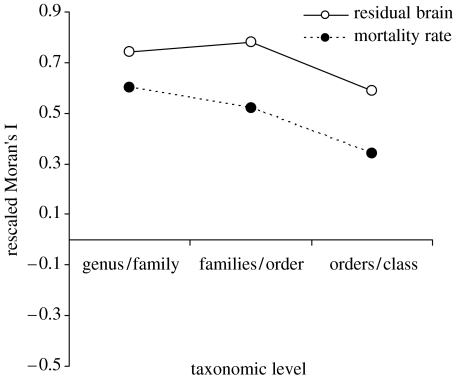
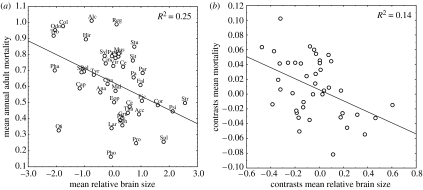
Most diversification in residual brain size occurred early in the avian radiation (Nealen & Ricklefs 2001), with families and higher taxonomic levels accounting for over 78% of variation estimated with a nested ANOVA (figure 3). Consequently, the negative relationship between residual brain size and mortality rate found at the population level should also be clearly detected at the highest taxonomic levels. Our results revealed that substantial variation (although less than for residual brain size) in mortality rate was found at the family or higher taxonomic levels (53.6%; figure 3) and such variation was associated with differences between lineages in residual brain size (least square regression: F1,41=9.45, p=0.003, figure 4a; independent contrasts, F1,41=10.99, p<0.002; figure 4b).
Figure 3.
Phylogenetic correlogram illustrating the results of a Moran's I procedure conducted on residual brains (n=1974 species) and mortality rates (n=236 species). All values are significantly higher than 0, according to a randomization test, indicating phylogenetic autocorrelation. The y-axis represents rescaled Moran's I (where I is rescaled for comparability across taxonomic levels); the x-axis is the taxonomic level, where genus/family indicates genera within families, and so on).
Figure 4.
Relationships between mean residual brain size and mean rate of annual adult mortality at the family level (a) without and (b) with phylogenetic corrections using independent contrasts. Codes refer to family names: Odo, Odontophoridae; Pha, Phasianidae; Ana, Anatidae; Pic, Picidae; Alc, Alcedinidae; Ce, Cerylidae; Psi, Psittacidae; Tro, Trochilidae; Str, Strigidae; Cap, Caprimulgidae; Col, Columbidae; Ral, Rallidae; Oti, Otididae; Gru, Gruidae; Sco, Scolopacidae; Cha, Charadriidae; Lar, Laridae; Fal, Falconidae; Acc, Accipitridae; Sul, Sulidae; Pha, Phalacrocoracidae; Pho, Phoenicopteridae; Cic, Ciconiidae; Sph, Spheniscidae; Pro, Procellariidae; Tha, Thamnophilidae; Tyr, Tyrannidae; Mal, Maluridae; Pa, Pardalotidae; Eop, Eopsaltriidae; Vir, Vireonidae; Cor, Corvidae; Cin, Cinclidae; Mus, Muscicapidae; Stu, Sturnidae; Sit, Sittidae; Cer, Certhiidae; Par, Paridae; Hir, Hirundinidae; Reg, Regulidae; Syl, Sylviidae; Pas, Passeridae and Fri, Fringillidae.
4. Discussion
Our results clearly demonstrate that larger brains, relative to body size, are associated with higher survival in nature, consistent with the hypothesis that enlarged brains allow a cognitive buffer whereby individuals are able to flexibly respond to environmental variation. Although the cognitive buffer hypothesis is the only clear mechanism that proposes benefits in terms of survival to counterbalance the costs associated with enlarged brains, the reverse causality that low mortality permits individuals to delay their maturity and grow larger brains (and learn how to use them) could also be true. Indeed, some life-history models argue that age-specific mortality is a major driver of variation in other life-history traits among species (Charnov 1991), predicting that a decrease in adult mortality should favour a reduction in reproductive effort (Saether 1988), a slowdown of maturation (Martin 2002) and a longer lifespan (Stearns 1992; Deaner et al. 2000). In brain evolution theory, ‘slow’ life histories (i.e. slow body growth rate, delayed maturity and long lifespan; see Saether 1988) are generally believed to favour selection for enlarged brains. A slower development is expected to increase the time available for brain growth and juveniles to learn those skills that are critical for survival, whereas a longer lifespan will increase the probability that an individual encounters a life-threatening situation, increasing the fitness value of more cognitive lifestyles (Allman et al. 1993; Allman 2000; Deaner et al. 2002). If these theories were correct, any reduction in mortality caused by changes in the environment or by the evolution of adaptations that buffer individuals from environmental challenges could favour the evolution of enlarged brains.
Whereas comparative analyses per se cannot establish an unambiguous causal relationship between large brains and reduced mortality (Bennett & Owens 2002), and experiments on this issue are obviously impossible, we tackled this limitation in two ways. First, we tested the effect of relative brain size on mortality rates while accounting for a number of alternative explanations. The association between residual brain size and survival was independent of a number of environmental conditions and species-level attributes suggesting that the relationship was not a mere consequence of enhanced survival caused by other factors. Second, we used nested ANOVAs and Moran's I indices to demonstrate that brain size has more phylogenetic signal than mortality rates. This implies that substantial variation in residual brain size preceded the establishment of current mortality patterns, and therefore cannot solely be its consequence.
One interesting theoretical possibility is that enlarged brains may both affect and be affected by life-history strategies. Such a causal scenario would imply a positive feedback in which big brains favour situations where the species develop slowly but has a higher chance of surviving, which would in turn favour further increases in brain size. If the existence of a positive feedback was confirmed, the implications for understanding brain evolution would be important, providing a new perspective to understand why the brain has evolved so fast in a variety of independent lineages such as hominids, cetaceans, corvids and parrots.
Our finding that a large brain is associated with higher survival in nature adds to a growing literature on brain evolution and enhanced cognitive skills to deal with environmental challenges (Lefebvre et al. 2004; Sol et al. 2005a). In general, behaviourally flexible animals that have to learn to (i) avoid predators, (ii) find edible food, or (iii) select habitats suitable for living and reproduction (decisions that take time and may be risky) should be more vulnerable during early stages of development than those that do so by means of instinctive behaviours (Reader 2004). Nevertheless, once acquired, these learned skills may be critical for surviving various ecological stresses, making the individual less vulnerable to extrinsic factors. Through learning, individuals may track the changes in resources, improve efficiency in exploiting available sources, gain access to novel opportunities and develop new responses to enemies (Dukas 1998; Sol 2003; Lefebvre et al. 2004). Thus, a major adaptive function of enlarged brains may be to reduce the risk of death when facing environmental challenges, a hypothesis that is widely accepted but for which the empirical evidence is scanty (Shultz et al. 2005; Sol et al. 2005a,b).
Our findings also suggest that large-brained animals might be better prepared to cope with environmental challenges such as global warming and habitat destruction, a possibility that is supported by recent findings in birds that colonization of new regions (Sol et al. 2005a), year-round residence in seasonally changing environments (Sol 2003; Winkler et al. 2004; Sol et al. 2005b) and long-term population trends (Shultz et al. 2005) are all positively associated with the large brains. Finally, our results lend additional support to the ‘behavioural drive’ model of evolution (Wyles et al. 1983; Price et al. 2003; Sol et al. 2005c), suggesting that an enlarged brain could help promote evolutionary divergence by facilitating survival in new adaptive zones.
Acknowledgments
We thank Eric Charnov, Juan Moreno, Gray Stirling, Neeltje Boogert and Bruce Hungate for their comments and discussion. This work was supported by a ‘Ramón y Cajal’ fellowship and a ‘Proyecto de Investigación’ (ref. CGL2005-07640/BOS) from the Ministerio de Educación y Ciencia (Spain) to D.S., a grant from the Natural Sciences and Engineering Research Council of Canada (Canada) to L.L. and a ‘Bolyai Janos’ fellowship by the Hungarian Academy of Sciences to A.L.
Supplementary Material
References
- Allman J. Scientific American Library; New York, NY: 2000. Evolving brains. [Google Scholar]
- Allman J.M, McLaughlin T, Hakeem A. Brain-weight and life-span in primate species. Proc. Natl Acad. Sci. USA. 1993;90:118–122. doi: 10.1073/pnas.90.1.118. doi:10.1073/pnas.90.1.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P.M, Harvey P.H. Relative brain size and ecology in birds. J. Zool. Lond. 1985;207:151–169. [Google Scholar]
- Bennett P.M, Owens I.P.F. Oxford University Press; Oxford, UK: 2002. Evolutionary ecology of birds: life histories, mating systems and extinction. [Google Scholar]
- Blackburn T.M, Duncan R.P. Determinants of establishment success in introduced birds. Nature. 2001;414:195–197. doi: 10.1038/35102557. doi:10.1038/35102557 [DOI] [PubMed] [Google Scholar]
- Byrne R.W, Corp N. Neocortex size predicts deception rate in primates. Proc. R. Soc. B. 2004;271:1693–1699. doi: 10.1098/rspb.2004.2780. doi:10.1098/rspb.2004.2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov E.L.R. Evolution of life history variation among female mammals. Proc. Natl Acad. Sci. USA. 1991;88:1134–1137. doi: 10.1073/pnas.88.4.1134. doi:10.1073/pnas.88.4.1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaner R.O, Barton R.A, van Schaik C.P. Primate brains and life histories: renewing the connection. In: Kappeler P.M, Pereira M.E, editors. Primates life histories and socioecology. The University of Chicago Press; Chicago, IL: 2002. pp. 233–265. [Google Scholar]
- Deaner R.O, Nunn C.L, van Schaik C.P. Comparative tests of primate cognition: different scaling methods produce different results. Brain Behav. Evol. 2000;55:44–52. doi: 10.1159/000006641. doi:10.1159/000006641 [DOI] [PubMed] [Google Scholar]
- Dukas R. Evolutionary ecology of learning. In: Dukas R, editor. Cognitive ecology: the evolutionary ecology of information processing and decision making. University of Chicago Press; Chicago, IL: 1998. pp. 129–174. [Google Scholar]
- Dunbar R.I.M. Neocortex size as a constraint on group size in primates. J. Hum. Evol. 1992;20:469–493. doi:10.1016/0047-2484(92)90081-J [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985;125:1–15. doi: 10.1086/703055. doi:10.1086/284325 [DOI] [PubMed] [Google Scholar]
- Garamszegi L.Z, Eens M, Erritzøe J, Møller A.P. Sperm competition and sexually size dimorphic brains in birds. Proc. R. Soc. B. 2005;272:159–166. doi: 10.1098/rspb.2004.2940. doi:10.1098/rspb.2004.2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garamszegi L.Z, Møller A.P, Erritzoe J. Coevolving avian eye size and brain size in relation to prey capture and nocturnality. Proc. R. Soc. B. 2002;269:961–967. doi: 10.1098/rspb.2002.1967. doi:10.1098/rspb.2002.1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T, Harvey P.H, Ives A.R. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 1992;41:18–32. doi:10.2307/2992503 [Google Scholar]
- Gittleman J.L. Female brain size and parental care in carnivores. Proc. Natl Acad. Sci. USA. 1994;91:5495–5497. doi: 10.1073/pnas.91.12.5495. doi:10.1073/pnas.91.12.5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittleman J.L, Anderson C.G, Kot M, Luh H.-K. Comparative tests of evolutionary lability and rates using molecular phylogenies. In: Harvey P.H, Brown A.J.L, Maynard Smith J, Nee S, editors. New uses for a new phylogenies. Oxford University Press; Oxford, UK: 1996. pp. 289–307. [Google Scholar]
- Harvey P.H, Pagel M.D. The comparative method in evolutionary biology. Oxford University Press; Oxford: 1991. [Google Scholar]
- Iwaniuk A.N, Nelson J.E. Can endocranial volume be used as an estimate of brain size in birds? Can. J. Zool. 2002;80:16–23. doi:10.1139/z01-204 [Google Scholar]
- Iwaniuk A.N, Nelson D.A. Developmental differences are correlated with relative brain size in birds: a comparative analysis. Can. J. Zool. 2003;81:1913–1928. doi:10.1139/z03-190 [Google Scholar]
- Jerison H.J. Academic Press; New York, NY: 1973. Evolution of the brain and intelligence. [Google Scholar]
- Lefebvre L, Reader S.M, Sol D. Brains, innovations and evolution in birds and primates. Brain Behav. Evol. 2004;63:233–246. doi: 10.1159/000076784. doi:10.1159/000076784 [DOI] [PubMed] [Google Scholar]
- Lefebvre L, Whittle P, Lascaris E, Finkelstein A. Feeding innovations and forebrain size in birds. Anim. Behav. 1997;53:549–560. doi:10.1006/anbe.1996.0330 [Google Scholar]
- Lessells C.M, Boag P.T. Unrepeatable repeatabilities: a common mistake. Auk. 1987;104:116–121. [Google Scholar]
- Li C.C. Boxwood Press; Pacific Grove, CA: 1975. Path analysis—a primer. [Google Scholar]
- Liker A, Székely T. Mortality costs of sexual selection and parental care in natural populations of birds. Evolution. 2005;59:890–897. doi:10.1554/04-560 [PubMed] [Google Scholar]
- Madden J. Sex, bowers and brains. Proc. R. Soc. B. 2001;268:833–838. doi: 10.1098/rspb.2000.1425. doi:10.1098/rspb.2000.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T.E. A new view of avian life-history evolution tested on an incubation paradox. Proc. R. Soc. B. 2002;269:309–316. doi: 10.1098/rspb.2001.1879. doi:10.1098/rspb.2001.1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealen P.M, Ricklefs R.E. Early diversification of the avian brain: body relationship. J. Zool. Lond. 2001;253:391–404. doi:10.1017/S095283690100036x [Google Scholar]
- Owens I.P.F, Bennett P.M. Mortality costs of parental care and sexual dimorphism in birds. Proc. R. Soc. B. 1994;257:1–8. [Google Scholar]
- Pagel M.D, Harvey P.H. The taxon-level problem in the evolution of mammalian brain size: facts and artifacts. Am. Nat. 1988;132:344–359. doi:10.1086/284857 [Google Scholar]
- Price T.D, Qvarnstrom A, Irwin D.E. The role of phenotypic plasticity in driving evolution. Proc. R. Soc. B. 2003;270:1433–1440. doi: 10.1098/rspb.2003.2372. doi:10.1098/rspb.2003.2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis A, Rambaut A. Comparative analysis by independent contrasts (CAIC)–an Apple-Macintosh application for analyzing comparative data. Comp. Applic. Biosci. 1995;11:247–251. doi: 10.1093/bioinformatics/11.3.247. [DOI] [PubMed] [Google Scholar]
- Reader S.M. Don't call me clever. New Sci. 2004;183:34. [Google Scholar]
- Reader S.M, Laland K.N. Social intelligence, innovation, and enhanced brain size in primates. Proc. Natl Acad. Sci. USA. 2002;99:4436–4441. doi: 10.1073/pnas.062041299. doi:10.1073/pnas.062041299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saether B.-E. Pattern of covariation between life-history traits of European birds. Nature. 1988;331:616–617. doi: 10.1038/331616a0. doi:10.1038/331616a0 [DOI] [PubMed] [Google Scholar]
- Sax D.F, Brown J.H. The paradox of invasion. Global Ecol. Biogeogr. 2000;9:363–371. doi:10.1046/j.1365-2699.2000.00217.x [Google Scholar]
- Shultz S, Bradbury R, Evans K, Gregory R.D, Blackburn T.M. Brain size and resource specialisation predict long-term population trends in British birds. Proc. R. Soc. B. 2005;272:2305–2311. doi: 10.1098/rspb.2005.3250. doi:10.1098/rspb.2005.3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C.G, Ahlquist J.E. Yale University Press; New Haven, CT: 1990. Phylogeny and classification of birds: a study in molecular evolution. [Google Scholar]
- Sibley C.G, Monroe B. Yale University Press; New Haven, CT: 1990. Distribution and taxonomy of birds of the world. [Google Scholar]
- Sol D. Behavioural flexibility: a neglected issue in the ecological and evolutionary literature? In: Reader S.M, Laland K.N, editors. Animal innovation. Oxford University Press; Oxford, UK: 2003. pp. 63–82. [Google Scholar]
- Sol D, Duncan R.P, Blackburn T.M, Cassey P, Lefebvre L. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA. 2005a;102:5460–5465. doi: 10.1073/pnas.0408145102. doi:10.1073/pnas.0408145102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol D, Lefebvre L, Rodriguez-Teijeiro J.D. Brain size, innovative propensity and migratory behaviour in temperate Palearctic birds. Proc. R. Soc. B. 2005b;272:1471–2954. doi: 10.1098/rspb.2005.3099. doi:10.1098/rspb.2005.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol D, Stirling D.G, Lefebvre L. Behavioral drive or behavioral inhibition in evolution: subspecific diversification in Holarctic passerines. Evolution. 2005c;59:2669–2677. doi:10.1554/05-196.1 [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Winkler H, Leisler B, Bernroider G. Ecological constraints on the evolution of avian brains. J. Ornithol. 2004;145:238–244. doi:10.1007/s10336-004-0040-y [Google Scholar]
- Wyles J.S, Kunkel J.G, Wilson A.C. Birds, behavior and anatomical evolution. Proc. Natl Acad. Sci. USA. 1983;80:4394–4397. doi: 10.1073/pnas.80.14.4394. doi:10.1073/pnas.80.14.4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.