Abstract
Background
Stress is an important factor in the development and maintenance of anxiety disorders. Stress also potentiates anxiety-like response in animals, but empirical evidence for a similar effect in humans is still lacking.
Methods
To test whether stress increases anxiety in humans, we examined the ability of a social stressor (speech and a counting task) to potentiate the facilitation of startle in the dark. Measures of subjective distress and of hypothalamic-pituitary-adrenal axis and autonomic nervous system activity (e.g., salivary cortisol, alpha-amylase, blood pressure and hear rate) were also taken to confirm the effectiveness of the stress manipulation.
Results
Startle was significantly facilitated in the dark. This effect was potentiated by prior exposure to the social stressor. The social stressor induced increases in salivary cortisol and alpha amylase, as well as increases in blood pressure, heart rate, and subjective distress.
Conclusion
The findings indicate that stress potentiates anxiety. Animal studies suggest that such an effect may be mediated by glucocorticoid effects on corticotropin-releasing hormone in limbic structures.
Keywords: Fear-potentiated startle, anxiety, stress, cortisol
Introduction
Despite abundant evidence of a role of stress in mood and anxiety disorders (1; 2), the underlying mechanisms remain elusive. Preclinical studies provide potential insight into such mechanisms. In animals, stress exacerbates or sensitized subsequent anxiety-like responses in a number of anxiety models involving severe or chronic the stressor (3-6), but sensitized anxiety can be found even immediately after a single acute stressor (6-8).
Despite the wealth of preclinical data on the stress sensitization of anxiety, empirical evidence for a similar effect in humans is lacking. Stress facilitates fear conditioning (9) and eyeblink conditioning (10) in humans, like is does in animals (11; 12), but this facilitation is mediated by a stress-induced effect on associative learning mechanisms (9; 13) rather than on fear/anxiety. Given the relevance of stress-sensitization of anxiety to psychopathology, our main objective was to examine whether stress increases unconditioned fear in humans.
The startle reflex is a sensitive tool to evaluate anxiety-like responses; it is potentiated by aversive events in humans and animals (14). Darkness increases the startle reflex in humans, an effect attributed to anxiety rather than attention (15), suggesting that darkness is unconditionally aversive. We have suggested that the facilitation of startle in the dark (FSD) in humans, a diurnal species, is equivalent to the “light-enhanced startle” in rats (16), a nocturnal species naturally afraid of brightly illuminated environments (15). In the rat, light-enhanced startle is mediated by corticotropin releasing hormone (CRH) receptors in the bed nucleus of the stria terminalis (BNST) (17; 18). Because glucocorticoid potentiation of CRH at extra-hypothalamic sites may be responsible for the stress-induced sensitization of anxiety (19), we hypothesized that FSD would be facilitated by a prior social stressor, which activates hypothalamic-pituitary-adrenal (HPA) activity (20). We also measured salivary cortisol, alpha-amylase, and autonomic reactivity to investigate the potential link between stress-related increase in FSD and autonomic nervous system (ANS) and HPA activation.
Methods and Materials
Participants were 20 medically and psychiatrically healthy volunteers (9 males) ages 28.1 years (SD = 8.3 years) who gave written informed consent.
The FSD was investigated in two sessions a week apart, one after a social stressor (stress) and the other after no stressor (control) in a between-subject design counterbalanced across subjects. The 10-min social stressor consisted of delivering a speech followed by a backward counting task (see Fig. 1 for details). The FSD test started twenty-five minutes after the end of the social stressor and consisted three alternating 60-sec blocks of startle stimuli delivered under lighted conditions or in complete darkness, counterbalanced across subjects. There were three startle stimuli per block, two of a high intensity and one of a low intensity.
Figure 1.
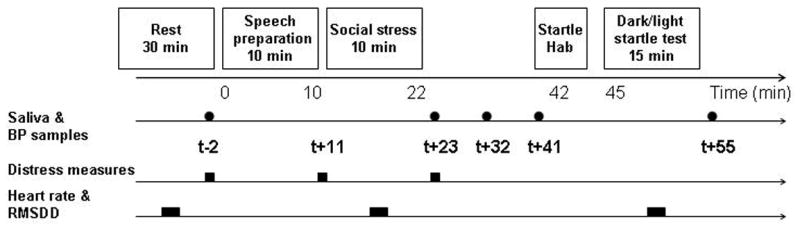
Schematic representation of the procedure. Following a 10-min preparation, participants in the social stressor condition gave an 8-min unstructured speech on abortion after which they counted backwardly from 1000 in decrements of 13 for 2 min in front of a male and a female “judge” in white lab coats (total duration of social stressor = 10 min). A video camera relayed the speaker's image to a TV screen that the speaker could see while talking. In the control condition, participants rested for about 20 min. The startle test was initiated twenty minutes after the end of the social stressor. It started with six habituation startle stimuli, immediately followed by the FSD test. The FSD test started with an additional six startle stimuli (under lighted conditions) followed by three alternating 60-sec blocks of startle stimuli delivered under lighted conditions or in complete darkness, counterbalanced across subjects. The saliva samples were collected and BP was measured at the five following time points; prior to speech preparation (t -2 min), immediately after and 9 min after the social stressor (t +23 min and t + 32 min), before startle habituation (t +41 min), and after the dark/light startle test (t + 55 min). In addition, subjects were asked to indicate their level of distress on a Likert scale ranging from 1 (not at all distressed) to 10 (extremely distressed) prior to the speech preparation (t -2 min), just before (t +11 min) and after (t +23 min) the social stressor.
The saliva samples and the blood pressure (BP) were taken at the five time points specified in Fig 1. Subjective distress was measured at two time points. Heart rate (HR) was averaged within three 5-min periods, at baseline, during the stress challenge, and during the FSD test.
The startle stimuli were 40-ms duration high (103 dB(A)) or low (96 dB(A)) intensity white noise presented through headphones. The eyeblink reflex was recorded with electrodes placed under the left eye. Amplifier bandwidth was set to 30-500 Hz. HR was monitored with two electrodes placed on each side of the chest. Blood pressure was measured by an automatic BP measurement device (Dinamap, Critikon, USA). Saliva samples were collected with the use of plain cotton Salivettes (Sarstedt, Leicester, UK) (see the Appendix for details).
Peak magnitude of the blink reflex was determined in the 20-120-ms time frame following stimulus onset and were averaged within light and dark conditions. Because preliminary analyses indicated no difference between stress or illumination conditions between startle intensities and because there was no order effect for the stress/no stress condition, startle intensity and condition order were not considered in the statistical analysis. The amylase data were square root transformed to reach normality. The data were analyzed with analyses of variance (ANOVAs) with repeated measures. Greenhouse–Geisser epsilon corrections were implemented when appropriate.
Pearson's correlations were calculated in the stress condition to examine correlations 1) between FSD (percent change from light to dark) and neuroendocrine measures (difference between baseline and stress levels of salivary cortisol, alpha-amylase, systolic and diastolic BP, and HR) and 2) within neuroendocrine measures.
Results
Startle was facilitated by darkness and this facilitation increased after stress (Fig. 2). A Stress Condition (2) × Illumination (2) × Sex (2) ANOVA revealed an Illumination main effect (F(1,18)=15.9, p<.0009) and an Stress Condition × Illumination interaction (F(1,18)=6.4, p<.02). Follow up tests showed significant facilitation of startle in the dark in the control (F(1,19)=6.8, p<.02) and stress condition (F(1,19)=18.5, p<.0009).
Figure 2.
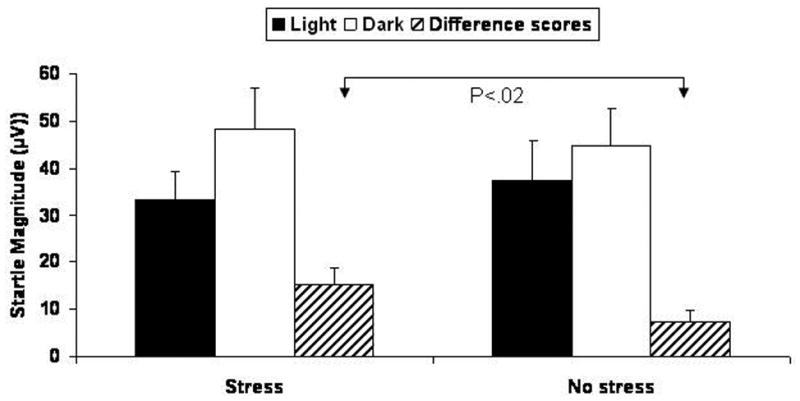
Startle magnitude and facilitation of startle in the dark (difference scores) during light and dark conditions following the stressor or no stressor.
The autonomic, endocrine, cardiovascular and subjective responses confirmed that the social stressor generated a stress response (Table 1). There was an increase in cortisol (time +32, +41, +55) and a sharp increase in alpha amylase (time 2). These data were analyzed with Stress Condition (2) × Time (5) × Sex (2) ANOVAs. Cortisol levels increased after the speech (Condition × Time quadratic trend;(F(1,18)=4.7, p<.04), showing a trend for higher cortisol in the stress condition at t +32, F(1,19)=3.7, p<.07), and, t +41 (F(1,19)=3.6, p<.07). For alpha amylase, there was a Condition × Time interaction (F(4,72)=7.5, p<.0009, epsilon=1) due to significantly elevated alpha amylase at t +23 after the social stressor (F(1,18)=14.5, p<.0009).
Table 1.
Mean (SEM) salivary cortisol concentration (μg/dL) and alpha-amylase activity (U/ml)), systolic and diastolic blood pressure (BP; mm Hg), heart rate (beats per minute), and distress scores at predetermined time points before and after the social stressor or the control condition.
| Measures | Conditions | Baseline∧ | T +11 min
After speech preparation |
T +16 min
During stress |
T +23 min
Immediately after stress |
T +32 min
10 min after stress |
T +41 min
Before startle habituation |
T +51 min
During dark/light startle test |
|---|---|---|---|---|---|---|---|---|
| Cortisol | No stress | .20 (.03) | - | - | .18 (.03) | .15 (.02) | .13 (.02) | - |
| Stress | .22 (.03) | .21 (.02) | .22 (.03)# | .18 (.02)# | ||||
|
| ||||||||
| Alpha-amylase | No stress | 8.2 (.8) | - | - | 8.3 (.9) | 8.0 (.8) | 7.9 (.8) | - |
| Stress | 8.0 (.7) | 11.6 (1.0)* | 8.3 (.6) | 8.1 (.7) | ||||
|
| ||||||||
| Systolic BP | No stress | 112.7 (3.6) | - | - | 108.9 (3.7) | 111.6 (3.1) | 110.6 (3.4) | - |
| Stress | 113.8 (3.9) | 136.2 (3.1)* | 116.8 (4.5) | 114.2 (4.4) | ||||
|
| ||||||||
| Diastolic BP | No stress | 68.6 (2.1) | - | - | 69.0 (2.2) | 68.9 (2.5) | 69.9 (1.9) | - |
| Stress | 68.0 (2.4) | 86.6 (2.1)* | 70.5 (2.2) | 69.4 (2.3) | ||||
|
| ||||||||
| Distress | No stress | 1.3 (.3) | 1.4 (.3) | - | 1.4 (.3) | - | - | - |
| Stress | 1.3 (.3) | 4.0 (.5)* | 3.7 (.5)* | |||||
|
| ||||||||
| Heart rate | No stress | 73.7 (2.8) | - | 72.8 (2.8) | - | - | - | 68.1 (2.4) |
| Stress | 74.3 (2) | 93 (3.1)* | 71.8 (1.8) | |||||
p<.07
p<.001
Baseline taken at T-2 min for cortisol, alpha-amylase, BP, distress, and at T-10 min for heart rate
There was a strong cardiovascular reactivity to the stressor (Table 1). The BP data were analyzed using the same ANOVA as the cortisol and alpha amylase data. The HR data were analyzed with a Condition (2) × Time (3) × Sex (2) ANOVA. For systolic and diastolic BP and for HR, there was a significant Condition × Time interaction (all p<.0009) due to increased systolic and diastolic BP after the social stressor (t+23) and increased HR during the social stressor (t+16; all p<.0009).
Subjects felt substantially distressed during the social stressor (Table 1). A Condition (2) × Time (2) × Sex (2) ANOVA revealed a significant Condition × Time interaction (F(2,36)=14.4, p<.0009, epsilon=1) due to greater distress at t +11, (F(1,19)=17.0, p<.001), and t +23, (F(1,19)=21.1, p<.0009). None of the stress reactivity measures differed between males and females.
No significant correlations were found between the potentiation of startle in the dark and neuroendocrine activation during stress (Table 2). Extensive correlations were observed between measures of neuroendocrine activation themselves. In particular, cortisol levels correlated positively with measures of ANS activation, including alpha amylase, and alpha amylase correlated with other measures of ANS activation.
Table 2.
Pearson correlations calculated in the stress condition A) between facilitation of startle in the dark (percent change from light to dark) and measures of neuroendocrine activation (difference between baseline and stress levels of salivary cortisol, alpha-amylase, systolic and diastolic blood pressure, heart rate) and B) within neuroendocrine measures.
| Cortisol | Alpha-amylase | Systolic
BP |
Diastolic BP | Heart rate | ||
|---|---|---|---|---|---|---|
| A | Startle | - 0.22 | - 0.18 | - 0.09 | - 0.02 | - 0.14 |
| B | Cortisol | . | 0.48 * | 0.57 * | 0.44 | 0.66 ** |
| Alpha-amylase | . | . | 0.47 * | 0.23 | 0.53 * | |
| Systolic BP | . | . | . | 0.62 ** | 0.44 | |
| Diastolic BP | . | . | . | . | 0.08 | |
| Heart rate | . | . | . | . | . |
p < 0.05.
p < 0.01.
Discussion
To our knowledge this is the first report showing that unconditioned anxiety is enhanced by prior stress. Consistent with animal data (7; 8), anxiety as measured with FSD was sensitized in humans exposed to a social stressor.
The light-enhanced startle effect in the rat is mediated by CRH in the BNST (18), suggesting that the effect of changes in background illumination on startle (i.e., FSD) is also mediated by CRH acting on receptors in the BNST. Sensitized FSD by stress in humans may ultimately rely on an enhancement of CRH effects in the BNST. Indeed, CRH antagonists can abolish sensitized anxiety in rodents (3).
What are the potential mechanisms for the sensitization of CRH effects? Prime candidates are glucocorticoids. Evidence for the role of glucocorticoids comes from two sources; 1) the stress-sensitization of anxiety in rats is believed to depend on glucocorticoids (3) and 2) glucocorticoids can potentiate fear via feed-forward regulation of CRH by glucocorticoids in the amygdala and in the BNST (19). Indeed, corticosterone (the principal glucocorticoid in rats), despite its well-known inhibitory effects on subsequent release of hypothalamic CRH, also has excitatory effects on CRH at extra-hypothalamic sites (21), including the BNST (22).
An alternative possibility is the involvement of the stress-sensitive noradrenergic input (23) into the BNST (24). Acute stress increases noradrenaline in the lateral BNST (25; 26), possibly via stimulation of CRH in the BNST (3; 27). In the present study, noradrenergic activity may have promoted alertness and arousal following the social stressor, leading to sensitized FSD via its action on CRH in the BNST.
Our stressor activated two major stress systems, the HPA axis and the ANS. The stress-induced increase in cortisol significantly correlated with increases in HR, systolic BP and salivary alpha-amylase, indicating a coordinated activation of both HPA axis and sympathetic ANS. The absence of correlation between cortisol and the magnitude of FSD may be due to the fact that salivary cortisol does not reflect accurately cortisol in the BNST or may reflect our inability to determine the cortisol response to the stressor because of individual differences in baseline cortisol caused by anticipatory anxiety.
The use of an experimental model and a measure of anxiety derived from animal research provide us with a fairly good understanding of the mechanisms underlying FSD. Whether glucocorticoids mediate the stress-induced sensitization of FSD is speculative, but it is a testable hypothesis. The availability of glucocorticoid receptor antagonists such as mifepristone will help test the role of glucocorticoids in mediating or modulating these responses.
Supplementary Material
Acknowledgments
This study was supported by the Intramural Research Program of the National Institutes of Mental Health.
Footnotes
Financial disclosures: The authors report no conflicts of interest, financial or otherwise, arising from this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Millon T, Davis RD. Developmental pathogenesis. In: Millon T, Davis RD, editors. Oxford Textbook of Psychopathology. New York: Oxford University Press; 1999. pp. 29–48. [Google Scholar]
- 2.Young EA, Abelson JL, Curtis GC, Nesse RM. Childhood adversity and vulnerability to mood and anxiety disorders. Depress Anxiety. 1997;5:66–72. [PubMed] [Google Scholar]
- 3.Korte SM, De Boer SF. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 2003;463:163–175. doi: 10.1016/s0014-2999(03)01279-2. [DOI] [PubMed] [Google Scholar]
- 4.McGregor IS, Dielenberg RA. Differential anxiolytic efficacy of a benzodiazepine on first versus second exposure to a predatory odor in rats. Psychopharmacology (Berl) 1999;147:174–181. doi: 10.1007/s002130051158. [DOI] [PubMed] [Google Scholar]
- 5.Adamec RE, Blundell J, Collins A. Neural plasticity and stress induced changes in defense in the rat. Neurosci Biobehav Rev. 2001;25:721–744. doi: 10.1016/s0149-7634(01)00053-7. [DOI] [PubMed] [Google Scholar]
- 6.MacNeil G, Sela Y, McIntosh J, Zacharko RM. Anxiogenic Behavior in the Light-Dark Paradigm Following Intraventricular Administration of Cholecystokinin-8S, Restraint Stress, or Uncontrollable Footshock in the CD-1 Mouse. Pharmacology Biochemistry and Behavior. 1997;58:737–746. doi: 10.1016/s0091-3057(97)00037-3. [DOI] [PubMed] [Google Scholar]
- 7.Zangrossi Hl, File SE. Behavioral consequences in animal tests of anxiety and exploration of exposure to cat odor. Brain Research Bulletin. 1992;29:381–388. doi: 10.1016/0361-9230(92)90072-6. [DOI] [PubMed] [Google Scholar]
- 8.Korte MS. Fear-potentiation in the elevated plus-maze test depends on stressor controllability and fear conditioning. Stress. 1999;3:27–40. doi: 10.3109/10253899909001110. [DOI] [PubMed] [Google Scholar]
- 9.Jackson ED, Payne JD, Nadel L, Jacobs WJ. Stress differentially modulates fear conditioning in healthy men and women. Biological psychiatry. 2006;59:516–522. doi: 10.1016/j.biopsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Duncko R, Cornwell B, Cui L, Merikangas KR, Grillon C. Acute exposure to stress improves performance in trace eyeblink conditioning and spatial learning tasks in healthy men: role of the HPA axis and sympathetic nervous system. Learning & Memory. 2007;14:329–335. doi: 10.1101/lm.483807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conrad CD, Magarinos AM, LeDoux JE, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behavioral Neuroscience. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 12.Shors T, Weiss C, Thompson R. Stress-induced facilitation of classical conditioning. Science. 1992;257:537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- 13.Shors TJ. Learning during stressful times. Learn Mem. 2004;11:137–144. doi: 10.1101/lm.66604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grillon C, Baas JM. A review of the modulation of startle by affective states and its application to psychiatry. Clinical Neurophysiology. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 15.Grillon C, Pellowski M, Merikangas KR, Davis M. Darkness facilitates the acoustic startle in humans. Biological Psychiatry. 1997;42:453–460. doi: 10.1016/S0006-3223(96)00466-0. [DOI] [PubMed] [Google Scholar]
- 16.Walker DL, Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biological psychiatry. 1997;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- 17.Walker D, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. Journal of Neuroscience. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jongh R, Groenink L, van der Gugten J, Olivier B. Light-enhanced and fear-potentiated startle: temporal characteristics and effects of alpha-helical corticotropin-releasing hormone. Biol Psychiatry. 2003;54:1041–1048. doi: 10.1016/s0006-3223(03)00468-2. [DOI] [PubMed] [Google Scholar]
- 19.Schulkin J, Morgan MA, Rosen JB. A neuroendocrine mechanism for sustaining fear. Trends in neurosciences. 2005;28:629–635. doi: 10.1016/j.tins.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 21.Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. J Comp Neurol. 1989;285:413–435. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- 22.Makino S, Gold PW, Schulkin J. Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain research. 1994;657:141–149. doi: 10.1016/0006-8993(94)90961-x. [DOI] [PubMed] [Google Scholar]
- 23.Svensson TH. Peripheral, autonomic regulation of locus coeruleus noradrenergic neurons in brain: putative implications for psychiatry and psychopharmacology. Psychopharmacology (Berl) 1987;92:1–7. doi: 10.1007/BF00215471. [DOI] [PubMed] [Google Scholar]
- 24.Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- 25.Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 26.Kilts CD, Anderson CM. The simultaneous quantification of dopamine, norepinephrine and epinephrine in micropunched rat brain nuclei by on-line trace enrichment HPLC with electrochemical detection distribution of catecholamines in the limbic system. Neurochem Int. 1986;9:437–445. doi: 10.1016/0197-0186(86)90086-0. [DOI] [PubMed] [Google Scholar]
- 27.Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Research Reviews. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


