Introduction
In mammalian myocardium the force of contraction increases with stimulation frequency. This important regulatory mechanism was first described in the year 1871; Henry Bowditch noted that the contractile force of the frog heart increased when it was paced at increasing frequency [1]. Since the first description of this force frequency relationship (FFR), the underlying mechanism has been a target for experimental investigation[2–7]. Although several contributing factors and modifiers of the FFR have been identified, our current understanding of frequency dependent activation is far from complete. The cardiac beat is a very dynamic process, and the various factors that activate and de-activate the myocardium are in constant flux. Transmembrane transport of various ions, intracellular release and re-uptake of calcium ions, calcium binding to troponin, extent of overlap between the thick and thin myofilaments, and the attachment and detachment of cross-bridges are processes that never reach equilibrium between the first and last beat of a heart. As a result the investigation of frequency-dependent activation is hampered by the complex dynamic nature of the system, whereby isolation of a certain process likely puts it out of context, and its relevance to the overall frequency-dependent activation cannot be unambiguously determined. Still, despite the fact that the precise underlying mechanism remains unclear, much progress has been made in the last few decades as new tools have emerged to probe cardiac function. In addition to its physiological importance, clinically there is much interest in frequency-dependent activation. A loss, or severe blunting, of the FFR is a hallmark of the end-stage failing myocardium. Most often, regardless of the underlying etiology that ultimately caused end-stage failure, the FFR is severely blunted, flat, or even negative in failing myocardium. Investigation of this pathological phenomenon has led to a further understanding of the mechanisms underlying frequency dependent activation, but significant gaps in our understanding of this important regulatory mechanism exist.
Force frequency response in small and large mammals
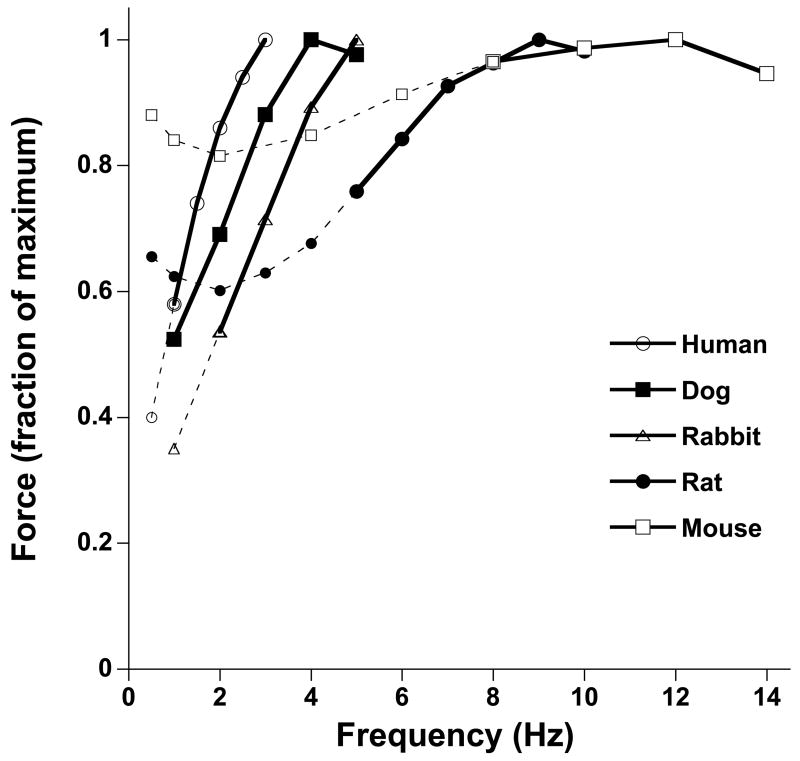
Species differences are prominent in frequency-dependent contractile strength. Across species, this regulatory mechanism is different mainly in its quantitative aspects. First, the range of frequencies used in vivo needs to be taken into consideration when examining frequency-dependent behavior. In adult humans, the resting heart rate is near 1 Hz (60 beats per minute), while at peak exercise this roughly rises to 3 Hz (180 beats per minute), reflecting an increase of 200% from the resting rate. In rabbits, the resting heart rate is near 2.5 Hz, and is elevated to about 5 Hz during exercise, reflecting an increase of only 100%. In mice this increase in heart rate is even more reduced, and amounts to roughly only 40% (about 10 to 14 Hz). Thus, the range of acceleration of the heartbeat is prominent in large mammals, and considerably less in small mammals. Second, the gain in contractile strength is greater in larger mammals as well. In human myocardium, within the in vivo frequency range, force development of cardiac contractile tissue easily can be doubled, if not tripled. In rabbits, this response is not as pronounced, but still a significant increase is observed in contractile strength in the in vivo frequency range. In rats, the FFR is still positive, but much more blunted compared to human or rabbit, and in mice this relationship becomes severely blunted, often to such extent that it is flat or even slightly negative over the in vivo frequency range. In Figure 1, the typical response is given over a range of frequencies that encompasses the in vivo range for that species. These were assessed under near identical experimental conditions at 37 °C, in isometric contractions, so pre- and after-load were not a variable. As can be seen in Figure 1, within the physiological range highlighted for each species, the force increases with stimulation frequency, and the increase is most pronounced in larger species.
Figure 1.
Comparison of the force frequency relationships in various species. All data are representative averages obtained in isometric contractions of small and thin ventricular trabeculae at 37 °C under near identical conditions (n=4–12/species). Within their respective in vivo frequency range (solid lines), the force frequency relationship is positive in all species, but the enhancement of force upon increase in frequency is much more pronounced in larger mammals compared to small rodents. Outside the in vivo frequency range, a flat or negative relationship can be obtained depending on the frequency range studied. In human, dog, and rabbit, the relative increases in force within the in vivo range are similar (nearly 100%) so contractile force generally doubles between resting and exercise heart rate, whereas in the rat the increase is only about 50%, and in the mouse only about 10%.
Considering the range of in vivo frequencies used, and the magnitude of the response, it is clear that the FFR is of much greater importance to cardiac output regulation in larger mammals than it is in the rat and mouse. Cardiac output is the product of stoke volume and heart rate, and in humans cardiac output therefore can change by 6–10 fold from rest to maximum exercise, whereas in the mouse this cardiac output will at most double. In humans, the increase in contractile strength with increasing frequency significantly contributes to the increase in cardiac output, whereas in the mouse this increase in cardiac output is almost exclusively due to an increase in the heart rate.
Role of experimental conditions on the force frequency response
Assessment of the FFR has been done under a wide variety of experimental conditions, that can drastically impact on the resulting data, in a qualitative and quantitative manner. A prime example is that rats and mice are reported to have a negative force-frequency response, but this however has recently been shown to be true mainly, or only, for non-physiological conditions. Under near physiological conditions, and within their respective in vivo heart rate range, the FFR in small rodents is slightly positive or nearly flat, but not negative[2, 8, 9]. There are two main experimental reasons that have led to the still widely held misconception that the FFR is negative in rats and mice.
First, in the past, many studies employing isolated cardiac muscle preparations have been conducted at room temperature. At room temperature, contractions are much slower, and thus the physiological frequency range cannot generally be attained. At increasing stimulus frequency at room temperature, diastolic fusion (i.e. incomplete relaxation) of the individual contractions occurs at a much lower frequency than it would at body temperature. As a result, at low temperature diastolic fusion of the individual contractions leads to a reduced developed force, at frequencies well below the in vivo range. With diastolic fusion of twitch contractions, any further increase in stimulation frequency will result in a decrease in developed force (systolic minus diastolic force), rather than the expected increase seen at body temperature. Thus, as temperature increases, the frequency at which maximum active tension is developed shifts to the right (higher frequency) on the spectrum. Whereas the active force development in rat myocardium is maximal at around 2 Hz at room temperature, it will shift to 4 or 6 Hz at 30 °C, to 8 Hz at body temperature, and even above that under hyperthermic conditions[10], that can occur in vivo during a fever. When at non-physiological temperature one measures force at various frequencies, the results do not necessarily reflect the FFR, or processes underlying physiological frequency-dependent activation as they occur in vivo. Even at body temperature, examining the FFR in a frequency spectrum that falls outside the in vivo range often provides data that do not necessarily reflect frequency-dependent processes as they occur in vivo. In the rat and mouse, in the range of 0.1 to 2 Hz, often a negative relationship between force and stimulation rate is observed (see Figure 1). If for example force decreases from 0.1 to 2 Hz, this is indicative of negative inotropy, whereas under otherwise identical conditions (pH, temperature, sarcomere length) in the same experiment the developed force would increase in the range of physiological heart rates. Thus, the conclusions drawn regarding frequency-dependent regulation from such experiments may be entirely opposite than under in vivo conditions. Hence, extreme caution is needed in interpreting frequency-dependent processes if the frequencies examined fall outside the in vivo range.
The second factor that led to reported negative FFRs’ in rats and mice is that rapid stimulation to mimic the very high frequency ranges as they occur in vivo can easily limit oxygen delivery and/or removal of waste products from the myocardium. At room temperature, at low frequency, Schouten and ter Keurs[11] argued that muscle with diameters in excess of 250 μm unavoidably suffer from a hypoxic core. A more recent study directly correlated muscle diameter with force development in rat trabeculae stimulated at in vivo rates at 37 °C, and observed a sharp drop-off in developed force in muscles exceeding 150 μm[12]. Because both an increase in temperature and an increase in frequency reduce this critical diameter, in the mouse, due to the even higher prevailing heart rates, core hypoxia may be likely hamper force development in the upper heart rate range in isolated muscles exceeding 100 μm in thickness. Given that experiments are often done with adult murine papillary muscles that are typically well exceeding 250 μm, and up to 600 μm, it is likely that the unavoidable experimentally-induced hypoxic core, rather than true myocardial properties, limit force development at these high (but physiological) stimulation rates. Hence, conclusions drawn from experiments with isolated muscles above this critical diameter must be interpreted with caution. Even when two groups of experiments are compared under identical conditions, differences in muscle function may be more indicative of differences in ability of the various groups to handle core-hypoxia rather than an indication that basic contractile properties of the groups are different.
In summary, the FFR is positive in all mammals, and furthermore there is a positive correlation between the contribution of the FFR to cardiac output regulation and the size of the species.
Molecular mechanisms
Despite well over a century of research into the underlying mechanism of frequency-dependent activation, it is still incompletely understood which molecular processes are responsible for regulation of cardiac force with an increase in heart rate. Nonetheless, over the past decades, novel tools, including the use of transgenic animals, have elucidated the FFR. It is generally accepted that changes in calcium handling play a crucial role in the FFR. It has been shown by many independent laboratories that when frequency increases, the amplitude of the calcium transient increases, as does the sarcoplasmic reticulum (SR) calcium load[6]. With an increase in the number of beats per given time period, there is more calcium influx through the L-type calcium channel during that given time period. This increase in calcium entry causes the SR Ca2+ ATPase (SERCA) to transport more calcium back into the SR, and thus at subsequent beats, more calcium can be released from the SR. Clearly, trans-membrane calcium extrusion is also enhanced at higher frequency[6]. When a switch from a given frequency to a higher one occurs, initially more calcium enters the cell than leaves the cell, until a new steady-state has developed. In this new steady-state, the total integrated L-type calcium channel calcium influx equals the integrated trans-membrane calcium extrusion, which occurs mainly via the sodium-calcium exchanger (NCX), and to a minor extent via the plasma membrane bound calcium pump. At this new steady-state, SR calcium load is increased, and as a result the calcium transient amplitude is increased.
An intervention in any of the processes that led to the new steady-state could impact the level of SR calcium load at the new steady-state. As a result, alterations in the SR Ca2+-ATPase function, alterations in phospholamban level and/or phosphorylation, as well as changes in activity or expression of other calcium handling proteins, such as NCX, can impact on the force-frequency relationship by affecting the new steady-state that develops after a change in stimulation rate. Since actual expression levels of proteins are not changing upon a change in frequency, various post-translational modifications could play a role in the modification of the SR calcium load and calcium transient.
Role of phospholamban in force frequency response
Several investigations have focused on the role of phospholamban (PLB) in modulation of frequency-dependent contractility. Phospholamban, in its unphosphorylated state, is an inhibitor of the SR Ca2+-ATPase. Phospholamban has 2 phosphorylation sites, a serine located at amino acid 16 (Ser16), and a threonine at adjacent amino acid 17 (Thr17). The Ser16 site is a prominent target of PKA, whereas the Thr17 site is a target for CAMKII. When phospholamban is phosphorylated, its inhibitory effect on the SR Ca2+-ATPase is relieved, enhancing activation of the SR Ca2+-ATPase[13]. Thus, it is conceivably that phosphorylation of phospholamban that is known to aid in increasing the SR calcium load with β-adrenergic stimulation, plays a similar role in the regulation of calcium handling with changes in frequency[14, 15]. However, some studies have refuted this hypothesis, and did not find a role for phospholamban phosphorylation in frequency dependent activation[16, 17]. An increase in cardiac contractility in vivo is primarily dependent upon adrenergic stimulation and PLB phosphorylation, which suggests that PLB phosphorylation is the primary mechanism for increased Ca2+ transport. Although phosphorylation of the SR Ca2+-ATPase itself could perceivably play a role in regulation of frequency dependent activation, to date there is no firm evidence that SERCA pump can be phosphorylated by CaMKII or by any other kinase.
The role of PLB in cardiac contractility and force frequency response has been investigated in PLB-KO mouse hearts[18, 19]. Isolated left ventricular papillary muscles were stimulated between 2 and 6 Hz, an increase in stimulation frequency resulted in an acceleration of both time to peak tension and half-relaxation time in WT muscles. However, this acceleration was greatly diminished in PLB-KO hearts indicating that the frequency-mediated response is largely facilitated by SERCA/PLB interaction. The frequency response of cardiac contractility was also determined by dF/dtmax and dF/dtmin in the same preparations. As expected the WT muscles showed an increase in dF/dtmax and dF/dtmin with increasing frequency of stimulation. On the other hand, both dF/dtmax and dF/dtmin decreased as frequency increased in PLB-KO muscles. These studies concluded that the positive force frequency response in WT muscles is largely due to PLB, where as muscles without PLB lack the ability to potentiate force frequency response. Of note however is that the reported forces for these muscles were very low, most likely due to size limitations as discussed earlier, and were only investigated at stimulation rates below the in vivo range. Thus, although a role of phospholamban can likely be extrapolated from these studies to the in vivo situation, the quantitative impact cannot unambiguously be derived.
A comparison of PLB-KO mice and PLB-overexpressing mice with their isogenic wild-types [20] revealed that the critical heart rate (defined as the heart rate where the maximum of the first derivative of pressure was the highest), shifted to higher rates in the PLB-KO mice, and to lower ones in the PLB-overexpressing mice. A somewhat confounding factor is that these critical heart rates (340–440 beats per minute) fall below the normal resting heart rate of the mouse, but this was discussed to be likely due to the effects on anesthesia. Still, the large differences in the critical heart rate suggest that PLB plays an important role in frequency-dependent activation. Meyer and coworkers[4] used adenoviral gene transfer of PLB and SERCA2a into rabbit cardiac myocytes to test the idea that more PLB leads to more frequency potentiation. Overexpression of PLB prolonged relaxation and reduced baseline contractility[4]. Conversely, the frequency-dependent shortening response was significantly increased in PLB-OE myocytes compared to control myocytes. These studies argued that PLB is a critical determinant of frequency-dependent activation. The lack of frequency potentiation in PLB-KO hearts could also be due to the fact that these muscles are contracting at their maximal rate and an increase in frequency cannot further accelerate the contractile parameters. The increased force response is primarily due to an increase in SR Ca2+ load and release that is enhanced during frequency potentiation. However, if the SR is already functioning at its maximal store capacity, an increase in frequency is not sufficient to further increase this load/release process. This interpretation is supported by the findings that a large increase in SERCA pump activity, achieved by SERCA overexpression similarly can show a negative or blunted force frequency response[4, 21, 22]. Thus, such muscles are already contracting at maximal rate and may not be responsive to increased frequency. It is important to point out that the isoproterenol response is blunted in PLB-KO and SERCA overexpression mice, which also supports the idea that hearts functioning at maximal SR Ca2+ load/transport will not respond to the force frequency response.
Role of sarcoplasmic reticulum Ca2+ ATP-ase in force frequency response
The expression of SERCA and PLB, and ratio of PLB to SERCA, are subject to regulation throughout the life of an animal, and under a variety of pathophysiological conditions. Most notably the expression level of PLB and SERCA2a are highly sensitive to thyroid hormone status. PLB is decreased in hyperthyroid animals whereas it is increased in hypothyroidism, while SERCA2a pump shows an opposite regulation[23]. As a result, the Ca2+ transport and contractile kinetics are significantly faster in hyperthyroidic hearts compared to hypothyroidic animals. The levels of PLB and SERCA are altered in a variety of disease conditions including diabetic cardiomyopathy and heart failure[24–27]. A decrease in SERCA protein and PLB phosphorylation has been correlated with a negative force frequency relationship in human hearts[24]. The role of the SERCA pump has been studied by several laboratories both in genetically manipulated animal models[28–30] or gene transfer either in vivo or in cultured myocytes[21, 31–33], as well as in the context of human heart failure[34]. In general, a decrease in SERCA pump levels is correlated with a negative FFR. We have investigated how decreases in SERCA pump level could affect the force frequency relationship using SERCA2 (+/−) mice[30], in which a single allele encoding for SERCA2a is mutated. SERCA2 (+/−) mice have decreased (~35%) SERCA2a pump levels, but do not exhibit any significant cardiac pathology. SERCA2 (+/−) mice show a moderate reduction in baseline cardiac contractility, but when challenged with isoproterenol respond with increased contractility similar to WT-control mice. The response of these hearts to increased stimulation frequency was studied using the isolated work performing heart[35]. Unlike in the wild-type mouse, the frequency response, assessed via dP/dtmax, in SERCA (+/−) hearts was flat and cardiac contractility decreased drastically at higher (~550 beats/min) frequencies. These studies pointed out that a reduction in SERCA pump numbers is likely responsible for the inability of the SR to sequester calcium at higher stimulation rates. As stated above, an increase in SERCA activity well beyond the normal spectrum may also result in negative frequency-dependent behavior, because already at low frequency the baseline SR load is higher, and thus there is less room for further enhancement. In addition, if re-uptake of calcium becomes too fast, the cytoplasmic calcium concentration near the myofilaments will decline very rapidly, preventing appropriate activation of the myofilaments, hindering adequate force development[4, 21, 22]. Combined, these studies indicate that SERCA activity has an optimal range, rather than a direct linear correlation to contractile performance. The above studies have delineated that SERCA pump level is an important player in the FFR.
Frequency dependent acceleration of relaxation
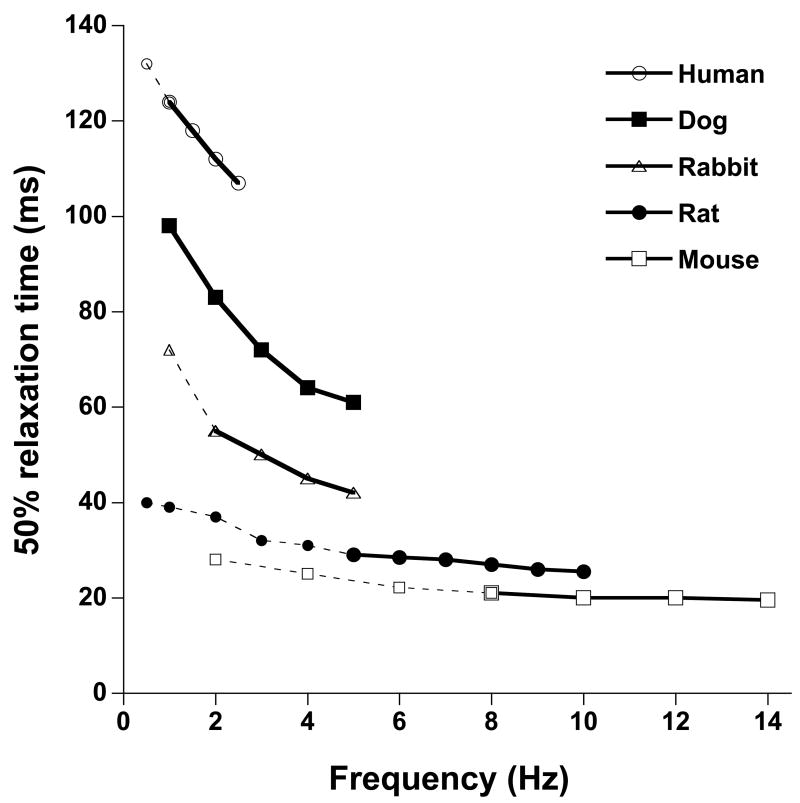
Not only does the heart generally beat stronger when it is stimulated to contract faster, the kinetics of contraction and relaxation are also accelerated. The physiological need for this is obvious, if the heart does not relax in timely fashion before the next beat is initiated, filling, and thus ultimately cardiac output, will be severely hampered. In addition, it is in diastole that coronary flow is maximal, and a decrease in the duration that the heart spends in diastole will impair proper perfusion of the heart muscle itself. Frequency dependent acceleration of relaxation (FDAR) occurs across all species, and unlike maximal contractile force, exhibits a monophasic behavior. Regardless of species and initial frequency, under otherwise identical conditions the relaxation of the myocardium is always faster when heart rate increases (Fig 2). FDAR occurs at the level of the single cell, and is therefore an intrinsic property of the myocardium rather than a sole effect of chamber geometry. However, since cardiac relaxation is significantly influenced by the prevailing loading conditions, secondary effects of load and/or chamber geometry may play a modulating role in FDAR.
Figure 2.
Comparison of frequency dependent acceleration of relaxation in various species. All data are representative averages obtained in isometric contraction/relaxations of small and thin ventricular trabeculae at 37 °C under near identical conditions (n=4–12/species). Over the entire frequency range, encompassing the in vivo frequency range (solid lines), relaxation (indicated by time form peak tension to 50% relaxation) was accelerated when frequency increased. Both in absolute times as well as relative to the lowest rate, within the in vivo range of the species, FDAR is most pronounced in the larger mammals, but can clearly be observed even in small rodents.
Logically, the majority of studies investigating FDAR have focused on similar pathways that are responsible for the FFR. Thus, changes in SR calcium handling have been the focal point of numerous investigations. Indeed, it has been clearly shown that the kinetics of the calcium decline are accelerated as frequency increases, and it has been hypothesized that this faster reduction in the intracellular calcium transient is responsible for FDAR. The molecular mechanism underlying FDAR has been the focus of many studies in the past decade. CaMKII is an attractive candidate to be mainly responsible for modulation of FDAR because it is activated by elevated calcium and can increase SERCA pump activity by phosphorylating PLB. Studies from Bassani et al.[36] and De Santiago and coworkers[17] indicated that FDAR is dependent on CaMKII activation, and studies in isolated myocytes suggested that phosphorylation of the Thr17 residue of PLN is responsible for FDAR[15]. However there are other studies that failed to show either an FDAR dependence on CaMKII activation[2, 37, 38] or a significant increase in PLB phosphorylation[16, 37]. The role of CaMKII in FDAR was investigated using isolated trabeculae and/or myocytes from PLB-KO mouse and rat hearts[17, 39]. These studies relied on CaMKII inhibitors (1 μm KN-93 or 20 μm altocamtide-2 peptide AIP). In the presence of CaMKII inhibitors, FDAR was tested by increasing stimulation frequency from 0.2–8 Hz either at 23 or 35 °C. Interestingly, CaMKII inhibitors suppressed both FDAR, Ca2+ transients, and twitch force. Similarly, AIP, a peptide CaMKII inhibitor, inhibited FDAR and Ca2+ transients in rat ventricular myocytes. These studies, using PLB KO animals, indicated that FDAR was not dependent on PLB but rather depended on CaMKII activity. In a recent study[40] the role of CaMKII was tested using TG mice that express four concatenated repeats of the CaMKII inhibitory peptide AIP (Auto Inhibitor Peptide). These mice show a moderate reduction in cardiac function and a decreased response to β-adrenergic stimulation. Furthermore, targeting of the AIP to the cardiac SR inhibited both basal and β-adrenergic stimulated PLB phosphorylation at Thr17 but not Ser16. Increasing stimulation frequency produced typical FDAR in wild-type myocytes, whereas it was significantly reduced in TG mice expressing AIP. These studies further showed that FDAR was dependent on increased SR Ca2+ uptake (Vmax), and this was, at least in part, affected by CaMKII inhibition. However, the exact mechanism by which CaMKII modulates frequency dependent effects in these hearts is not clear. The relevance of these findings is difficult to interpret because chronic expression of AIP in the TG mouse model could exert its inhibitory effect at multiple levels including L-type Ca2+channel activity and chronic inhibition of CaMKII signaling in the heart may trigger a new functional state of the myocardium.
The role of PLB phosphorylation in FDAR has been studied by several groups. Valverde and coworkers[16] studied the role of PLB phosphorylation at Ser16 and Thr17 in FDAR and compared their data with those produced by isoprenaline. Isoprenaline (or isoproterenol) is known to accelerate relaxation (IDAR) via PLB phosphorylation. They showed that IDAR is associated with a significant increase in the phosphorylation of Ser16 and Thr17 of PLB, whereas FDAR occurs without significant changes in the phosphorylation of PLB residues in the intact heart and cat papillary muscles. In addition they report that FDAR was not associated with any significant change in the CaMKII-dependent phosphorylation of SERCA2a, and was not affected by the presence of the CaMKII inhibitor, KN-93. These studies argue that CaMKII phosphorylation pathways are not involved in FDAR and that FDAR and IDAR do not share a common underlying mechanism. Instead they propose a CaMKII-independent mechanism, whereby increasing stimulation frequency would disrupt the SERCA2a–PLB interaction, leading to an increase in SR Ca2+ uptake and myocardial relaxation. This idea is supported by the findings that elevated Ca2+ may disrupt physical interaction between PLB and SERCA2a[41] and promote dissociation of PLB–SERCA2a heterodimer dissociation, thereby increasing SERCA2a activity.
A recent study[42] examined the role of CaMKII in phosphorylation of the SR Ca2+-handling proteins phospholamban and the ryanodine receptor channel in fluo-4 loaded rat ventricular myocytes. They showed that FDAR occurs abruptly when frequency is raised from 0.1 to 2 Hz and that under these conditions the effect is essentially complete within a few beats. In addition, they documented a small increase in PLB Thr17 and RyR Ser2814 phosphorylation, (PLB<5%, RyR approximately 8%), but the time-course was much slower and did not coincide with FDAR. Essentially, this study shows that although CAMKII can phosphorylate several targets within the myocyte as a result of the increased frequency, these effects occur secondary to the actual acceleration of relaxation, and are thus not primarily responsible for FDAR. These studies conclude that FDAR does not rely on phosphorylation of PLB or RyR; these studies actually rule out the possibility that the CaMKII effect on PLB or RyR is the primary player in the FDAR.
Role of myofilaments in FDAR
Thus, evidence is mounting against a primary role of CAMKII in FDAR, at least via effect on PLB or RyR, which brings up the question, what is the primary mechanism? Part of the answer may be found in the studies that actually argued in favor of a role of CAMKII. The bulk of these studies were done in isolated myocytes, often in combination with experimental conditions far removed from the in vivo situation, i.e. low temperature, or at frequencies well below the in vivo spectrum of the species studied. Similar to the role of species in frequency-dependent modulation of force, FDAR is more prominent in larger species. Moreover, FDAR may critically depend on the loading condition of the myocardium. Isolated myocytes in the context of FDAR have exclusively been studied unloaded, and thus at sarcomere lengths ranging between 1.9 and 1.6 μm, which fall well outside the physiological range of about 2.0–2.3 μm[43]. At these short sarcomere lengths, in absence of pre- and after-load, the rate-limiting process that governs relaxation may be significantly different than in the loaded in vivo situation. As a result, the re-lengthening of the cardiac myocyte under unloaded conditions may be critically governed by calcium removal from the cytosol. Under these conditions, the myofilament matrix is very insensitive to calcium[44], and in striated muscle, including cardiac muscle, unloaded cross-bridge cycle much faster than loaded ones[45, 46]. As a result of these factors, the calcium decline may critically and predominantly modulate relaxation in unloaded myocytes, and thus manipulation of calcium decline will directly affect relaxation kinetics when load is absent. Indeed, in the unloaded isolated myocyte at sub-physiological pacing frequencies, inhibition of CAMKII targeted to the SR blunts FDAR[17, 40]. However, relaxation of the myocardium under loaded conditions as it occurs in vivo is likely governed in a significantly different manner. Several studies indicate that the myofilament properties play a prominent role in governing the rate of myocardial relaxation[9, 47]. Moreover, recent work by us[48], and others[38] argues in favor of a significant involvement of the myofilaments in FDAR. In isolated rabbit trabeculae, contracting isometrically at body temperature within the in vivo range of the rabbit, myofilament calcium sensitivity greatly decreased when stimulation frequency was increased[48]. This frequency dependent myofilament desensitization correlated with TnI phosphorylation, which is known to de-sensitize the myofilaments for activator Ca2+ in skinned fibers[13] as well as in intact myocardium[49]. These results draw a significant parallel to IDAR. It is well known that isoprenaline (or isoproterenol) phopshorylates TnI, and that this TnI phosphorylation desensitizes the myofilaments for calcium[13]. In IDAR, this TnI phosphorylation is thought to be responsible, at least for a significant part, for the faster relaxation. A likely role for TnI in frequency-dependent processes is supported by the observations of Takimoto et al. [7] and Bilchick et al. [50]. These studies observed an effect of TnI phosphorylation via PKA as well as PKC that affected FFR and FDAR in intact murine hearts. Thus, FDAR may also depend significantly, and perhaps predominantly, on a desensitization and/or other properties of the myofilament matrix. Although this explanation may elucidate the physiological mechanism of accelerated relaxation, the underlying molecular steps that encompass this process are currently unknown.
Post-rest potentiation and extra-systolic behavior
Frequency-dependent processes are mainly investigated with respect to steady-state behavior. However, many of the processes discussed above are involved in beat-to-beat regulation of contractility. For instance, post-rest potentiation (PRP) is critically influenced by SR calcium load and function, which in turn is critically impacted upon by the prevailing stimulation frequency. When, from a given steady contractile state, the interval between successive beats is prolonged, the first twitch after reinstatement of stimulation is generally larger in amplitude, and the increase in twitch force compared to the pre-resting state is dependent on the duration of the rest [51, 52]. Because the initial force is dependent on the initial frequency, the potential magnitude of the post-rest potentiation is greatly influenced by the initial frequency via two mechanisms. First, if the initial frequency is low, the resulting steady state force is low, and the potential for gain is large. Second, and less well understood, is that the processes underlying frequency dependent behavior are geared to provide an increase in contractility, and thus may impact on the temporal resolution via which PRP is achieved. Further evidence in that PRP and FFR are closely linked is found in the studies on failing myocardium, where both PRP [53] and FFR [3, 54] seem to be blunted and hallmarks of the contractile phenotype of failing myocardium.
In addition, extra-systolic contractions are also likely highly influenced by the mechanisms governing frequency dependent activation. The amplitude of extra systolic beats critically depends on the time that has passed between the extra systolic beat and the previous regular beat[55]. At different baseline frequencies, the amplitude and duration of extra-systolic beats are likely to be dependent on the prevailing SR calcium load[56], which is critically influenced by baseline frequency.
Conclusions
The force frequency response of cardiac muscle can be observed in all species, and it relates to an innate survival mechanism that allows the heart to increase its contractile function and cardiac output during fight or flight response. The current knowledge clearly documents that the ability of the SR to transport increasing amounts of Ca2+ and enhance its load is critical for the increased force frequency response. There is a considerable amount of data to suggest that both SERCA and PLB are critical determinants, since the interaction between these two molecules regulate the rate and amount of Ca2+ sequestered under all conditions. Further, this concept is well established by the observation that β-adrenergic mediated inotropy is mediated by PLB phosphorylation and an increase in SR Ca2+ handling. The accelerated relaxation that occurs with an increase in stimulation frequency remains less well understood, but recent studies suggest that not only the faster intracellular calcium decline, but also the myofilament responsiveness plays a critical role. Given the fact that the altered frequency-dependent response is a critical modulator of cardiac function, and this modulating function is severely impaired in various cardiomyopathies[57, 58], a better understanding of the mechanisms underlying frequency-dependent modulation of contractility and relaxation, including post-rest potentiation and extra-systolic beat behavior, may be paramount in development of treatment of these cardiomyopathies.
Acknowledgments
This work was supported by grants NIH R01 HL-64140 (MP), NIH R01-73816 (PMLJ), NIH K02-83957 (PMLJ), and American Heart Association Established Investigator Award 740040N (PMLJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bowditch HP. Ueber die Eigenthuemlichkeiten der Reizbarkeit, welche die Muskelfasern des Herzens zeigen. Ber Sachs Ges (Akad) Wiss. 1871;23:652–89. [Google Scholar]
- 2.Layland J, Kentish JC. Positive force- and [Ca2+]i-frequency relationships in rat ventricular trabeculae at physiological frequencies. Am J Physiol Heart Circ Physiol. 1999;276(1 Pt 2):H9–H18. doi: 10.1152/ajpheart.1999.276.1.H9. [DOI] [PubMed] [Google Scholar]
- 3.Gwathmey JK, Slawsky MT, Hajjar RJ, Briggs GM, Morgan JP. Role of intracellular calcium handling in force-interval relationships of human ventricular myocardium. J Clin Invest. 1990;85(5):1599–613. doi: 10.1172/JCI114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer M, Bluhm WF, He H, Post SR, Giordano FJ, Lew WY, et al. Phospholamban-to-SERCA2 ratio controls the force-frequency relationship. Am J Physiol. 1999;276(3 Pt 2):H779–85. doi: 10.1152/ajpheart.1999.276.3.H779. [DOI] [PubMed] [Google Scholar]
- 5.Pieske B, Kretschmann B, Meyer M, Holubarsch C, Weirich J, Posival H, et al. Alterations in intracellular calcium handling associated with the inverse force-frequency relation in human dilated cardiomyopathy. Circulation. 1995;92(5):1169–78. doi: 10.1161/01.cir.92.5.1169. [DOI] [PubMed] [Google Scholar]
- 6.Endoh M. Force-frequency relationship in intact mammalian ventricular myocardium: physiological and pathophysiological relevance. 2004;500(1–3):73–86. doi: 10.1016/j.ejphar.2004.07.013. 2004/10/01/ [DOI] [PubMed] [Google Scholar]
- 7.Takimoto E, Soergel DG, Janssen PM, Stull LB, Kass DA, Murphy AM. Frequency-and afterload-dependent cardiac modulation in vivo by troponin I with constitutively active protein kinase A phosphorylation sites. Circ Res. 2004;94(4):496–504. doi: 10.1161/01.RES.0000117307.57798.F5. Epub 2004 Jan 15. [DOI] [PubMed] [Google Scholar]
- 8.Stull LB, Leppo M, Marban E, Janssen PML. Physiological determinants of contractile force generation and calcium handling in mouse myocardium. J Mol Cell Cardiol. 2002;34(xx):1367–76. doi: 10.1006/jmcc.2002.2065. [DOI] [PubMed] [Google Scholar]
- 9.Janssen PML, Stull LB, Marban E. Myofilament properties comprise the rate-limiting step for cardiac relaxation at body temperature in the rat. Am J Physiol Heart Circ Physiol. 2002;282:H499–H507. doi: 10.1152/ajpheart.00595.2001. [DOI] [PubMed] [Google Scholar]
- 10.Hiranandani N, Varian KD, Monasky MM, Janssen PML. Frequency-dependent contractile response of isolated cardiac trabeculae under hypo-, normo-, and hyperthermic conditions. J Appl Physiol. 2006 May;100(5):1727–32. doi: 10.1152/japplphysiol.01244.2005. [DOI] [PubMed] [Google Scholar]
- 11.Schouten VJ, ter Keurs HE. The force-frequency relationship in rat myocardium. The influence of muscle dimensions. Pflugers Arch. 1986;407(1):14–7. doi: 10.1007/BF00580714. [DOI] [PubMed] [Google Scholar]
- 12.Raman S, Kelley MA, Janssen PM. Effect of muscle dimensions on trabecular contractile performance under physiological conditions. Pflugers Arch. 2006 Feb;451(5):625–30. doi: 10.1007/s00424-005-1500-9. [DOI] [PubMed] [Google Scholar]
- 13.Kranias EG, Solaro RJ. Phosphorylation of troponin I and phospholamban during catecholamine stimulation of rabbit heart. Nature. 1982;298(5870):182–4. doi: 10.1038/298182a0. [DOI] [PubMed] [Google Scholar]
- 14.Zhao W, Uehara Y, Chu G, Song Q, Qian J, Young K, et al. Threonine-17 phosphorylation of phospholamban: a key determinant of frequency-dependent increase of cardiac contractility. J Mol Cell Cardiol. 2004 Aug;37(2):607–12. doi: 10.1016/j.yjmcc.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Hagemann D, Kuschel M, Kuramochi T, Zhu W, Cheng H, Xiao RP. Frequency-encoding Thr17 phospholamban phosphorylation is independent of Ser16 phosphorylation in cardiac myocytes. J Biol Chem. 2000 Jul 21;275(29):22532–6. doi: 10.1074/jbc.C000253200. [DOI] [PubMed] [Google Scholar]
- 16.Valverde CA, Mundina-Weilenmann C, Said M, Ferrero P, Vittone L, Salas M, et al. Frequency-dependent acceleration of relaxation in mammalian heart: a property not relying on phospholamban and SERCA2a phosphorylation. J Physiol. 2005 Feb 1;562(Pt 3):801–13. doi: 10.1113/jphysiol.2004.075432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeSantiago J, Maier LS, Bers DM. Frequency-dependent acceleration of relaxation in the heart depends on CaMKII, but not phospholamban. J Mol Cell Cardiol. 2002 Aug;34(8):975–84. doi: 10.1006/jmcc.2002.2034. [DOI] [PubMed] [Google Scholar]
- 18.Brittsan AG, Kiss E, Edes I, Grupp IL, Grupp G, Kranias EG. The effect of isoproterenol on phospholamban-deficient mouse hearts with altered thyroid conditions. J Mol Cell Cardiol. 1999;31(9):1725–37. doi: 10.1006/jmcc.1999.1010. [DOI] [PubMed] [Google Scholar]
- 19.Bluhm WF, Kranias EG, Dillmann WH, Meyer M. Phospholamban: a major determinant of the cardiac force-frequency relationship. Am J Physiol Heart Circ Physiol. 2000 Jan;278(1):H249–55. doi: 10.1152/ajpheart.2000.278.1.H249. [DOI] [PubMed] [Google Scholar]
- 20.Kadambi VJ, Ball N, Kranias EG, Walsh RA, Hoit BD. Modulation of force-frequency relation by phospholamban in genetically engineered mice. Am J Physiol. 1999 Jun;276(6 Pt 2):H2245–50. doi: 10.1152/ajpheart.1999.276.6.H2245. [DOI] [PubMed] [Google Scholar]
- 21.Teucher N, Prestle J, Seidler T, Currie S, Elliott EB, Reynolds DF, et al. Excessive sarcoplasmic/endoplasmic reticulum Ca2+-ATPase expression causes increased sarcoplasmic reticulum Ca2+ uptake but decreases myocyte shortening. Circulation. 2004 Dec 7;110(23):3553–9. doi: 10.1161/01.CIR.0000145161.48545.B3. [DOI] [PubMed] [Google Scholar]
- 22.Hiranandani N, Raman S, Kalyanasundaram A, Periasamy M, Janssen PML. Frequency-dependent contractile strength in mice over- and under-expressing the sarcoplasmic reticulum calcium ATPase. Am J Physiol Regul Integr Comp Physiol. 2007 Jan 25; doi: 10.1152/ajpregu.00508.2006. in press. [DOI] [PubMed] [Google Scholar]
- 23.Arai M, Otsu K, MacLennan DH, Alpert NR, Periasamy M. Effect of thyroid hormone on the expression of mRNA encoding sarcoplasmic reticulum proteins. Circ Res. 1991 Aug;69(2):266–76. doi: 10.1161/01.res.69.2.266. [DOI] [PubMed] [Google Scholar]
- 24.Hasenfuss G, Reinecke H, Studer R, Pieske B, Meyer M, Drexler H, et al. Calcium cycling proteins and force-frequency relationship in heart failure. Basic Res Cardiol. 1996;91(Suppl 2):17–22. doi: 10.1007/BF00795357. [DOI] [PubMed] [Google Scholar]
- 25.Belke DD, Dillmann WH. Altered cardiac calcium handling in diabetes. Curr Hypertens Rep. 2004 Dec;6(6):424–9. doi: 10.1007/s11906-004-0035-3. [DOI] [PubMed] [Google Scholar]
- 26.Periasamy M, Huke S. SERCA pump level is a critical determinant of Ca(2+)homeostasis and cardiac contractility. J Mol Cell Cardiol. 2001 Jun;33(6):1053–63. doi: 10.1006/jmcc.2001.1366. [DOI] [PubMed] [Google Scholar]
- 27.Frank KF, Bolck B, Brixius K, Kranias EG, Schwinger RH. Modulation of SERCA: implications for the failing human heart. Basic Res Cardiol. 2002;97(Suppl 1):172–8. doi: 10.1007/s003950200033. [DOI] [PubMed] [Google Scholar]
- 28.Baker DL, Hashimoto K, Grupp IL, Ji Y, Reed T, Loukianov E, et al. Targeted overexpression of the sarcoplasmic reticulum Ca2+-ATPase increases cardiac contractility in transgenic mouse hearts. Circ Res. 1998;83(12):1205–14. doi: 10.1161/01.res.83.12.1205. [DOI] [PubMed] [Google Scholar]
- 29.Ji Y, Loukianov E, Loukianova T, Jones LR, Periasamy M. SERCA1a can functionally substitute for SERCA2a in the heart. Am J Physiol. 1999;276(1 Pt 2):H89–97. doi: 10.1152/ajpheart.1999.276.1.H89. [DOI] [PubMed] [Google Scholar]
- 30.Ji Y, Lalli MJ, Babu GJ, Xu Y, Kirkpatrick DL, Liu LH, et al. Disruption of a single copy of the SERCA2 gene results in altered Ca2+ homeostasis and cardiomyocyte function. J Biol Chem. 2000 Dec 1;275(48):38073–80. doi: 10.1074/jbc.M004804200. [DOI] [PubMed] [Google Scholar]
- 31.Giordano FJ, He H, McDonough P, Meyer M, Sayen MR, Dillmann WH. Adenovirus-mediated gene transfer reconstitutes depressed sarcoplasmic reticulum Ca2+-ATPase levels and shortens prolonged cardiac myocyte Ca2+ transients. Circulation. 1997;96(2):400–3. doi: 10.1161/01.cir.96.2.400. [DOI] [PubMed] [Google Scholar]
- 32.Hajjar RJ, Kang JX, Gwathmey JK, Rosenzweig A. Physiological effects of adenoviral gene transfer of sarcoplasmic reticulum calcium ATPase in isolated rat myocytes. Circulation. 1997;95(2):423–9. doi: 10.1161/01.cir.95.2.423. [DOI] [PubMed] [Google Scholar]
- 33.Hajjar RJ, Schmidt U, Matsui T, Guerrero JL, Lee KH, Gwathmey JK, et al. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci U S A. 1998;95(9):5251–6. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.del Monte F, Harding SE, Schmidt U, Matsui T, Kang ZB, Dec GW, et al. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a [see comments] Circulation. 1999;100(23):2308–11. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huke S, Liu LH, Biniakiewicz D, Abraham WT, Periasamy M. Altered force-frequency response in non-failing hearts with decreased SERCA pump-level. Cardiovasc Res. 2003 Sep 1;59(3):668–77. doi: 10.1016/s0008-6363(03)00436-x. [DOI] [PubMed] [Google Scholar]
- 36.Bassani RA, Bassani JW, Bers DM. Relaxation in ferret ventricular myocytes: role of the sarcolemmal Ca ATPase. Pflugers Arch. 1995 Aug;430(4):573–8. doi: 10.1007/BF00373894. [DOI] [PubMed] [Google Scholar]
- 37.Hussain M, Drago GA, Colyer J, Orchard CH. Rate-dependent abbreviation of Ca2+ transient in rat heart is independent of phospholamban phosphorylation. Am J Physiol. 1997 Aug;273(2 Pt 2):H695–706. doi: 10.1152/ajpheart.1997.273.2.H695. [DOI] [PubMed] [Google Scholar]
- 38.Kassiri Z, Myers R, Kaprielian R, Banijamali HS, Backx PH. Rate-dependent changes of twitch force duration in rat cardiac trabeculae: a property of the contractile system. J Physiol (Lond) 2000;524(Pt 1):221–31. doi: 10.1111/j.1469-7793.2000.t01-3-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Chu G, Kranias EG, Bers DM. Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. Am J Physiol. 1998 Apr;274(4 Pt 2):H1335–47. doi: 10.1152/ajpheart.1998.274.4.H1335. [DOI] [PubMed] [Google Scholar]
- 40.Picht E, DeSantiago J, Huke S, Kaetzel MA, Dedman JR, Bers DM. CaMKII inhibition targeted to the sarcoplasmic reticulum inhibits frequency-dependent acceleration of relaxation and Ca2+ current facilitation. J Mol Cell Cardiol. 2007 Jan;42(1):196–205. doi: 10.1016/j.yjmcc.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asahi M, McKenna E, Kurzydlowski K, Tada M, MacLennan DH. Physical interactions between phospholamban and sarco(endo)plasmic reticulum Ca2+-ATPases are dissociated by elevated Ca2+, but not by phospholamban phosphorylation, vanadate, or thapsigargin, and are enhanced by ATP. J Biol Chem. 2000 May 19;275(20):15034–8. doi: 10.1074/jbc.275.20.15034. [DOI] [PubMed] [Google Scholar]
- 42.Huke S, Bers DM. Temporal dissociation of frequency-dependent acceleration of relaxation and protein phosphorylation by CaMKII. J Mol Cell Cardiol. 2007 Mar;42(3):590–9. doi: 10.1016/j.yjmcc.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez EK, Hunter WC, Royce MJ, Leppo MK, Douglas AS, Weisman HF. A method to reconstruct myocardial sarcomere lengths and orientations at transmural sites in beating canine hearts. Am J Physiol. 1992;263(1 Pt 2):H293–306. doi: 10.1152/ajpheart.1992.263.1.H293. [DOI] [PubMed] [Google Scholar]
- 44.Kentish JC, ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ Res. 1986;58(6):755–68. doi: 10.1161/01.res.58.6.755. [DOI] [PubMed] [Google Scholar]
- 45.Edman KA. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol (Lond) 1979;291(7):143–59. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ter Keurs HE, de Tombe PP. Determinants of velocity of sarcomere shortening in mammalian myocardium. Adv Exp Med Biol. 1993;332:649–64. doi: 10.1007/978-1-4615-2872-2_58. [DOI] [PubMed] [Google Scholar]
- 47.Janssen PML, Hunter WC. Force, not sarcomere length, correlates with prolongation of isosarcometric contraction. Am J Physiol Heart Circ Physiol. 1995;269(2 Pt 2):H676–85. doi: 10.1152/ajpheart.1995.269.2.H676. [DOI] [PubMed] [Google Scholar]
- 48.Varian KD, Janssen PML. Frequency Dependent Acceleration of Relaxation Involves Decreased Myofilament Calcium Sensitivity. Am J Physiol Heart Circ Physiol. 2007 Jan 5;292:H2212–H9. doi: 10.1152/ajpheart.00778.2006. [DOI] [PubMed] [Google Scholar]
- 49.Varian KD, Raman S, Janssen PML. Measurement of myofilament calcium sensitivity at physiological temperature in intact cardiac trabeculae. Am J Physiol Heart Circ Physiol. 2006;290:H2092–H7. doi: 10.1152/ajpheart.01241.2005. [DOI] [PubMed] [Google Scholar]
- 50.Bilchick KC, Duncan JG, Ravi R, Takimoto E, Champion HC, Gao WD, et al. Heart failure-associated alterations in troponin I phosphorylation impair ventricular relaxation-afterload and force-frequency responses and systolic function. Am J Physiol Heart Circ Physiol. 2007 Jan;292(1):H318–25. doi: 10.1152/ajpheart.00283.2006. [DOI] [PubMed] [Google Scholar]
- 51.Koch-Weser J, Blinks JR. The Influence of the Interval between Beats on Myocardial Contractility Physical Factors in the Analysis of the Actions of Drugs on Myocardial Contractility. Pharmacol Rev. 1963;15:601–52. [PubMed] [Google Scholar]
- 52.Bers DM. Ca influx and sarcoplasmic reticulum Ca release in cardiac muscle activation during postrest recovery. Am J Physiol. 1985 Mar;248(3 Pt 2):H366–81. doi: 10.1152/ajpheart.1985.248.3.H366. [DOI] [PubMed] [Google Scholar]
- 53.Pieske B, Sutterlin M, Schmidt-Schweda S, Minami K, Meyer M, Olschewski M, et al. Diminished post-rest potentiation of contractile force in human dilated cardiomyopathy. Functional evidence for alterations in intracellular Ca2+ handling. J Clin Invest. 1996;98(3):764–76. doi: 10.1172/JCI118849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mulieri LA, Hasenfuss G, Leavitt B, Allen PD, Alpert NR. Altered myocardial force-frequency relation in human heart failure. Circulation. 1992;85(5):1743–50. doi: 10.1161/01.cir.85.5.1743. [DOI] [PubMed] [Google Scholar]
- 55.Burkhoff D, Yue DT, Franz MR, Hunter WC, Sunagawa K, Maughan WL, et al. Quantitative comparison of the force-interval relationships of the canine right and left ventricles. Circ Res. 1984;54(4):468–73. doi: 10.1161/01.res.54.4.468. [DOI] [PubMed] [Google Scholar]
- 56.Hoit BD, Kadambi VJ, Tramuta DA, Ball N, Kranias EG, Walsh RA. Influence of sarcoplasmic reticulum calcium loading on mechanical and relaxation restitution. Am J Physiol Heart Circ Physiol. 2000 Mar;278(3):H958–63. doi: 10.1152/ajpheart.2000.278.3.H958. [DOI] [PubMed] [Google Scholar]
- 57.Hasenfuss G, Holubarsch C, Hermann HP, Astheimer K, Pieske B, Just H. Influence of the force-frequency relationship on haemodynamics and left ventricular function in patients with non-failing hearts and in patients with dilated cardiomyopathy. Eur Heart J. 1994;15(2):164–70. doi: 10.1093/oxfordjournals.eurheartj.a060471. [DOI] [PubMed] [Google Scholar]
- 58.Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, et al. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ Res. 1994;75(3):434–42. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]